Anemia is a common comorbidity in patients with acute coronary syndromes (ACS), and is associated with higher risk for both bleeding and ischemic complications. We aimed to assess the predictive ability of bleeding risk scores (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines [CRUSADE], Mehran and Acute Coronary Treatment and Intervention Outcomes Network [ACTION]) in ACS patients with anemia.
MethodsAll consecutive ACS patients were prospectively included. The primary outcome was in-hospital major bleeding according to the CRUSADE, Mehran and ACTION definitions. Anemia was defined as hemoglobin <130 g/l in men and <120 g/l in women. The predictive ability of the bleeding risk scores was assessed by binary logistic regression, calculating receiver operating characteristic (ROC) curves and their corresponding area under the curve (AUC).
ResultsWe included 2255 patients, mean age 62.4 years. Anemia was present in 550 patients (24.4%). Patients with anemia had a significantly higher prevalence of comorbidities. The three bleeding risk scores adequately predicted major bleeding in the whole cohort.
No significant differences were observed regarding the predictive ability of each of the scores in patients with and without anemia (CRUSADE: AUC 0.73 without anemia vs. 0.74 with anemia, p=0.913; ACTION: AUC 0.68 without anemia vs. 0.73 with anemia, p=0.353; Mehran: AUC 0.69 without anemia vs. 0.61 with anemia, p=0.210). Only the Mehran score showed significantly lower predictive ability in patients with hemoglobin <11 g/dl (AUC 0.51, p=0.044).
ConclusionsAnemia was a common comorbidity in patients with ACS from our series. Currently available bleeding risk scores showed an adequate predictive ability in patients with mild anemia.
A anemia é uma comorbilidade frequente em doentes com síndromes coronárias agudas (SCA) e associa-se tanto a um maior risco de hemorragia, como de complicações isquémicas. O nosso objetivo foi avaliar a capacidade preditiva de scores de risco de hemorragia (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines [CRUSADE], Mehran e Acute Coronary Treatment and Intervention Outcomes Network [ACTION]) em doentes com SCA e anemia.
MétodosTodos os doentes consecutivos com SCA foram prospetivamente incluídos. O resultado primário foi a hemorragia intra-hospitalar major, de acordo com as definições CRUSADE, Mehran e ACTION. A anemia foi definida como uma concentração de hemoglobina <130 g/L em homens e <120 g/L em mulheres. A capacidade preditiva dos scores de risco de hemorragia foi avaliada pelo método de regressão logística binária, calculando curvas ROC e a sua área correspondente sob a curva (AUC).
ResultadosForam incluídos 2255 doentes. A média de idades foi de 62,4 anos. A anemia estava presente em 550 doentes (24,4%). Doentes com anemia apresentaram uma prevalência significativamente maior de comorbilidades. Os três scores de risco previram corretamente hemorragia major no conjunto da coorte.
Não se observaram diferenças significativas em relação à capacidade preditiva de cada um dos scores de risco de hemorragia em pacientes com e sem anemia (CRUSADE AUC 0,73 sem anemia versus 0,74 com anemia; p<0,913; ACTION AUC 0,68 sem anemia versus 0,73 com anemia; p<0,353; Mehran AUC 0,69 sem anemia versus 0,61 com anemia p<0,210). Apenas o score Mehran mostrou uma capacidade preditiva significativamente menor nos doentes com hemoglobina <11 g/dL (AUC 0,51, p<0,044).
ConclusõesNa nossa amostra, a anemia foi uma comorbilidade frequente em doentes com SCA. Os scores de risco de hemorragia estudados mostraram uma capacidade de previsão adequada em doentes com anemia leve.
The incidence of major bleeding in patients with acute coronary syndromes (ACS) ranges from 3 to 5%.1–5 Major bleeding events are associated with worse outcomes in this clinical setting.6–8 Several bleeding risk scores9–11 have been developed in recent years in order to properly predict bleeding complications in patients with ACS, and their use is recommended in the current clinical guidelines.12 However, it has been suggested that these scores have lower predictive ability in the elderly13 and in patients with comorbidities.
