Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have been shown to reduce mortality after myocardial infarction (MI). Current guidelines recommend their prescription in all patients after MI. Limited data are available on whether ACEIs/ARBs still improve prognosis in the contemporary era of non-ST elevation MI (NSTEMI) management. We aimed to evaluate the mortality benefit of ACEIs/ARBs in NSTEMI patients treated successfully with percutaneous coronary intervention (PCI).
MethodsWe analyzed 2784 patients with NSTEMI treated successfully with in-hospital PCI. Two groups were formed based on ACEI/ARB prescription at discharge. Two propensity score (PS) analyses were performed to control for differences in covariates: one with adjustment among the entire cohort, and the other with PS matching (n=1626). The outcome variable was all-cause mortality at four-year follow-up.
ResultsThere were 1902 (68.3%) patients prescribed ACEIs/ARBs at discharge. When adjusted by PS, ACEI/ARB use was associated with a hazard ratio (HR) for mortality of 0.75 (0.60-0.94; absolute risk reduction [ARR] 4.0%) in the whole cohort (p=0.01).
After one-to-one PS matching (n=813 in each group), the mortality rate was significantly lower in patients prescribed ACEIs/ARBs, with HR of 0.77 (0.63-0.94; ARR 3.8%) (p=0.03).
ConclusionsIn this observational study of patients with NSTEMI, all of them treated successfully by PCI, the use of ACEIs/ARBs was significantly associated with a lower risk of four-year all-cause mortality.
Os inibidores da enzima de conversão da angiotensina (IECA) ou os antagonistas do recetor da angiotensina (ARA) mostraram reduzir a mortalidade após enfarte do miocárdio (EM). As guias internacionais atuais recomendam a sua prescrição a todos os doentes após EM. Existem poucos dados disponíveis relativamente a se os IECA/ARA continuam a melhorar o prognóstico na abordagem atual do EM sem elevação do segmento ST (EMSEST). O nosso objetivo foi avaliar o benefício em termos de mortalidade dos IECA ou ARA em doentes com EMSEST, tratados com intervenção coronária percutânea (ICP) com sucesso.
MétodosAnalisámos 2784 doentes com EMSEST tratados, de forma bem-sucedida, com ICP durante o internamento hospitalar. Foram constituídos dois grupos com base na prescrição de IECA/ARA aquando da alta. Foram efetuadas duas análises de propensity score para controlar diferenças nas co variáveis: uma com ajuste entre toda a coorte e outra com propensity score matching (n=1,626). A variável resultado foi a mortalidade por qualquer causa, após quatro anos de seguimento.
ResultadosHouve 1902 (68,3%) de doentes com IECA/ARA prescritos aquando da alta. Quando ajustado para o propensity score, o uso de IECA/ARA associou-se a um hazard ratio de 0,75 para mortalidade (0,60-0,94; redução do risco absoluto [RRA] 4,0%) em toda a coorte (p=0,01).
Após o one-to-one propensity score matching (n=813 em cada grupo), a taxa de mortalidade foi significativamente menor em doentes sob tratamento com IECA/ARA, com um hazard ratio de 0,77 (0,63-0,94; RRA 3,8%) (p=0,03).
ConclusõesNeste estudo observacional de doentes com EAMSEST, todos tratados com ICP com sucesso, o uso de IECA/ARA esteve associado a um risco significativamente menor de mortalidade por todas as causas aos quatro anos.
angiotensin-converting enzyme inhibitor
acute coronary syndrome
angiotensin receptor blocker
absolute risk reduction
coronary artery bypass grafting
coronary artery disease
electrocardiogram
hazard ratio
left ventricular ejection fraction
myocardial infarction
non-ST elevation myocardial infarction
percutaneous coronary intervention
propensity score
ST-elevation myocardial infarction
Thrombolysis In Myocardial Infarction
Many randomized trials have demonstrated that inhibition of the renin-angiotensin system with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) significantly improves short- and long-term prognosis after an acute myocardial infarction (MI).1–16 In these trials, the vast majority of patients had ST-elevation MI (STEMI), and the data on non-ST elevation MI (NSTEMI) patients are limited.
Contemporary management of patients with NSTEMI based on the results of large-scale randomized controlled trials demonstrates survival benefits with early invasive strategies17 and secondary prevention treatments including antiplatelets,18 beta-blockers19 and statins20 started soon after the index event. However, no studies have examined the independent impact of ACEIs/ARBs on clinical outcomes in NSTEMI. Despite this, the European21 and North American22 guidelines recommend long-term use of ACEIs/ARBs for all patients with NSTEMI (class I, level of evidence B).
