There have been no prospective randomized trials that enable the best strategy and timing to be determined for revascularization in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) and multivessel coronary artery disease (CAD).
ObjectivesTo compare short- and long-term adverse events following multivessel vs. culprit-only revascularization in patients with NSTE-ACS and multivessel CAD.
MethodsThis was a retrospective observational study that included all patients diagnosed with NSTE-ACS and multivessel CAD who underwent percutaneous coronary intervention (PCI) between January 2010 and June 2013 (n=232). After exclusion of patients with previous coronary artery bypass grafting (n=30), a multivessel revascularization strategy was adopted in 35.1% of patients (n=71); in the others (n=131, 64.9%), only the culprit artery was revascularized. After propensity score matching (PSM), two groups of 66 patients were obtained, matched according to revascularization strategy.
ResultsDuring follow-up (1543±545 days), after PSM, patients undergoing multivessel revascularization had lower rates of reinfarction (4.5% vs. 16.7%; log-rank p=0.018), unplanned revascularization (6.1% vs. 16.7%; log-rank p=0.048), unplanned PCI (3.0% vs. 13.6%; log-rank p=0.023) and the combined endpoint of death, reinfarction and unplanned revascularization (16.7 vs. 31.8%; log-rank p=0.046).
ConclusionsIn real-world patients presenting with NSTE-ACS and multivessel CAD, a multivessel revascularization strategy was associated with lower rates of reinfarction, unplanned revascularization and unplanned PCI, as well as a reduction in the combined endpoint of death, reinfarction and unplanned revascularization.
Atualmente, não existem estudos prospetivos aleatorizados que permitam definir a estratégia de revascularização ideal quanto ao tipo e timing da sua realização, em doentes com síndrome coronária aguda (SCA) sem supradesnivelamento do segmento ST (SCAsSST) e doença coronária multivaso (DCMV).
ObjetivosComparar os eventos adversos a curto e a longo prazo da revascularização multivaso versus revascularização apenas da artéria culprit em doentes com SCAsSST e DCMV.
MétodosEste estudo observacional retrospetivo incluiu todos os doentes com SCAsSST e DCMV submetidos a intervenção coronária percutânea (ICP), entre janeiro de 2010 e junho de 2013 (n=232). Após exclusão dos doentes com história de cirurgia de revascularização miocárdica (n=30), a estratégia de revascularização multivaso foi adotada em 35,1% (n=71) dos doentes; nos restantes 64,9% (n=131) procedeu-se à revascularização apenas da artéria culprit. Após propensity score matching (PSM), obtiveram-se dois grupos de 66 doentes, emparelhados de acordo com a estratégia de revascularização.
ResultadosDurante o seguimento (1543±545dias), após PSM, os pacientes submetidos a revascularização multivaso tiveram menores taxas de reenfarte (4,5 versus 16,7%; log-rank p=0,018), revascularização não planeada (RVNP; 6,1 versus 16,7%; log-rank p=0,048), ICP não planeada (3,0 versus 13,6%; log-rank p=0,023) e do endpoint combinado de morte, reenfarte e RVNP (16,7 versus 31,8%; log-rank p=0,046).
ConclusãoNesta população de pacientes do mundo real com SCAsSST e DCMV, a revascularização multivaso associou-se a menores taxas de reenfarte, RVNP e ICP não planeada, bem como a uma redução do endpoint combinado de morte, reenfarte e RVNP.
The prevalence of significant multivessel coronary artery disease (CAD) in patients with non-ST-segment elevation acute coronary syndrome (ACS) (NSTE-ACS) has been reported as around 50%.1 According to current guidelines, patients with NSTE-ACS and multivessel CAD should preferably undergo complete revascularization, although the consensus that incomplete revascularization has a negative prognostic impact is only based on observational studies.2–4 In patients with complex coronary anatomy, complete revascularization, whether percutaneous or surgical, may carry excessive risk and the patient's age, comorbidities and general clinical status should therefore be taken into consideration.
There have been no prospective randomized trials that enable the best strategy (multivessel vs. culprit-only, complete vs. incomplete) and timing (one-stage vs. multi-staged) to be determined for revascularization in patients with NSTE-ACS and multivessel CAD.2 Although it is not possible to make a direct association, recently published randomized clinical trials have shown a beneficial effect of complete revascularization compared to culprit-only revascularization in patients with ST-elevation acute coronary syndrome (STE-ACS) and multivessel CAD,5–8 which suggests that this strategy should at least be assessed prospectively in patients with NSTE-ACS.
