The objectives of this study were to assess the neuropsychological performance (NP) of adolescents and young adults with congenital heart disease (CHD), comparing them with a group of healthy controls, to determine whether there are different neurocognitive phenotypes in CHD, and to identify their relation to sociodemographic, neonatal, clinical and psychological adjustment variables.
MethodsA total of 217 CHD patients (116 male, aged 15.73±2.68 years) and 80 controls (35 male, age 16.76±2.22 years) underwent an extensive neuropsychological assessment and analysis of psychological adjustment.
ResultsCHD patients had significantly poorer NP than healthy controls in all neurocognitive domains. Three different phenotypes of NP in CHD patients were identified: non-impaired (NI), moderately impaired (MI) and globally impaired (GI). They differed in all dimensions of NP. The GI cluster showed fewer years of schooling (p=0.025) and lower neonatal indicators such as head circumference (p=0.019), 1-min Apgar score (p=0.006), birth weight (p=0.05) and length (p=0.034) than the NI cluster. In the MI and GI clusters, there were more cyanotic forms of disease, including tetralogy of Fallot and transposition of the great arteries. The GI cluster presented more difficulties with psychological adjustment, including social (p=0.038), attention (p=0.001) and aggressive (p=0.003) problems.
ConclusionsCHD patients have poorer NP than controls. NP in the CHD group can be classified in three clusters that reflect different levels of neuropsychological functioning, which is sensitive to social, neonatal and psychological adjustment variables.
Os objetivos deste estudo foram avaliar o desempenho neuropsicológico (DN) de adolescentes e jovens adultos com Cardiopatia Congénita (CC), comparando-os com um grupo de controlos saudáveis e determinar se existem diferentes fenótipos neurocognitivos na CC, tentando identificar a sua relação com variáveis demográficas, neonatais, clínicas e de ajustamento psicológico.
Métodos217 pacientes com CC (116 de sexo masculino, 15,73 anos ± 2,68) e 80 controlos (35 de sexo masculino, 16,76 anos ± 2,22) foram submetidos a uma avaliação neuropsicológica extensa e a uma avaliação de ajustamento psicológico.
ResultadosOs pacientes com CC apresentaram um DN significativamente pior do que os controlos saudáveis em todos os domínios neurocognitivos. Identificamos três fenótipos: não afetado (NA), moderadamente afetado (MA) e globalmente afetado (GA), diferentes em todas as dimensões de DN. O grupo GA apresentava menos anos de escolaridade (p = 0,025) e piores indicadores neonatais, como perímetro cefálico (p = 0,019), Apgar 1 (p = 0,006), peso ao nascer (p = 0,05) e comprimento (p = 0,034) quando comparado com o cluster NA. Nos grupos MA e GA, havia mais formas cianóticas de doença, incluindo Tetralogia de Fallot e Transposição das Grandes Artérias. O cluster GA apresentava mais problemas de ajustamento psicológico, problemas sociais (p = 0,038), de atenção (p = 0,001) e comportamentos agressivos (p = 0,003).
ConclusõesOs pacientes com CC têm pior DN do que os controlos. O DN nos doentes com CC pode ser classificado em três clusters que refletem diferentes níveis de funcionamento neuropsicológico, sensível às variáveis neonatais e de ajustamento psicológico.
Advances in pediatric cardiac care have resulted in an increasing number of adults with congenital heart disease (CHD) being followed in tertiary care centers. This has generated interest in adult CHD as a new subspecialty of cardiology; there are now more adults than children in the world population affected with CHD.1,2 The increasing longevity of this population has led to increases in lifelong medical, psychosocial and behavioral problems, raising concerns about these patients’ well-being and perceived quality of life.3,4 Many of them also have deficits in neurocognitive development that lead to greater difficulty in adapting to educational and occupational tasks.