We address the area of uncertainty surrounding the effect of ACEIs/ARBs on long-term mortality after successful percutaneous coronary intervention (PCI) in a contemporary population of patients with NSTEMI.
MethodsStudy populationThe CardioCHUS-SCA (Síndrome Coronario Agudo en Cardiología del Complejo Hospitalario Universitario de Santiago) registry was a retrospective study in which demographic, clinical and angiographic data, as well as information relating to management and in-hospital complications, were collected prospectively and recorded in an electronic database by the department's cardiologists in the admission ward and coronary care unit.23,24 Subjects were all patients with a definitive primary diagnosis of acute coronary syndrome (ACS) admitted consecutively to the cardiology department of our center between December 2003 and December 2012. The diagnosis of ACS was validated, through retrospective chart review, if the patient had new-onset symptoms suggestive of myocardial ischemia and any of the following criteria: cardiac biomarkers above the upper normal limit; ST-segment deviation on the electrocardiogram (ECG); in-hospital stress testing showing ischemia; or known history of coronary disease. Patients were classified as having STEMI or non-ST elevation ACS (NSTEMI or unstable angina). The diagnosis of NSTEMI required the presence of suggestive symptoms together with cardiac troponin levels above the upper normal limit.
The aim of this study was to describe the effect of ACEIs/ARBs on mortality in patients surviving hospitalization for NSTEMI and successfully treated with PCI. The initial study sample was 5504 patients. Patients with STEMI (n=1685) or unstable angina (n=504) and those whose NSTEMI was treated medically or with in-hospital coronary artery bypass grafting (CABG) (n=388) were excluded, as were patients with unsuccessful in-hospital PCI (n=105). Patients lacking data on vital status during follow-up (n=38) were also excluded.
The final study population thus consisted of 2784 patients with NSTEMI treated successfully with in-hospital PCI.
Successful PCI was defined as achievement of Thrombolysis In Myocardial Infarction (TIMI) 3 flow and final residual stenosis <30% in the treated coronary arteries. Left ventricular ejection fraction (LVEF) was determined by echocardiography during admission using Simpson's rule.
Endpoint and follow-upThe primary endpoint was all-cause mortality. After discharge, patients were followed in a clinic specializing in coronary artery disease (CAD), as well as in primary care. Follow-up was performed by reviewing the electronic medical records of the region of Galicia (population: two million), using the IANUS system, which integrates data on primary care and specialized care sectors. Reports from primary care as well as hospital records were reviewed. In some cases patients were contacted by phone to collect data regarding the primary endpoint. Median follow-up was four years (interquartile range: 2.9-4.0).
Statistical analysisThe statistical analysis was performed with SPSS 21.0 and STATA 13.0. Cox proportional hazards regression models were used to compare mortality rates between treatment groups. For the follow-up time (median four years), event rates, and matched sample size (the smaller cohort), we had >80% power to detect a 15% reduction (hazard ratio [HR] 0.85) in mortality risk with ACEIs/ARB. Due to the inability to reach 80% power to detect small benefits (HR >0.90), no subgroup analysis was performed.25
Association between angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use and mortalityThe survival analysis was based on the intention-to-treat principle regardless of subsequent ACEI/ARB use. We used three different Cox regression models to assess the adjusted effect of ACEI/ARB use on post-discharge mortality: (1) multivariate Cox proportional hazards regression adjusted for characteristics associated with mortality; (2) Cox proportional hazards regression with propensity score (PS) matching; and (3) Cox proportional hazards regression with PS adjustment.
Multivariate model risk adjustmentThis analysis was performed on the entire sample (n=2784). Mortality rates of patients who did or did not receive ACEIs/ARBs were adjusted for associated characteristics on the basis of clinical plausibility or p<0.05 in the bivariate Cox analyses with post-NSTEMI mortality: age, gender, year of admission, history of smoking, hypertension, diabetes, history of MI, prior PCI, prior CABG, history of heart failure, history of atrial fibrillation, chronic obstructive pulmonary disease, history of peripheral arterial disease and/or stroke, prior malignancy, ≥1 mm ST depression or transient (<30 min) ST elevation on the qualifying ECG, baseline hemoglobin values and estimated glomerular filtration rate, multivessel (≥2 vessels with >70% stenosis) vs. single-vessel CAD, LVEF (as a continuous covariable), in-hospital TIMI serious (minor or major) bleeding, or treatment at discharge with vs. without ACEIs/ARBs, aspirin, clopidogrel, vitamin K antagonists, statins, beta-blockers, loop diuretics, digoxin, aldosterone receptor blockers, and/or calcium channel blockers.