The main objective of this study was to compare prognosis and short- and long-term adverse events following multivessel vs. culprit-only revascularization before and after propensity score matching (PSM) in a real-world population of patients with NSTE-ACS and multivessel CAD.
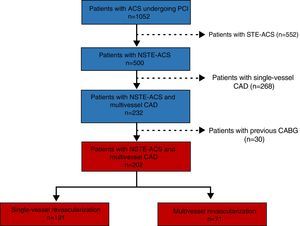
MethodsThis was a retrospective, longitudinal and observational study that included all patients (n=1052) with a diagnosis of ACS who underwent percutaneous coronary intervention (PCI) in the catheterization laboratory of Braga Hospital and who were registered in the Portuguese Registry of Acute Coronary Syndromes between January 2010 and June 2013, enabling a clinical follow-up of at least three years. Patients’ clinical presentation was divided more or less evenly between NSTE-ACS (n=500, 47.5%) and STE-ACS (n=552, 52.5%). Of the patients with NSTE-ACS, about 94% (n=470) presented with non-ST-segment elevation myocardial infarction (MI), and the other 6% (n=30) presented with unstable angina. Among patients with NSTE-ACS, the prevalence of multivessel CAD was 46.4% (n=232); the other 53.6% (n=268) had single-vessel disease.
Study population and follow-upIn order to analyze the impact of multivessel vs. culprit-only or single-vessel revascularization, patients with multivessel CAD and a previous history of coronary artery bypass grafting (CABG) (n=30) were excluded due to their excessive anatomical complexity (Figure 1). The study population consisted of 202 patients with NSTE-ACS and multivessel CAD, of whom 35.1% (n=71) underwent multivessel revascularization (70 by PCI and only one by CABG) and 64.9% (n=131) underwent culprit-only revascularization (all by PCI). The decision to perform non-culprit revascularization and its timing were determined by the interventional cardiologist and the clinical cardiologist, or by the heart team when appropriate.
PCI was performed according to the guidelines of the European Society of Cardiology and the clinical practice of the interventional clinicians and included administration of low molecular weight or unfractionated heparin, use of glycoprotein IIb/IIIa inhibitors and, in a few cases, aspiration thrombectomy. Antiplatelet therapy included aspirin and a P2Y12 inhibitor (clopidogrel in most cases).
Information regarding the revascularization strategy was collected, including the type (multivessel vs. single-vessel; complete vs. incomplete), ischemia testing, the reasons for deciding whether to perform multivessel revascularization, the timing of revascularization, and arteries revascularized or to be revascularized.
Demographic, clinical, laboratory, echocardiographic and angiographic data were collected and entered into an electronic database. This information was obtained from patients’ medical records, using medical information management software (Glintt®, SimmaCardio®, SClínico® and PDS®). Data on short-term (in-hospital mortality, residual or new-onset angina, reinfarction, second degree or higher atrioventricular block, stroke, in-stent thrombosis, and mechanical complications) and long-term (mortality, reinfarction, unplanned revascularization, unplanned PCI, stroke and heart failure) prognosis and adverse events were also obtained and flagged.
Follow-up was conducted by reviewing patients’ medical records, using the above software. All medical assessments and hospital records were reviewed. The mean follow-up was 1542±554 days, median 1520 days and interquartile range 704 days.
DefinitionsThe diagnosis of NSTE-ACS was made in accordance with the current guidelines.2 Multivessel CAD was defined as the presence of at least two lesions deemed angiographically significant (≥50% stenosis of the intraluminal diameter) in different coronary artery territories. The culprit artery was defined by the interventional cardiologist according to a combination of clinical, electrocardiographic, echocardiographic and angiographic data. Culprit-only or single-vessel revascularization was defined as an intervention on the culprit artery only, while multivessel revascularization was defined as intervention (percutaneous or surgical) on two or more lesions in different coronary artery territories, during the initial procedure (first stage) or planned in the next 30 days (second stage). Heart failure during follow-up was defined by the presence of dyspnea on exertion corresponding to New York Heart Association (NYHA) functional class II or higher9 or hospital admission due to heart failure. The Killip classification and the Global Registry of Acute Coronary Events (GRACE) and Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) risk scores were used, according to the definitions described in the literature.10–13 Creatinine clearance was calculated by the Cockcroft-Gault formula. Major adverse cardiovascular events (MACE) were defined as mortality, MI, heart failure and stroke.