There are many well-known reasons for neurocognitive impairment in CHD, all related to hypoxia. Recent studies indicate that cerebral damage may occur during fetal life in certain forms of severe CHD related to reduced delivery of nutrients and oxygen, responsible for cerebral insult, mostly during the third trimester of pregnancy, due to abnormal cerebral circulation resulting from altered flow patterns in the aortic arch, and others have studied the parameters of cardiac and circulatory dysfunction that influence fetal brain development.5–8
In 2012, after reviewing the literature on surveillance, screening, evaluation, and management strategies for children with CHD, the American Heart Association published a scientific statement9 which made recommendations to optimize neurodevelopmental outcomes in the pediatric CHD population.
It is therefore important to study the neurocognitive performance of this patient population, as well as their psychosocial and emotional status and educational and occupational achievements.
The main objectives of this study were to assess the neuropsychological performance of adolescents and young adults with CHD, comparing them with a group of healthy controls, to determine whether there are different neurocognitive phenotypes in CHD through the establishment of clusters of cognitive function, and to identify their relation to sociodemographic, neonatal, clinical and psychological adjustment variables.
MethodsStudy populationThe participants were recruited consecutively at the outpatient clinics of the departments of pediatric cardiology and adult cardiology of a tertiary hospital. Only patients who were between 12 and 30 years old at the time of the interview, who had a basic educational level that enabled them to understand and complete the written questionnaires, and for whom complete medical records were available, were included. Of all patients approached, only nine refused to participate. Given the requirement for complete information on neurocognitive variables and neonatal markers of fetal development, only 217 of the 335 patients enrolled in the study completed the entire protocol, of whom 116 were male, 101 female, 82 cyanotic and 135 acyanotic, ranging in age between 12 and 30 years (mean 15.73±2.68 years), with mean schooling of 9.41±2.14 years, and with diverse CHD diagnoses. With regard to parents’ educational level, fathers had completed a mean of 10.13±3.70 years schooling and mothers had completed a mean of 10.54±3.64 years.
One relative of each patient was also invited to participate in the study and 190 accepted.
The control group consisted of 80 healthy age- and gender-matched youngsters (35 male, 45 female) recruited from various schools and universities in the Porto area. Their ages ranged between 12 and 30 years (mean 16.76 ± 2.22 years) and mean schooling was 10.13±2.32 years. Fathers had completed a mean of 9.66±3.45 years schooling and mothers had completed a mean of 9.45±3.28 years. Subjects with any diagnosis of chronic illness were excluded.
The CHD and control groups were compared to ensure that the groups were matched in the main demographic variables (age, gender, years of schooling and parents’ schooling). No statistically significant differences were found between the groups (age: p=0.278; schooling: p=0.06; gender: p=0.06; father's schooling: p=0.726; mother's schooling: p=0.08). Table 1 details the demographic characteristics of CHD patients and controls.
Comparison of demographic variables between congenital heart disease patients and control groups.
| Controls (n=80) | CHD patients (n=217) | p | |
|---|---|---|---|
| Age, yearsa (mean ± SD) | 16.76±2.22 | 15.73±2.68 | 0.492 |
| Gender (F/M) | 45/35 | 101/116 | 0.344 |
| Schooling, yearsb (mean ± SD) | 10.13±2.32 | 9.41±2.14 | 0.260 |
| Father's schooling, yearsc (mean ± SD) | 9.66±3.45 | 10.13±3.70 | 0.151 |
| Mother's schooling, yearsd (mean ± SD) | 9.45±3.28 | 10.54±3.64 | 0.116 |
CHD: congenital heart disease; F: female; M: male; max: maximum; min: minimum; SD: standard deviation.
Cyanotic and acyanotic patients were also compared regarding parents’ schooling. No statistical differences were found between the groups (father's schooling: p=0.648; mother's schooling: p=0.551). Table 2 details the demographic characteristics of cyanotic and acyanotic patients.