Propensity score matchingBecause of differences in key baseline characteristics (Table 1), we used PS matching to assemble a cohort in which all the measured covariates would be well balanced across the comparator group. Propensity scores were estimated using a non-parsimonious multivariate logistic regression model, with the dependent variables being treatment with ACEIs/ARBs and the following 18 characteristics entered as covariates: age, gender, year of admission, hypertension, diabetes, prior MI, prior PCI, prior CABG, history of heart failure, history of atrial fibrillation, history of peripheral arterial disease and/or stroke, ≥1 mm ST depression or transient (<30 min) ST elevation on the qualifying ECG, peak cardiac troponin I, baseline hemoglobin, estimated glomerular filtration rate, multivessel vs. single-vessel CAD, LVEF (as a continuous covariable), and treatment at discharge with aspirin, clopidogrel, statins, beta-blockers, loop diuretics, ARBs, and/or calcium channel blockers.
Pre- and post-matching characteristics of the study population by treatment subgroups.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| ACEIs/ARBs (n=1902) | No ACEIs/ARBs (n=882) | SD, % | ACEIs/ARBs (n=813) | No ACEIs/ARBs (n=813) | SD, % | |
| Demographic data | ||||||
| Age (years) | 68.4±12 | 67.6±13 | 7% | 66.5±12 | 67.1±14 | 5% |
| Female, % | 22.2 | 24.0 | 6% | 22.9 | 23.7 | 3% |
| Weight (kg) | 78.0±17 | 75.0±15 | 18% | 76.1±16 | 74.8±15 | 8% |
| Past medical history | ||||||
| Smoking, % | 30.2 | 31.2 | 3% | 68.3 | 69.6 | 3% |
| Hypertension, % | 57.6 | 43.2 | 32% | 45.4 | 44.4 | 2% |
| Diabetes, % | 28.8 | 21.5 | 22% | 22.4 | 21.6 | 3% |
| Dyslipidemia, % | 45.3 | 44.4 | 2% | 45.1 | 45.3 | 1% |
| PAD, % | 8.7 | 8.6 | 0% | 9.5 | 8.1 | 8% |
| Stroke, % | 6.0 | 5.6 | 4% | 5.5 | 5.4 | 4% |
| MI, % | 17.2 | 21.1 | 14% | 20.7 | 20.4 | 1% |
| CHF, % | 2.5 | 2.7 | 4% | 2.8 | 2.3 | 9% |
| PCI, % | 7.3 | 8.4 | 8% | 9.0 | 8.2 | 6% |
| CABG, % | 3.3 | 3.7 | 7% | 2.1 | 2.4 | 9% |
| COPD, % | 8.9 | 9.6 | 5% | 9.5 | 9.2 | 2% |
| AF, % | 6.1 | 5.0 | 12% | 5.5 | 4.9 | 7% |
| Prior malignancy, % | 5.8 | 8.2 | 21% | 6.9 | 8.0 | 9% |
| Data on admission | ||||||
| Class IA indication for ACEIs/ARBs, % | 72.2 | 55.2 | 41% | 42.9 | 43.7 | 1% |
| Killip class ≥II, % | 15.3 | 12.2 | 15% | 88.1 | 88.1 | 0% |
| ST deviation, % | 16.9 | 17.1 | 1% | 16.0 | 17.2 | 4% |
| Peak cardiac troponin I (ng/dl) | 44.0±80 | 31.36±60 | 17% | 33.9±70 | 32.4±62 | 2% |
| LVEF (%) | 54.7±11.1 | 57.1±9.3 | 23% | 56.9±10.0 | 57.2±9.2 | 3% |
| LVEF ≤40%, % | 16.4 | 9.0 | 38% | 9.7 | 8.5 | 8% |
| Multivessel disease, % | 47.4 | 46.7 | 2% | 48.0 | 46.4 | 2% |
| Hemoglobin (g/dl) | 14.3±2 | 14.1±2 | 12% | 14.2±2 | 14.1±2 | 5% |
| GFR <60 ml/min/1.73 m2, % | 25.8 | 29.9 | 11% | 26.1 | 26.9 | 2% |
| In-hospital complications | ||||||
| CHF, % | 4.7 | 5.0 | 4% | 4.2 | 4.8 | 8% |
| MI, % | 2.5 | 1.7 | 22% | 2.1 | 1.9 | 6% |
| AF, % | 4.4 | 4.9 | 6% | 3.8 | 4.3 | 7% |
| Treatment at discharge | ||||||
| Aspirin, % | 98.6 | 92.6 | 95% | 96.8 | 97.2 | 8% |
| Clopidogrel, % | 97.2 | 92.7 | 55% | 95.8 | 96.3 | 7% |
| Beta-blockers, % | 86.0 | 78.4 | 29% | 83.7 | 82.3 | 6% |
| Statins, % | 91.3 | 85.0 | 34% | 90.3 | 89.3 | 6% |
| Loop diuretics, % | 15.2 | 12.1 | 15% | 12.3 | 12.3 | 0% |
| Aldosterone blockers, % | 7.0 | 2.6 | 57% | 3.4 | 2.8 | 2% |
| CCBs, % | 14.1 | 21.1 | 27% | 19.9 | 20.7 | 3% |
| Digoxin, % | 1.8 | 1.7 | 3% | 1.4 | 1.6 | 8% |