Statistical analysisThe statistical analysis was performed using IBM SPSS® version 23.0. A p-value ≤0.05 was considered to indicate statistically significant differences.
Frequencies and respective percentages were calculated for categorical variables. The chi-square test was used, continuity correction factors being reported in 2×2 contingency tables, and the Pearson's chi-square was used for contingency tables larger than 2×2. Fisher's exact test was used whenever the proportion of cells in the table with expected frequency less than five was higher than 20%.14
For continuous variables, medians and standard deviation were calculated using a parametric test and medians and interquartile range were calculated with a non-parametric test. In order to compare continuous variables between the two groups (multivessel vs. culprit-only revascularization), the following tests were performed: the t test for independent samples (parametric) and the Mann-Whitney test (non-parametric), respectively, depending on whether or not the dependent variable showed a normal distribution.
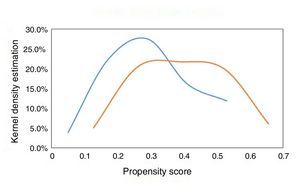
Given that this was a non-randomized, observational, single-center study, and considering the multiple factors that can influence the choice of revascularization type in patients with NSTE-ACS and multivessel CAD, it was decided that PSM should be performed to match the study populations (patients undergoing multivessel vs. culprit-only revascularization) and to reduce bias caused by confounding variables that could have influenced treatment decisions and clinical outcomes. The propensity score was used to assess the likelihood of each individual being selected for a revascularization strategy according to their baseline characteristics. This was followed by propensity score matching, a statistical technique that matches the characteristics of the groups according to defined variables, thus enabling the effect of one variable to be analyzed, in this case revascularization strategy (multivessel vs. single-vessel). For this case, the nearest neighbor matching without replacement technique was used, setting the optimal standard deviation as 0.03. The analysis was performed using binary logistic regression, with revascularization strategy (multivessel vs. culprit artery) as the dependent variable and the following explanatory variables: age, gender, body mass index, diabetes, history of MI, previous PCI, left ventricular ejection fraction, peak troponin I, creatinine clearance (using the Cockcroft-Gault formula), maximum Killip class, minimum hemoglobin, vascular access for coronary angiography, and significant left main, left anterior descending, circumflex, and right coronary artery disease. The degree of overlap of the propensity score between the two groups was high, as shown in Figure 2. Two groups of 66 patients matched for decision on revascularization type (multivessel vs. single-vessel) were thereby obtained. The predictive capacity of the model used to generate the propensity score was 0.69, with adequate calibration (Hosmer-Lemeshow p=0.81).
In the matched cohort, Kaplan-Meier curves were obtained comparing the groups with the log-rank test to analyze clinical event-free survival during follow-up (death, reinfarction, unplanned revascularization, unplanned PCI and MACE).
ResultsBaseline population characteristicsThe study population consisted of 202 patients with NSTE-ACS and multivessel CAD. Mean age was 65.3±12.5 years and 77.2% were male. Regarding cardiovascular risk factors, 34.7% had diabetes, 71.3% hypertension, 59.4% dyslipidemia, 23.3% were current smokers and 22.3% former smokers. With regard to history, 16.3% reported a previous history of MI, 20.3% angina and 12.4% PCI.
Patients who underwent multivessel revascularization tended to be younger than those who underwent culprit-only revascularization (64.1±12.5 vs. 66.0±12.4 years), with no statistically significant difference (p=0.639) (Table 1). In addition, those who underwent multivessel revascularization tended to have a higher body mass index than those undergoing culprit-only revascularization (28.9±3.9 vs. 27.7±4.2 kg/m2), at the threshold of statistical significance (p=0.051) and with a small effect.