Comparison of demographic variables between cyanotic and acyanotic patients.
| Cyanotic patients | Acyanotic patients | p | |
|---|---|---|---|
| Age, yearsa (mean ± SD) | 16.11/2.80 | 15.50/2.58 | 0.106 |
| Gender (F/M) | 35/47 | 66/69 | 0.377 |
| Schooling, yearsb (mean ± SD) | 9.52/2.29 | 9.35/2.05 | 0.558 |
| Father's schooling, yearsc (mean ± SD) | 10.31/3.73 | 10.04/3.69 | 0.648 |
| Mother's schooling, yearsd (mean ± SD) | 10.77/3.90 | 10.41/3.50 | 0.551 |
CHD: congenital heart disease; F: female; M: male; max: maximum; min: minimum; SD: standard deviation.
All the assessment measures were obtained on a single occasion. Clinical data were collected retrospectively using each patient's clinical record, with assistance from hospital medical staff.
Prospective participants were contacted before or after scheduled hospital appointments. Subjects were asked to participate after being fully informed of the objectives and procedures of the investigation. Patients (or parents of those aged under 18 years) who agreed completed an informed consent form approved by the hospital's ethics committee which followed international conventions guaranteeing the patients’ rights. The interview and assessment were completed on the same day as the patient's scheduled hospital appointment.
Instruments for neuropsychological assessmentIn order to assess the performance in different neurocognitive functions, all participants underwent a neuropsychological assessment that included clinical measures of memory, executive function, processing speed, attention and visuoconstructive ability.10–14 These tests were selected to cover the major precognitive functions that may have been compromised in CHD.15,16 The whole protocol took 30-45 min to complete.
All 217 participants underwent the test battery. Table 3 shows detailed information on the neuropsychological tests included in each of the neurocognitive domains, as well as on the individual functions measured.
Neurocognitive domains and abilities measured and assessment instruments used.
| Neurocognitive domain | Ability | Neuropsychological task |
|---|---|---|
| Memory | Verbal | Wechsler logical memory10 |
| Visuoconstructive | Rey complex figure (memory reproduction)11 | |
| Processing speed | Speed of execution | Wechsler coding10 |
| Processing speed | Stroop words and colors13 | |
| Attention | Auditory and verbal immediate attention | Wechsler digits forward10 |
| Attention, visual scanning | Trail Making Test part A14 | |
| Executive function | Effective planning | Key Search Test12 |
| Response inhibition | Stroop interference13 | |
| Divided attention and cognitive flexibility | Trail Making Test part B14 | |
| Working memory | Wechsler digits backward10 | |
| Visuoconstructive | Visuoconstructive ability | Rey Complex Figure (copy)11 |
The raw scores for all neuropsychological tests were converted to adjusted z-scores using the means and standard deviations of the sample, in order to place the tests and the neurocognitive domains on a common metric. Neurocognitive domains were calculated by the mean adjusted z-scores of tests within each domain.
Assessment of neonatal parametersWeight, length and head circumference at birth were assessed, as these biometric parameters are considered good indices of fetal brain development.17–20 The 1- and 5-min Apgar scores were also analyzed.
Clinical, psychosocial and sociodemographic assessmentPersonal and demographic data were collected from each patient, including marital status, educational level and occupation, as well as all relevant information from their medical history, including diagnosis, severity and category of heart disease, surgical interventions, pharmacological therapy and presence of residual lesions. Clinical data were collected retrospectively using each patient's medical record, with assistance from hospital medical staff. A semi-structured interview and the self-report and observational questionnaires of the Achenbach System of Empirically Based Assessment (ASEBA) to assess psychosocial adjustment (Adult Self-Report Form for patients aged 18-59 years, Youth Self-Report Form for patients aged 11-18 years, Adult Behaviour Checklist and Child Behaviour Checklist) were applied.21–25
The participants underwent a semi-structured interview covering social support, family educational style, environment, self-image, functional limitations, educational background (e.g. educational achievements and retentions), and emotional adjustment.