| VKAs, % | 5.3 | 5.3 | 0% | 5.4 | 5.4 | % |
| Propensity score | 0.717±0.167 | 0.611±0.167 | 83% | 0.652±0.130 | 0.645±0.130 | 5% |
ACEIs/ARBs: angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; AF: atrial fibrillation; CABG: coronary artery bypass grafting; CCB: calcium channel blockers; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; SD: standardized difference; VKAs: vitamin K antagonists.
The discriminative power of the previous model was 0.704 (95% confidence interval 0.683-0.715; p<0.001), with a Hosmer-Lemeshow goodness-of-fit test p=0.43, indicating a good fit.
Matching was performed using the post-matching algorithm in STATA (version 13.0), with 1:1 nearest-neighbor matching without replacement and with a caliper width of 0.05 of the standard deviation of all PSs. Standard mean differences were estimated for all covariates before and after matching to assess pre-matching imbalance and post-matching balance; standard mean differences of <10% for a given covariate indicate adequate balance.26,27
The mortality risk in patients taking ACEIs/ARBs vs. not taking ACEIs/ARBs was estimated using a Cox regression model stratified on the matched pairs.
In the PS-matched analysis, many patients remained unmatched (Figure 1) and were thus excluded from the analysis (which may have slightly reduced efficiency). A regression adjustment with the propensity score (as a continuous variable) was therefore also performed in which all the patients in the cohort were analyzed.28
Mortality curves are drawn by the Kaplan-Meier method, and compared by the log-rank test and the stratified log-rank test in the pre- and post-matching cohorts, respectively. The proportional hazards assumption was tested using Schoenfeld residuals.
All tests with a two-tailed p value of less than 0.05 were considered statistically significant.
ResultsAs shown in Table 1, before matching (n=2784), most of the baseline characteristics of patients in the present study were significantly different (standardized differences >10%). There were no significant differences between the two groups in age or gender. However, compared with patients not prescribed ACEIs/ARBs at discharge, those prescribed ACEIs/ARBs more frequently had hypertension, diabetes, and prior MI.
Overall, patients prescribed ACEIs/ARBs more often had clinical conditions fulfilling class IA recommendations1,22 (hypertension, diabetes, LVEF ≤40%, and/or glomerular filtration rate <60 ml/min/1.73 m2). The medications recommended in the guidelines for NSTEMI (aspirin, clopidogrel, statins, and beta-blockers) were used in the vast majority of patients. The usage rates of these medications were significantly different between the two study groups, while the usage rates of digoxin and vitamin K antagonists were similar.
Matching made the groups similar with no significant differences remaining in the characteristics compared (Table 1); standardized differences in baseline characteristics did not exceed 9%.
All-cause mortalityUnadjusted and PS matching-adjusted Kaplan-Meier estimates for mortality are shown in Figure 2.
In the pre-matching cohort, 362 patients died during follow-up: 220/1902 (11.6%) in patients prescribed ACEIs/ARBs and 142/882 (16.1%) in the others (log-rank test, p=0.001).
The PS-matching adjusted mortality rates were 11.9% (n=97) in the ACEI/ARB group, and 15.7% (n=128) in the no ACEI/ARB group (stratified log-rank test, p=0.03).