Demographic, clinical, laboratory, echocardiographic and angiographic characteristics of the study groups before and after matching.
| Characteristics | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Multivessel RV (n=71) | Culprit-only RV (n=131) | p | Multivessel RV (n=66) | Culprit-only RV (n=66) | p | |
| Demographic | ||||||
| Age, years | 64.1±12.5 | 66.0±12.4 | 0.304 | 64.6±12.3 | 65.6±12.5 | 0.639 |
| Male | 78.9% (56) | 76.3% (100) | 0.814 | 77.3% (51) | 72.7% (48) | 0.668 |
| BMI, kg/m2 | 28.9±3.9 | 27.7±4.2 | 0.051 | 28.6±3.82 | 28.8±4.50 | 0.725 |
| Clinical | ||||||
| CV risk factors | ||||||
| Diabetes | 36.6% (26) | 33.6% (44) | 0.781 | 36.4% (24) | 36.4 (24) | 1.000 |
| Hypertension | 74.6% (53) | 69.5% (91) | 0.539 | 74.2% (49) | 75.8% (50) | 1.000 |
| Dyslipidemia | 56.3% (40) | 61.1% (80) | 0.615 | 57.6% (38) | 60.6% (40) | 0.859 |
| Smoking | 19.7% (14) | 25.2% (33) | 0.481 | 18.2% (12) | 22.7% (15) | 0.666 |
| History | ||||||
| CRF | 7.0% (5) | 6.1% (8) | 0.772a | 6.1% (4) | 7.6% (5) | 1.000a |
| MI | 11.3% (8) | 19.1% (25) | 0.217 | 12.1% (8) | 12.1% (8) | 1.000 |
| Angina | 19.7% (14) | 20.6% (27) | 1.000 | 19.7% (13) | 21.2% (14) | 1.000 |
| Stroke/TIA | 5.6% (4) | 7.6% (10) | 0.774a | 4.5% (3) | 9.1% (6) | 0.492a |
| PAD | 2.8% (2) | 3.8% (5) | 1.000a | 1.5% (1) | 3.0% (2) | 1.000a |
| Carotid disease | 0% (0) | 2.3% (3) | 0.553a | 0% (0) | 4.5% (3) | 0.244a |
| PCI | 11.3% (8) | 13.0% (17) | 0.898 | 10.6% (7) | 12.1% (8) | 1.000 |
| Previous medication | ||||||
| Aspirin | 32.4% (23) | 29.0% (38) | 0.734 | 33.3% (22) | 28.8% (19) | 0.707 |
| ACEI/ARB | 52.1% (37) | 51.1% (67) | 1.000 | 54.5% (36) | 42.4% (28) | 0.223 |
| Beta-blocker | 23.9% (17) | 27.5% (36) | 0.705 | 24.2% (16) | 25.8% (17) | 1.000 |
| Statin | 36.6% (26) | 43.5% (57) | 0.423 | 37.9% (25) | 42.4% (28) | 0.723 |
| Diuretic | 22.5% (16) | 22.1% (29) | 1.000 | 22.7% (15) | 24.2% (16) | 1.000 |
| Max. Killip class | 0.751 | 0.496 | ||||
| I | 73.2% (52) | 74.0% (97) | 74.2% (49) | 66.7% (44) | ||
| II | 18.3% (13) | 19.8% (26) | 16.7% (11) | 25.8% (17) | ||
| III | 7.0% (5) | 3.8% (5) | 7.6% (5) | 4.5% (3) | ||
| IV | 1.4% (1) | 2.3% (3) | 1.5% (1) | 3.0% (2) | ||
| CRUSADE score | 25.9±18.4 | 28.4±18.7 | 0.547 | 25.5±18.1 | 27.2±19.0 | 0.713 |
| GRACE score | 127±40.8 | 134±44.8 | 0.247 | 128±41.3 | 134±45.2 | 0.446 |
| Laboratory | ||||||
| Peak TnI, ng/ml | 4.88 (22.65) | 6.56 (15.46) | 0.571 | 5.32 (21.90) | 6.56 (10.96) | 0.915 |
| Peak Cr, mg/dl | 0.90 (0.40) | 0.90 (0.40) | 0.882 | 0.90 (0.40) | 0.90 (0.40) | 0.579 |
| ClCr, ml/min | 93.7±38.6 | 86.3±36.2 | 0.181 | 91.6±37.5 | 90.8±37.2 | 0.909 |
| proBNP, pg/ml | 691 (1966) | 1088 (2108) | 0.024 | 729 (2029) | 937 (2074) | 0.187 |
| Min. Hb, g/dl | 14.1±1.67 | 13.7±1.66 | 0.183 | 14.1±1.66 | 13.8±1.57 | 0.217 |
| Echocardiographic | ||||||
| LVEF, % | 50.1±9.6 | 48.7±9.3 | 0.293 | 50.0±9.83 | 50.0±9.67 | 0.831 |
| Angiographic | ||||||
| Vascular access | 0.499a | 1.000a | ||||
| Radial | 97.2% (69) | 93.9% (123) | 97.0% (64) | 95.5% (63) | ||
| Femoral | 2.8% (2) | 6.1% (8) | 3.0% (2) | 4.5% (3) | ||
| Significant CAD | ||||||
| LM | 2.8% (2) | 3.8% (5) | 1.000a | 3.0% (2) | 1.5% (1) | 1.000a |
| LAD | 88.7% (63) | 80.2% (105) | 0.174 | 87.9% (58) | 87.9% (58) | 1.000 |
| Cx | 81.7% (58) | 76.3% (100) | 0.483 | 80.3% (53) | 78.8% (52) | 1.000 |
| RCA | 76.1% (54) | 71.0% (93) | 0.544 | 74.2% (49) | 74.2% (49) | 1.000 |
Fisher's exact test.