Statistical analysisThe statistical analysis was carried out using IBM SPSS Statistics for Windows, version 21 (IBM SPSS, Chicago, IL, USA). To obtain an overall performance index on the neuropsychological tests, the z-scores for each participant in each test were summed (inverting the Trail Making Test part A and part B data, for which a higher score reveals poorer performance, unlike the other tests). The control and CHD group z-scores were compared by the Student's t test. Cluster analysis (k-means) was also carried out to identify groups of subjects based on the adjusted z-scores in each neurocognitive domain. The number of clusters was determined based on the determination coefficients (r2) and number of participants in each cluster. Cluster analysis is considered a gold standard procedure for exploring data when the sample is not homogeneous.26,27
Once the neurocognitive clusters were obtained, the performance of participants in the three clusters was compared to controls. Performance on neurocognitive domains, sociodemographic characteristics (age and schooling), and neonatal and psychosocial variables were compared between clusters using one-way analysis of variance with post-hoc comparisons (Scheffé’s test). The clusters were compared in terms of gender and clinical diagnosis using the chi-square test.
Results were considered significant with p<0.05.
ResultsStatistically significant differences were found between patients with CHD and the control group of healthy participants in all areas of neurocognitive performance, with better results for the latter. Table 4 shows the results of neuropsychological testing and comparisons between controls and CHD patients.
Comparison of neurocognitive performance between congenital heart disease patients and controls.
| Neurocognitive domain | Controls | CHD patients | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Memory | 0.40 | 0.7 | −0.13 | 0.83 | <0.001 |
| Processing speed | 0.45 | 0.67 | −0.18 | 0.84 | <0.001 |
| Attention | 0.41 | 0.53 | −0.16 | 0.81 | <0.001 |
| Executive function | 0.24 | 0.63 | −0.09 | 0.43 | <0.001 |
| Visuoconstructive | 0.53 | 0.34 | −0.19 | 1.11 | <0.001 |
| Global cognitive function | 0.42 | 0.43 | −0.16 | 0.62 | <0.001 |
CHD: congenital heart disease; SD: standard deviation.
Solutions of two (r2=0.368), three (r2=0.508) and four (r2=0.575) clusters were tested. The three-cluster solution was considered optimal since the determination coefficient (r2) was only slightly lower than in the four-cluster solution, while the latter had a small number of participants (n=9) in one of the clusters. Therefore, the three-cluster analysis provided groups of CHD patients that could be analyzed with suitable sample sizes for further statistical procedures.
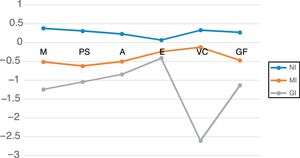
Table 5 shows the mean neurocognitive performance for the three clusters. Their neurocognitive profiles are represented in Figure 1. Univariate effects were significant for all neurocognitive domains: memory (F=76.61; p<0.001); processing speed (F=65.12; p<0.001); attention (F=44.12; p<0.001); executive function (F=21.9; p<0.001); visuoconstructive ability (F=240.56; p<0.001); and global cognitive function (F=159.08; p<0.001).
Descriptive statistics, analysis of variance and post-hoc comparisons between neurocognitive clusters.
| Neurocognitive domain | NI | MI | GI | F | p | NI vs. MI | NI vs. GI | MI vs. GI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Memory | 0.38 | 0.59 | -0.51 | 0.61 | -1.24 | 0.6 | 76.61 | <0.001 | <0.001 | <0.001 | <0.001 |
| Processing speed | 0.31 | 0.61 | -0.62 | 0.62 | -1.04 | 0.89 | 65.12 | <0.001 | <0.001 | <0.001 | 0.044 |
| Attention | 0.23 | 0.64 | -0.5 | 0.63 | -0.84 | 1.02 | 44.12 | <0.001 | <0.001 | <0.001 | 0.145 |
| Executive function | 0.07 | 0.4 | -0.24 | 0.37 | -0.41 | 0.4 | 21.9 | <0.001 | <0.001 | <0.001 | 0.457 |
| Visuoconstructive | 0.33 | 0.48 | -0.12 | 0.6 | -2.6 | 1 | 240.55 | <0.001 | <0.001 | <0.001 | <0.001 |
| Global cognitive function | 0.27 | 0.32 | -0.47 | 0.31 | -1.13 | 0.67 | 159.08 | <0.001 | <0.001 | <0.001 | <0.001 |
GI: globally impaired cluster; MI: moderately impaired cluster; NI: non-impaired cluster; SD: standard deviation.