In the pre-matching cohort, ACEI/ARB use was significantly associated with 29% mortality risk reduction (absolute risk reduction [ARR] 4.5%, p=0.001). The mortality benefit associated with ACEI/ARB treatment persisted after controlling for multiple possible confounders, using standard Cox regression analysis (ARR 4.3%, p=0.008), adjustment for PS (ARR 4.0%, p=0.01), and after adjustment for PS matching survivor bias (ARR 3.8%, p=0.03) (Table 2).
Unadjusted and adjusted absolute risk reductions and hazard ratios for mortality associated with use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
| Risk-adjustment methods for mortality | ARR, % | p | HR | 95% CI |
|---|---|---|---|---|
| Unadjusted | 4.5% | 0.001 | 0.71 | 0.58-0.88 |
| Adjusted for 31 covariablesa | 4.3% | 0.008 | 0.73 | 0.58-0.92 |
| Propensity score adjusted | 4.0% | 0.01 | 0.75 | 0.60-0.94 |
| Propensity score matching adjusted | 3.8% | 0.03 | 0.77 | 0.63-0.94 |
AAR: absolute risk reduction; ACEIs/ARBs: angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; CI: confidence interval; HR: hazard ratio.
Adjusted for: age, gender, year of admission, history of smoking, hypertension, diabetes, history of myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, history of heart failure, history of atrial fibrillation, chronic obstructive pulmonary disease, history of peripheral arterial disease and/or stroke, prior malignancy, ≥1 mm ST depression or transient (<30 min) ST elevation on the qualifying ECG, baseline hemoglobin values and estimated glomerular filtration rate, multivessel vs. single-vessel CAD, LVEF, in-hospital TIMI serious bleeding, treatment at discharge with ACEIs/ARBs vs. no ACEIs/ARBs; aspirin, clopidogrel, vitamin K antagonists, statins, beta-blockers, loop diuretics, digoxin, aldosterone receptor blockers, and/or calcium channel blockers.
The main finding of the present study including patients with NSTEMI treated successfully with PCI is that treatment with ACEIs or ARBs prescribed at discharge is independently associated with reduction in long-term mortality risk.
To our knowledge, this is the first study examining the survival benefit of ACEIs/ARBs after successful PCI in a contemporary real-life cohort of NSTEMI patients.
Current international guidelines recommend the use of ACEIs/ARBs in all patients with NSTEMI. These recommendations are class IA, indicating a strong recommendation derived from multiple randomized clinical trials or meta-analyses, in patients with LVEF ≤40% and in patients with heart failure, diabetes, hypertension, or chronic kidney disease, unless contraindicated.21,22
For all other patients, current guidelines define the use of ACEIs/ARBs as class IB (based on limited study populations). However, the recommendations in the guidelines are based on evidence derived from relatively old post-MI studies1–16 and from trials on heart failure, stable CAD and even from trials including patients at high risk for but without established CAD.29,30
No trials have yet examined the effect of long-term ACEI/ARB treatment in patients with recent MI without heart failure or left ventricular dysfunction. The current guidelines are mainly based on the results of the HOPE and EUROPA trials, which included patients with stable CAD and no known heart failure or left ventricular dysfunction.29,30 In these trials, and in subsequent meta-analyses, ACEIs reduced both mortality and the rate of MI and stroke.
In the NSTEMI setting, the standard of care has changed over the past decade, now including early revascularization in addition to multiple evidence-based medications such as dual and potent antithrombotic therapy, high statin doses, and beta-blockers. The use of these medications after successful PCI might weaken or even nullify the long-term efficacy of ACEIs/ARBs use in patients with NSTEMI. Consequently, the current recommendation on long-term ACEI/ARB use in NSTEMI patients may no longer be justified. A large international registry recently questioned the long-term benefit on mortality of beta-blocker use, even in the presence of prior MI.31
In contrast to earlier studies,29,30 a later placebo-controlled trial32 indicated no benefit of ACEIs in patients with stable CAD and preserved LVEF, calling into question the routine prescription of ACEIs in all patients with CAD.
Moreover, a recent Australian longitudinal population-based study33 found no additional long-term survival benefit in patients with MI treated with beta-blockers and statins compared with those on beta-blockers, statins, and ACEIs/ARBs.