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; CAD: coronary artery disease; Cr: creatinine; CrCl: creatinine clearance; CRF: chronic renal failure; CV: cardiovascular; Cx: circumflex artery; Hb: hemoglobin; LAD: left anterior descending; LM: left main; LVEF: left ventricular ejection fraction; Max.: maximum; Min.: minimum; PAD: peripheral arterial disease; proBNP: pro-B-type natriuretic peptide; RCA: right coronary artery; RV: revascularization; TIA: transient ischemic attack; TnI: troponin I.
There were no statistically significant differences between the study groups in terms of clinical characteristics (Table 1).
Regarding laboratory characteristics, there were no significant differences between the groups before matching with the exception of pro-B-type natriuretic peptide levels, which differed significantly between patients undergoing multivessel revascularization and those undergoing culprit-only revascularization (1088 [2108] vs. 691 [1966], p=0.024).
There were also no statistically significant differences between the groups with regard to echocardiographic and angiographic characteristics (Table 1).
No significant differences were found between the groups in any of the above characteristics after matching.
Revascularization strategyAs can be seen in Table 2, multivessel revascularization was most often performed percutaneously during the same procedure (first stage) (66.2%, n=47), while 32.4% of the patients underwent second-stage percutaneous revascularization. Only one patient underwent CABG, although seven were rejected for surgery due to high surgical risk. In 18.3% (n=13) of the cases, the decision to revascularize more than one vessel was based on ischemia testing. Complete revascularization was achieved in 52.1% (n=37) of patients undergoing multivessel revascularization. Among the main reasons for incomplete revascularization were diffuse disease, small vessels or high anatomical complexity (e.g. chronic occlusions). The decision not to perform PCI of non-culprit arteries was influenced by various factors, the most common being the presence of moderate lesions, i.e. with less than 70% stenosis (n=46, 35.1%). The most commonly revascularized arteries in patients undergoing culprit-only revascularization were the circumflex (35.9%, n=47) and anterior descending artery (n=35.1%, n=46); in 35.9% (n=47) of these patients, more than one artery remained to be revascularized (Table 2).
Factors associated with revascularization strategy.