Mean performance of the three clusters in different neurocognitive domains. A: attention; EF: executive function; GF: global cognitive function; GI: globally impaired cluster; M: memory; MI: moderately impaired cluster; NI: non-impaired cluster; PS: processing speed; VC: visuoconstructive.
The NI cluster (non-impaired) included 114 patients. Their neurocognitive performance was significantly superior to that of the moderately impaired (MI) and globally impaired (GI) clusters. No significant differences were found between this patient group and controls across the neurocognitive domains: memory (p=0.997); processing speed (p=0.557); attention (p=0.306); executive function (p=0.099); visuoconstructive ability (p=0.1); and global cognitive function (p=0.063).
The MI cluster included 76 patients. Compared to the NI cluster, significantly poorer results were found in all cognitive domains and in global cognitive function.
The GI cluster included 27 patients. Significant differences were found in comparison to the MI cluster, with poorer performance in memory, processing speed, visuoconstructive ability and global cognitive function. There are no significant differences in attention or executive function.
Cluster characteristicsTables 6–8 show the characteristics of patients in the three clusters in terms of sociodemographic, neonatal, clinical and psychosocial parameters.
Characteristics of the clusters in terms of sociodemographic and neonatal variables.
| NI | MI | GI | F | p | NI vs. MI | NI vs. GI | MI vs. GI | |
|---|---|---|---|---|---|---|---|---|
| Age, years | 15.97±2.6 | 15.53±3.05 | 15.3±2.1 | 1.05 | 0.353 | 0.530 | 0.498 | 0.929 |
| Gender (male/female) | 60/54 | 42/34 | 14/13 | 0.924 | ||||
| Schooling, years | 9.89±1.95 | 8.96±2.23 | 8.67±2.25 | 6.54 | 0.002 | 0.011 | 0.025 | 0.821 |
| Head circumference, cm | 34.1±1.88 | 33.74±1.58 | 32.61±2.48 | 5.27 | 0.006 | 0.707 | 0.019 | 0.087 |
| 1-min Apgar score | 8.01±1.33 | 8.39±1.22 | 7.6±2.52 | 2.36 | 0.048 | 0.546 | 0.006 | 0.065 |
| 5-min Apgar score | 9.62±0.83 | 9.58±0.8 | 9.35±1.09 | 0.82 | 0.443 | 0.950 | 0.547 | 0.592 |
| Birth weight, kg | 3.27±0.88 | 3.09±0.54 | 2.83±0.78 | 3.22 | 0.042 | 0.392 | 0.05 | 0.355 |
| Birth length, cm | 48.35±2.8 | 48.8±2.83 | 46.16±6.35 | 4.77 | 0.01 | 0.738 | 0.034 | 0.011 |
GI: globally impaired cluster; MI: moderately impaired cluster; NI: non-impaired cluster.
Values presented are means and standard deviations except for gender.
Characteristics of the clusters in terms of diagnosis and type of congenital heart disease.
| NI | MI | GI | Chi-square | p | ||
|---|---|---|---|---|---|---|
| Clinical diagnosis | 10.73 | 0.030 | ||||
| TOF | n | 16 | 21 | 7 | ||
| AR | -2.4 | 2.0 | 0.8 | |||
| TGA | n | 13 | 15 | 5 | ||
| AR | -1.6 | 1.4 | 0.5 | |||
| Other | n | 85 | 40 | 15 | ||
| AR | 3.3 | -2.7 | -1.0 | |||
| Type of CHD | 13.47 | 0.001 | ||||
| Cyanotic | n | 30 | 38 | 14 | ||
| AR | -3.7 | 2.7 | 1.6 | |||
| Acyanotic | n | 84 | 38 | 13 | ||
| AR | 3.7 | -2.7 | -1.6 | |||
AR: adjusted residual; CHD: congenital heart disease; GI: globally impaired cluster; MI: moderately impaired cluster; NI: non-impaired cluster; TGA: transposition of the great arteries; TOF: tetralogy of Fallot.