Anemia is a common comorbidity among patients with ACS,14 and its prevalence is expected to increase due to the aging of the population. Anemia is associated with higher risk for both bleeding and ischemic complications in patients with ACS.15 Information on the predictive ability of bleeding risk scores in patients with anemia is scarce; no study has assessed the performance of bleeding risk scores according to hematocrit status in patients with ACS. The aim of this study was to assess the predictive ability of the most widely used bleeding risk scores according to anemia status in a series of consecutive patients with ACS from routine clinical practice.
MethodsStudy design and populationThis is an observational single-center registry, conducted at a tertiary care hospital in Spain (Hospital Universitari de Bellvitge, l’Hospitalet de Llobregat, Barcelona). All consecutive ACS patients admitted to the coronary care unit between October 2009 and April 2014 were prospectively included. Informed consent was provided by all patients before their inclusion in the study. Confidential patient data were protected according to current national directives. This manuscript was revised for publication by the Clinical Research Ethics Committee of Bellvitge University Hospital (IRB00005523).
The primary outcome was in-hospital major bleeding according to the CRUSADE,9 Mehran10 and ACTION11 definitions.
Definitions, data collection and managementNon-ST-segment elevation ACS was defined as the presence of chest pain during the previous 48 hours with ST-segment changes on the electrocardiogram indicating ischemia or a positive troponin test. ST-segment elevation ACS was defined as the presence of chest pain with persistent ST-segment elevation of at least 0.1 mV in at least two contiguous leads or new left bundle branch block. Patients were classified as having anemia using the definition of the World Health Organization (WHO): hemoglobin <13.0 g/dl in men, and <12.0 g/dl in women.16 Severe anemia was defined as hemoglobin <11.0 g/dl for the purpose of this study.
Data were prospectively collected on site by trained physicians using a standardized case report form. Baseline characteristics, medical history, biochemical and electrocardiographic findings, treatments administered during hospitalization, incidence of in-hospital bleeding events and their anatomic location were collected. All elements included in the CRUSADE,9 Mehran10 and ACTION11 bleeding risk scores were included in the case report forms. The development of these scores has been described in detail previously. The CRUSADE, Mehran and ACTION bleeding risk scores were all prospectively calculated for each patient.
In-hospital major bleeding events were recorded using the CRUSADE,9 TIMI,12 Mehran,10 ACTION11 and Bleeding Academic Research Consortium (BARC)17 definitions. For reasons of clinical relevance, BARC categories 3 and 5 were considered severe BARC bleeding for this study. Since the main aim of the present analysis was to identify the risk of bleeding unrelated to surgery, bleeding in patients who underwent coronary artery bypass graft surgery was included in the analysis only if it occurred before surgery. Thus, BARC category 4 was excluded.
All elements included in the BARC category 2 criteria (need for nonsurgical medical intervention, need for increased level of care, prompting evaluation, baseline and lowest recorded hemoglobin, need for transfusion or surgery, requirement for intravenous vasoactive drugs) were included in the case report form, the data thus being prospectively collected. However, since the BARC definition was not available until 2011, BARC bleeding events were retrospectively assigned. ACTION, CRUSADE and Mehran major bleeding events were prospectively adjudicated.
The quality of data collection was assessed by checking source documentation in random samples. Hemodynamic parameters (heart rate and systolic blood pressure) and Killip class were measured at admission. Creatinine clearance was calculated using the Cockcroft-Gault formula18 and body surface area by the Mosteller formula.19 Patients were managed according to current clinical guidelines.
Information on deaths was obtained from hospital records, death certificates, or telephone contact with relatives of the patients or their referring physician.
Statistical analysisQuantitative variables were expressed as mean and standard deviation. For baseline variables, the Student's t test was used for comparison of quantitative variables and the chi-square test or Fisher's exact test, when appropriate, were used for categorical variables (PASW Statistics 18, Chicago, IL, USA). The normality of distribution of variables was assessed using the Kolmogorov-Smirnov test.