| Unmatched groups | |||
|---|---|---|---|
| Multivessel RV (n=71) | Single-vessel RV (n=131) | p | |
| Type of RV | |||
| Single-vessel PCI | 0% (0) | 100% (131) | |
| 1st-stage multivessel PCI | 66.2% (47) | 0% (0) | |
| 2nd-stage multivessel PCI | 32.4% (23) | 0% (0) | |
| Planned CABG | 1.4% (1) | 0% (0) | |
| Ischemia testing | 0.013 | ||
| Positive | 18.3% (13) | 5.3% (7) | |
| Negative | 1.4% (1) | 2.3% (3) | |
| Not performed | 80.3% (57) | 92.4% (121) | |
| Reason not to perform multivessel revascularization | |||
| Negative ischemia test | 3.8% (5) | ||
| Moderate lesions (<70%) | 35.1% (46) | ||
| Anatomical complexity | 17.6% (23) | ||
| Chronic occlusion | 8.4% (11) | ||
| Age and comorbidities | 2.3% (3) | ||
| More than one of the above | 32.8% (43) | ||
| Reason to perform multivessel revascularization | |||
| Positive ischemia test | 18.3% (13) | ||
| Anatomical | 81.7% (58) | ||
| Complete revascularization | <0.001 | ||
| No | 47.9% (34) | 100% (131) | |
| Yes | 52.1% (37) | 0% (0) | |
| 1st-stage artery revascularized | <0.001 | ||
| LM | 0% (0) | 2.3% (3) | |
| LAD | 9.9% (7) | 35.1% (46) | |
| Cx | 8.5% (6) | 35.9% (47) | |
| RCA | 15.5% (11) | 24.4% (32) | |
| More than one | 66.2% (47) | 0% (0) | |
| 2nd-stage artery revascularized | |||
| LM | 0% (0) | ||
| LAD | 12.7% (9) | ||
| Cx | 7.0% (5) | ||
| RCA | 7.0% (5) | ||
| More than one | 7.0% (5) | ||
| Artery or branch to be revascularized | <0.001 | ||
| LM | 0% (0) | 0% (0) | |
| LAD | 12.7% (9) | 26.2% (34) | |
| Cx | 9.9% (7) | 17.6% (23) | |
| RCA | 14.1% (10) | 21.5% (28) | |
| More than one | 0% (0) | 35.1% (46) | |
| Stent characteristics | |||
| No. of stents per patient | 2.3±0.9 | 1.0±0.4 | <0.001 |
| Total length (mm) | 48±23 | 18±8 | <0.001 |
| % DES | 81.6% | 55.2% | <0.001 |
CABG: coronary artery bypass grafting; Cx: circumflex artery; DES: drug-eluting stents; LAD: left anterior descending; LM: left main; PCI: percutaneous coronary intervention; RCA: right coronary artery; RV: revascularization.
There were no statistically significant differences between the multivessel and culprit-only revascularization groups with regard to in-hospital adverse clinical events before and after PSM, as seen in Table 3.
In-hospital adverse clinical events before and after propensity score matching.
| Events | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Multivessel RV (n=71) | Culprit-only RV (n=131) | p | Multivessel RV (n=66) | Culprit-only RV (n=66) | p | |
| Death | 0% (0) | 1.5% (2) | 0.542a | 0% (0) | 1.5% (1) | 1.000a |
| Anginab | 7.0% (5) | 8.4% (11) | 0.946 | 7.6% (5) | 6.1% (4) | 1.000a |
| Reinfarction | 7.0% (5) | 4.6% (6) | 0.522a | 7.6% (5) | 4.5% (3) | 0.718a |
| ≥2 AV block | 4.2% (3) | 3.1% (4) | 0.698a | 4.5% (3) | 3.0% (2) | 1.000a |
| Stroke | 2.8% (2) | 0% (0) | 0.122a | 3.0% (2) | 0% (0) | 0.496a |
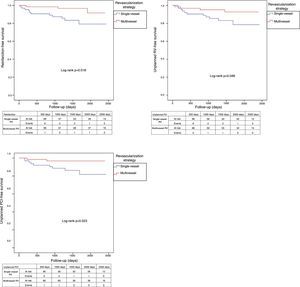
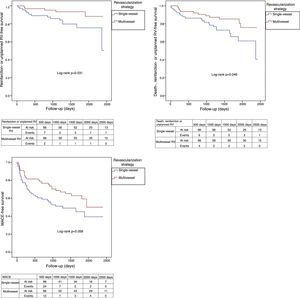
Adverse clinical events during follow-up are shown in Table 4. After PSM, patients undergoing multivessel revascularization had a lower incidence of reinfarction (4.5% vs. 16.7%; log-rank p=0.018; Figure 3), unplanned revascularization (6.1% vs. 16.7%; log-rank p=0.048; Figure 3), unplanned PCI (3.0% vs. 13.6%; log-rank p=0.023; Figure 3), the composite endpoint of reinfarction and unplanned revascularization (7.6% vs. 21.2%; log-rank p=0.031; Figure 4) and the composite endpoint of death, reinfarction and unplanned revascularization (16.7% vs. 31.8%; log-rank p=0.046; Figure 4). The incidence of MACE in these patients was significantly lower before, but not after, matching, although it also tended to be lower after matching (39.4% vs. 53.0%; log-rank p=0.056; Figure 4).