Values presented are number of participants.
Characteristics of the clusters in terms of psychosocial adjustment.
| F | p | Clusters | |||
|---|---|---|---|---|---|
| NI vs. MI | NI vs. GI | MI vs. GI | |||
| Parent-report measures | |||||
| Isolation | 1.2 | 0.30 | |||
| Somatic complaints | 0.5 | 0.60 | |||
| Anxiety/depression | 4.0 | 0.02 | 0.75 | 0.02 | 0.08 |
| Social problems | 3.4 | 0.04 | 0.63 | 0.04 | 0.21 |
| Altered thoughts | 0.6 | 0.50 | |||
| Attention problems | 9.3 | <0.01 | 0.10 | <0.01 | 0.03 |
| Delinquent behavior | 0.9 | 0.40 | |||
| Aggressive behavior | 1.4 | 0.30 | |||
| Internalization | 1.8 | 0.17 | |||
| Externalization | 3.3 | 0.09 | |||
| Self-report measures | |||||
| Isolation | 0.5 | 0.60 | |||
| Somatic complaints | 0.3 | 0.70 | |||
| Anxiety/depression | 0.5 | 0.60 | |||
| Social problems | 1.2 | 0.30 | |||
| Altered thoughts | 0.07 | 0.90 | |||
| Attention problems | 3.7 | 0.02 | 0.85 | 0.03 | 0.08 |
| Delinquent behavior | 0.4 | 0.70 | |||
| Aggressive behavior | 5.9 | 0.00 | 0.19 | 0.00 | 0.13 |
| Internalization | 1.4 | 0.26 | |||
| Externalization | 1.4 | 0.25 | |||
GI: globally impaired cluster; MI: moderately impaired cluster; NI: non-impaired cluster.
The clusters did not differ according to age and gender. Patients in the NI cluster had more years of schooling than those in the GI cluster.
Patients in the NI cluster presented higher 1-min Apgar scores and greater head circumference, birth weight and birth length than in the GI cluster. The MI cluster presented greater birth length than the GI cluster.
In the NI cluster there were fewer cases of tetralogy of Fallot and more acyanotic forms of CHD.
Patients in the GI cluster presented more anxiety and depression and more social and attention problems than the NI cluster and had more attention problems in parent report measures than the MI cluster. Patients in the GI cluster also self-reported more attention problems and aggressive behavior than patients in both NI and MI clusters (Table 8).
DiscussionThe aims of this study were to assess the neuropsychological performance of adolescents and young adults with CHD, comparing them with a group of healthy controls, to determine whether there are different neurocognitive phenotypes in CHD, and to identify their relation to sociodemographic, neonatal, clinical and psychological adjustment variables.
Our study demonstrated that CHD patients have consistently poorer neurocognitive performance than healthy controls, confirming data from several other published reports.28–32 There is growing evidence that, although they score within the average range on intelligence testing, CHD patients present poor performance in various neurocognitive abilities, with a consistent pattern of sequelae in gross and fine motor skills, attention, visuospatial ability, speech and language, executive function, social cognition and impulsive behavior. This pattern was confirmed in our study.