Cardiovascular mortality and morbidity have recently been considerably reduced with the implementation of evidence-based therapy in patients with cardiovascular disease. Accordingly, re-evaluation of the effectiveness of cardioprotective medications may be warranted in the contemporary PCI era, because the mortality benefit of such medications may have changed along with the decrease in mortality.34 Furthermore, ACEIs/ARBs are not free of adverse effects, and their tolerability may be less than ideal in some subgroups of patients,35 particularly in the elderly. Thus, the validity of the current guidelines regarding long-term use of ACEIs/ARBs, in the best scenario, is an area of significant uncertainty. In this regard, and in view of the financial, practical, and ethical challenges inherent in undertaking randomized trials, observational studies to compare the outcomes of a given therapy are of potential clinical interest.
Our data regarding the long-term benefit of ACEIs/ARBs in patients with NSTEMI are in agreement with a large Swedish observational study36 that examined ACEI treatment in patients with MI between 1995 and 2005. In that study, both STEMI and NSTEMI patients treated with ACEIs/ARBs had clear reductions in one-year mortality.
In contrast to the results from the Swedish registry, Amann et al.37 recently found that ACEIs/ARBs reduced all-cause mortality by 26% (HR 0.74) at five-year follow-up only among NSTEMI patients (no benefit was found in patients with STEMI), enrolled between 2000 and 2008, and all treated with beta-blockers and antiplatelet agents (69% PCI, 90% statins).
Moreover, Hara et al.38 reported a reduction in long-term mortality with the use of ACEIs/ARBs in Japanese patients with MI, although this benefit was observed only two years after discharge.
Of note, in comparison with the previous three studies examining the benefit of ACEIs/ARBs on long-term mortality in MI, our study supports the recommendations of the current international guidelines on long-term ACEI/ARB use in NSTEMI patients, even those treated successfully with PCI during the index episode.
Given the fact that NSTEMI is the acute coronary syndrome with the highest rates of mortality and morbidity, every effort should be made to improve prognosis in this setting. Therefore, physicians should be encouraged to “get with the guidelines” regarding long-term use of ACEIs/ARBs after NSTEMI after successful PCI.
LimitationsA major strength of our study is its setting in a population-based registry with patients consecutively hospitalized with a validated definitive primary diagnosis of NSTEMI. Data collection was performed by cardiologists soon after the NSTEMI during the hospitalization stage, and long-term follow-up was obtained in almost all patients, thanks to the availability of electronic medical records, shared between primary and specialized care, which covered a population of about two million. Our findings are limited by the observational nature of the study. However, because randomized trials cannot be undertaken in all situations in which evidence is needed to guide care, well-designed observational studies are still needed to assess effectiveness in a specific population and to extend the results to the general population. One of the main limitations is lack of complete knowledge of revascularization rates, almost 50% of patients having multivessel disease in both groups. The rate of incomplete revascularization could have biased the results on mortality. It should be kept in mind that PS and PS-based matching have the same limitations as multivariate risk adjustment model methods, and are no more likely to remove bias due to unmeasured confounding when strong selection bias exists, despite the fact that many variables were collected by the present registry.
Furthermore, our study may be criticized for being from a single center. However, this is countered by the unselected nature of our patients, who were representative of populations of patients with NSTEMI in routine clinical practice, and the large number of patients included and of the events observed. Finally, ACEI/ARB usage was categorized according to prescription at discharge, and we have no data on the influence of, duration of, or changes in treatment thereafter. However, as mentioned in the method section, our analysis was based on the intention-to-treat principle regardless of subsequent ACEI/ARB use, which is often the method used in randomized clinical trials.
ConclusionsThis observational study performed in the PCI era demonstrated a survival benefit over four years with the use of ACEIs/ARBs following successful PCI in NSTEMI patients.
Because NSTEMI is associated with a high mortality burden – even higher than that observed in other types of acute coronary syndrome – and bearing in mind that the aim of medical care is to reduce patients’ mortality and morbidity, medical therapy with ACEIs/ARBs after successful PCI should be considered as a complementary strategy for reducing mortality risk.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.








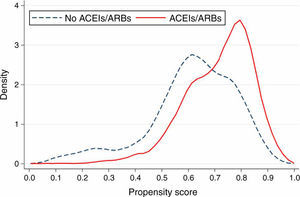 ACEIs/ARBs: angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.'/>
ACEIs/ARBs: angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.'/>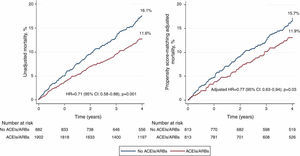 ACEIs/ARBs were prescribed at discharge.
ACEIs/ARBs were prescribed at discharge. 