Adverse clinical events during follow-up before and after propensity score matching.
| Events | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Multivessel RV (n=71) | Culprit-only RV (n=131) | Log-rank p | Multivessel RV (n=66) | Culprit-only RV (n=66) | Log-rank p | |
| Death | 11.3% (8) | 16.0% (21) | 0.307 | 12.1% (8) | 18.2% (12) | 0.248 |
| Reinfarction | 5.6% (4) | 16.8% (22) | 0.018 | 4.5% (3) | 16.7% (11) | 0.018 |
| Stroke | 5.6% (4) | 3.8% (5) | 0.619 | 6.1% (4) | 3.0% (2) | 0.495 |
| HF | 29.6% (21) | 34.4% (45) | 0.426 | 30.3% (20) | 31.8% (21) | 0.767 |
| Unplanned RV | 5.6% (4) | 15.3% (20) | 0.040 | 6.1% (4) | 16.7% (11) | 0.048 |
| Unplanned PCI | 2.8% (2) | 12.2% (16) | 0.022 | 3.0% (2) | 13.6% (9) | 0.023 |
| Unplanned CABG | 2.8% (2) | 3.1% (4) | 0.900 | 3.0% (2) | 3.0% (2) | 0.945 |
| Reinfarction or unplanned RV | 8.5% (6) | 19.8% (26) | 0.027 | 7.6% (5) | 21.2% (14) | 0.031 |
| Death, reinfarction or unplanned RV | 16.9% (12) | 31.3% (41) | 0.034 | 16.7% (11) | 31.8% (21) | 0.046 |
| MACE | 39.4% (28) | 53.4% (70) | 0.031 | 39.4% (26) | 53.0% (35) | 0.056 |
CABG: coronary artery bypass grafting; HF: heart failure; MACE: major adverse cardiovascular events (mortality, myocardial infarction, heart failure and stroke); PCI: percutaneous coronary intervention; RV: revascularization.
The prevalence of multivessel CAD in patients with NSTE-ACS in this study was 46.4%. This result is comparable to those of other studies and meta-analyses, in which the prevalence was about 50% for those undergoing angiography.15–20
The present study shows a protective effect of multivessel revascularization compared with culprit-only revascularization with regard to occurrence of long-term adverse clinical events, after adjustment of baseline characteristics by PSM. In this study of real-world patients, significant reductions are reported in reinfarction, unplanned revascularization, unplanned PCI, and the composite endpoints of reinfarction and unplanned revascularization and of death, reinfarction and unplanned revascularization. Other studies21–25 and a meta-analysis26 have also shown a reduction in need for revascularization during follow-up, and in some cases there has also been a reduction in MACE. In a PSM analysis of 1240 patients with ACS and multivessel CAD, Shishehbor et al.27 observed that complete revascularization was associated with a lower rate of the composite endpoint of death, reinfarction or revascularization. However, a recent meta-analysis20 does not confirm the reduction in revascularization during follow-up and even suggests that in some cases there is increased mortality, reinfarction or MACE with a multivessel revascularization strategy, which conflicts with our results and those of other studies.
In this study, the decision whether to perform multivessel revascularization was taken by the clinical cardiologist and the interventional cardiologist or by the heart team, as appropriate. This reflects real-world practice, in which decisions on how, when and which coronary arteries should be revascularized is individualized on the basis of the anatomical characteristics of the lesions themselves, symptoms, the results of ischemia testing, the myocardial territory at risk, left ventricular systolic function, the risk of complications, the experience of the site and operator, and the patient's age, comorbidities and preferences.28
Higher morbidity and mortality in patients with NSTE-ACS and multivessel CAD compared with those with single-vessel disease only29,30 may be due to various mechanisms, including multiple unstable plaques and abnormalities in myocardial perfusion or contractility, which may result in MI, arrhythmias and death. The potential advantages of multivessel revascularization in this context include preventing ischemia or reinfarction and associated complications, reducing the myocardial territory at risk, and improving myocardial function by increasing blood flow to the peri-infarct area.