A major strength of our study was that it enabled us to identify three neurocognitive phenotypes (non-impaired, moderately impaired and globally impaired) in our patients and the features associated with each. The MI cluster, compared to the NI cluster, presented deficiencies in memory, processing speed, attention and executive function, while the GI cluster showed striking deficiencies in memory, processing speed, executive function and visuoconstructive ability compared to the MI cluster. The NI cluster had larger head circumference and higher birth weight than the other two clusters, which is consistent with magnetic resonance imaging (MRI) findings in fetuses and infants. They also presented higher 1- and 5-min Apgar scores. As is to be expected, participants in the NI and MI clusters had more years of schooling than those in the GI cluster.
Interestingly, compared to the NI and MI clusters, the GI phenotype presented more aggressive behaviors on self-report measures, and their caregivers reported higher rates of attention and social problems on observational measures.
Furthermore, we found that patients with more severe impairment in neurocognitive function (MI and GI clusters) had a higher proportion of cyanotic forms of disease than the NI cluster. Our findings are also consistent with other published data showing that although all children with CHD are at increased risk for developmental disorders or delay, cyanotic heart lesions are associated with increased vulnerability for these conditions.9,30,33,34
Recent studies35,36 focusing on neurological sequelae in adult survivors of CHD reveal that severe forms of disease are associated with significant deficits in several cognitive domains, including attention, processing speed, and executive function. However, cognitive function in moderate and mild forms did not differ significantly from normal. These findings are consistent with data in our study showing that severe forms of disease, such as tetralogy of Fallot and transposition of the great arteries, are more frequent among patients with more severe cognitive impairment.
Many authors report that birth weight and head circumference are good predictors of neurodevelopment in children with severe CHD.37,38 Consistently, we found that head circumference, weight and length measured at birth and 1-min Apgar score in our patients were able to differentiate between the NI, MI and GI clusters, associating with different neurocognitive abilities in later life. This finding highlights the impact of the antenatal impairment in brain growth and development that occurs in CHD fetuses.
In previous studies, several mechanisms found in CHD implicated in poor neurocognitive development have been investigated, and their relative contributions analyzed, some arising in fetal life, others occurring after birth and especially during or after surgery (anoxia and perioperative ischemia). MRI scans and Doppler studies in fetuses have shown that some forms of CHD lead to derangements of fetal blood flow and decreased oxygen and nutrient delivery, resulting in impaired brain development and increased likelihood of sequelae.37–39 Data from a review38 of the mechanisms involved in the delay and impairment of brain maturation in complex CHD, unique to each form of illness, are consistent with the smaller head circumference found in neonates with complex CHD compared to normal babies.
Our findings are important because they may contribute to a new generation of studies focusing on better understanding of the ongoing processes of poor neurocognitive development and injury, that could enable decisions on neuroprotective strategies, early detection and rehabilitation from injury and disability.
In summary, our study analyzed various parameters in CHD patients, and generally confirmed findings of previously published reports. However, it is the first to examine so many different clinical, neurocognitive and psychosocial variables together, and this, we suggest, constitutes the originality and social importance of our research.
Our study also has limitations. Firstly, the number of participants was small for some of the comparisons between groups, and we were thus unable to draw robust conclusions. This resulted from the lack of data on some variables, such as fetal and neonatal measures, for some participants. Furthermore, the study's retrospective design, with all the measurements obtained in a single session, may have biased patient recruitment.
Another limitation of the study is that it does not assess the neurocognitive profile of babies born with low birth weight who do not have CHD, which would shed further light on the nature of such disabilities.
This study is of clinical value, helping to predict which children are at increased risk for neurocognitive disabilities and therefore to plan prevention and neuroprotective strategies. To our knowledge, it is the first study to provide data on all these variables in a Latin population.
ConclusionsIn conclusion, we gathered information on the impact of various clinical and psychosocial variables in CHD, providing evidence that is useful for prevention, neuroprotection and rehabilitation.
In our opinion, future research should compare CHD patients with low birth weight children who do not have CHD, and those with intrauterine growth restriction resulting in low birth weight, to improve understanding of the mechanisms that give rise to impaired neurocognitive development.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was presented in part at the Annual Meeting of the American Heart Association, November 2015, Orlando, FL, USA.