Patients with missing hemoglobin values and those lost to follow-up were excluded from the analysis. Baseline patient characteristics were analyzed in order to assess the impact of this exclusion. No significant differences were observed.
The ability of the CRUSADE, Mehran and ACTION bleeding risk scores to predict major in-hospital bleeding according to different definitions was assessed by binary logistic regression, calculating receiver operating characteristic (ROC) curves and their corresponding area under the curve (AUC). The non-parametric method described by DeLong20 was used to compare the predictive ability of the different bleeding risk scores.
ResultsDuring the study period 2255 patients were admitted with a diagnosis of ACS. Their mean age was 62.4 years, and almost 77% were male. Anemia was present in 550 patients (24.4%). Patients with anemia were significantly older, less often male and had a significantly higher prevalence of comorbidities (Table 1).
Baseline and clinical characteristics according to hematocrit status.
| Whole cohort (n=2255) | Anemia (n=550) | No anemia (n=1705) | p | |
|---|---|---|---|---|
| Male | 1727 (76.6) | 372 (67.6) | 1355 (795) | 0.001 |
| Age | 62.4 (13) | 68.9 (12) | 60.3 (13) | 0.001 |
| BMI (kg/m2) | 27.9 (4) | 27.8 (5) | 27.9 (4) | 0.532 |
| BSA (m2) | 1.9 (0.2) | 1.8 (0.2) | 1.9 (0.2) | 0.001 |
| Diabetes | 674 (29.9) | 260 (47.3) | 414 (24.3) | 0.001 |
| Hypertension | 1335 (59.2) | 407 (74) | 928 (54.4) | 0.001 |
| Dyslipidemia | 1302 (57.7) | 350 (63.6) | 952 (55.8) | 0.001 |
| Active smoking | 947 (42.7) | 127 (23.4) | 820 (48.9) | 0.001 |
| Previous MI | 315 (14) | 126 (22.9) | 189 (11.1) | 0.001 |
| Previous PCI | 264 (11.7) | 123 (22.4) | 141 (8.3) | 0.001 |
| Previous stroke | 157 (6.9) | 73 (13.3) | 84 (4.9) | 0.001 |
| PAD | 256 (11.4) | 121 (22) | 135 (7.9) | 0.001 |
| Hematocrit (%) | 41 (5) | 34 (4) | 43 (4) | 0.001 |
| Creatinine clearance (ml/min) | 91.7 (40) | 70.4 (36) | 98.5 (39) | 0.001 |
| Previous bleeding | 78 (3.5) | 37 (6.7) | 41 (2.4) | 0.001 |
| STEMI | 1461 (64.8) | 304 (55.3) | 1157 (67.9) | 0.001 |
| Killip class >I | 441 (19.6) | 173 (31.5) | 268 (15.7) | 0.001 |
| SBP (mmHg) | 130 (34) | 129 (28) | 130 (36) | 0.476 |
| HR (bpm) | 80 (17) | 81 (18) | 79 (17) | 0.090 |
| LVEF (%) | 51.9 (11) | 49.9 (11) | 52.5 (11) | 0.001 |
| CRUSADE score | 26 (16) | 39 (17) | 22 (14) | 0.001 |
| Mehran score | 17 (8) | 24 (7) | 14 (7) | 0.001 |
| ACTION score | 30 (8) | 35 (7) | 28 (7) | 0.001 |
| GRACE score | 124 (36) | 138 (37) | 118 (34) | 0.001 |
| Invasive strategy | 2170 (96.2) | 516 (93.8) | 1654 (97) | 0.001 |
| Radial approach | 1455 (67.1) | 296 (57.4) | 1159 (70.1) | 0.001 |
BMI: body mass index; BSA: body surface area; HR: heart rate; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; STEMI: ST-segment elevation myocardial infarction.
Categorical variables are expressed as n (%). Quantitative variables are expressed as mean (SD).
Patients with anemia also had poorer renal function at admission, a lower incidence of ST-segment elevation myocardial infarction and a higher percentage of signs of heart failure at presentation. Values of CRUSADE, Mehran and ACTION bleeding risk scores were all significantly higher in patients with anemia.