However, there may also be disadvantages in multivessel revascularization, including more prolonged procedures, requiring greater use of contrast and higher radiation dose, and more stent-associated complications (in-stent thrombosis and restenosis) due to more stents being implanted. Nonetheless, the use of new antiplatelet agents, radial access and new-generation drug-eluting stents may help enhance the safety of the procedure for experienced operators. In this study, patients undergoing multivessel revascularization showed a similar in-stent thrombosis rate to those undergoing single-vessel revascularization, as well as similar rates for other in-hospital adverse events. Therefore, in our study, multivessel revascularization was not associated with worse short-term prognosis relative to culprit-only revascularization, as also seen in other studies, in which there were no statistically significant differences between the groups (multivessel vs. single-vessel) in terms of periprocedural complications. It can thus be stated that multivessel revascularization is as safe as, and in some cases safer than, culprit-only revascularization.29,31,32
In this study, multivessel revascularization was mainly performed during the initial procedure (66.2%). Intervention on a non-culprit lesion during the initial procedure can result in unnecessary hemodynamic compromise, at a time when the patient may have significant regional myocardial dysfunction due to the infarction. Moreover, non-culprit lesions may be overestimated and their physiological significance may be difficult to assess, which may lead to PCI being performed unnecessarily.33 Elective revascularization enables better assessment of the physiological significance of non-culprit lesions, through invasive and non-invasive ischemia tests, and gives more time to discuss the revascularization strategy, which may thus be safer. However, in a recent randomized trial (SMILE34) comparing multivessel revascularization during the initial procedure with a staged revascularization strategy, a reduction in major cardiovascular events was found with the former, mainly due to fewer unplanned revascularizations.
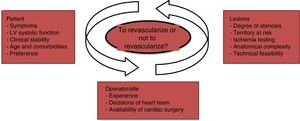
In summary, data from articles published in this field suggest that assessment of the risk-benefit balance associated with future invasive procedures should be based on analysis of the patient's general status and cardiovascular risk factors, the possibility of identifying the culprit artery, the technical feasibility of performing multivessel revascularization, and the location, degree of stenosis and severity of lesions. In addition, the patient's clinical stability, operator experience, the decisions of the heart team, and the availability of cardiac surgery facilities are also relevant to the choice of strategy32 (Figure 5).
In view of the conflicting results obtained in meta-analyses and observational studies, prospective randomized studies with large patient populations are needed to be able to identify the most appropriate revascularization strategy for patients with NSTE-ACS and multivessel CAD.
LimitationsThere are several limitations to be considered when interpreting this study. First, this was a retrospective, non-randomized observational study conducted at a single hospital, involving a relatively small number of patients, and thus presents the limitations and biases inherent to retrospective single-center studies. Although PSM was carried out between the groups, which increases the power of the statistical analysis, it is impossible to correct for unmeasured confounding factors and all the selection biases related to treatment decisions, which means definitive conclusions cannot be drawn. Second, definition of the type of lesion (culprit vs. non-culprit) did not follow a protocol, and it is therefore likely that this decision varied according to how the interventional cardiologist interpreted the angiographic results at the time of coronary angiography. Given how difficult it can be to identify the culprit artery, it cannot be ruled out that incorrect identification may have influenced the results, such as the culprit lesion being more likely to be erroneously identified in the group undergoing single-vessel revascularization. Third, there is no simple way to identify the factors that led to the decision to perform multivessel revascularization in each case, and so it is impossible to establish a standard approach for patients with NSTE-ACS and multivessel CAD. Fourth, data on the functional assessment of coronary stenoses were not collected for all patients undergoing multivessel revascularization. In practice, most of the non-culprit lesions were identified as significant using coronary angiography alone. Fifth, the treatment groups were defined after patients underwent PCI, and some patients who had been initially allocated to the multivessel revascularization group may have undergone culprit-only revascularization, due to technical or anatomical factors that may have precluded more complete revascularization.
ConclusionThe decision to perform multivessel vs. culprit-only revascularization in patients with NSTE-ACS and multivessel CAD is still the subject of heated debate and there is as yet no consensus.
However, this study supports the hypothesis that multivessel revascularization in patients with NSTE-ACS and multivessel CAD reduces the occurrence of adverse clinical events during follow-up, including reinfarction, unplanned revascularization, unplanned PCI, and the composite endpoints of reinfarction and unplanned revascularization and of death, reinfarction and unplanned revascularization, both before and after performing PSM.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Correia C, Galvão Braga C, Martins J, et al. Revascularização multivaso versus revascularização da artéria culprit em pacientes com síndrome coronária aguda sem supradesnivelamento do segmento ST e doença coronária multivaso. Rev Port Cardiol. 2018;37:143–154.