Significant differences were also observed regarding clinical management (Table 2). Patients with anemia were less often treated with clopidogrel and abciximab, and more often needed invasive procedures during hospitalization, such as intra-aortic balloon counterpulsation, renal replacement therapy, invasive mechanical ventilation and temporary pacing. Patients with anemia less often underwent an invasive strategy, and in patients undergoing angiography a radial approach was used less often than in patients without anemia.
Management and clinical course according to hematocrit status.
| Whole cohort (n=2255) | Anemia (n=550) | No anemia (n=1705) | p | |
|---|---|---|---|---|
| Anti-thrombotic treatments and in-hospital procedures | ||||
| Aspirin | 2186 (97.2) | 525 (96) | 1661 (97.5) | 0.057 |
| Clopidogrel | 2083 (92.6) | 489 (89.4) | 1594 (93.6) | 0.001 |
| Prasugrel | 36 (1.2) | 11 (2) | 25 (1.5) | 0.379 |
| Ticagrelor | 73 (3.2) | 11 (2) | 62 (3.6) | 0.061 |
| Enoxaparin | 1020 (45.3) | 259 (47.3) | 761 (44.7) | 0.276 |
| UFH | 1168 (51.9) | 261 (47.7) | 907 (53.3) | 0.024 |
| Bivalirudin | 236 (10.5) | 49 (9) | 187 (11) | 0.179 |
| Abciximab | 328 (14.6) | 47 (8.6) | 281 (16.5) | 0.001 |
| IABP | 156 (6.9) | 68 (12.4) | 88 (5.2) | 0.001 |
| Swan-Ganz catheter | 54 (2.5) | 22 (4.2) | 32 (2) | 0.005 |
| Hemodialysis | 29 (1.3) | 25 (4.5) | 4 (0.2) | 0.001 |
| Invasive mechanical ventilation | 124 (5.5) | 40 (7.3) | 84 (4.9) | 0.036 |
| Temporary pacemaker | 69 (3.1) | 28 (5.1) | 41 (2.4) | 0.001 |
| In-hospital clinical course | ||||
| AV block | 156 (7) | 54 (10.1) | 102 (6.1) | 0.002 |
| Atrial fibrillation | 158 (7) | 63 (11.5) | 95 (5.6) | 0.001 |
| Ventricular fibrillation | 138 (6.4) | 32 (6.2) | 106 (6.5) | 0.769 |
| Reinfarction | 17 (0.8) | 6 (1.1) | 11 (0.6) | 0.216 |
| VSD | 5 (0.2) | - | 5 (0.3) | 0.247 |
| CIN | 98 (4.3) | 48 (8.7) | 50 (2.9) | 0.001 |
| Ischemic MR | 10 (0.4) | 6 (1.1) | 4 (0.2) | 0.009 |
| Cardiac rupture | 14 (0.7) | 4 (0.7) | 10 (0.6) | 0.267 |
| Infections | 120 (5.3) | 57 (10.4) | 63 (3.7) | 0.001 |
| All bleeding | 164 (7.3) | 55 (10) | 109 (6.4) | 0.005 |
| Transfusion | 64 (2.8) | 44 (6) | 20 (1.2) | 0.001 |
| CRUSADE major bleeding | 115 (5.1) | 39 (7.1) | 76 (4.5) | 0.015 |
| Mehran major bleeding | 136 (6) | 49 (8.9) | 87 (5.1) | 0.001 |
| ACTION major bleeding | 116 (5.1) | 40 (7.3) | 76 (4.5) | 0.009 |
| BARC 3/5 bleeding | 61 (2.7) | 24 (4.4) | 37 (2.2) | 0.006 |
| In-hospital mortality | 90 (4.1) | 43 (8) | 47 (2.8) | 0.001 |
AV: atrioventricular block; BARC: Bleeding Academic Research Consortium; CIN: contrast-induced nephropathy; IABP: intra-aortic balloon pump; MR: mitral regurgitation; UF: unfractionated heparin; VSD: ventricular septal defect. Infections were defined as infectious complications requiring antibiotics.
Categorical variables are expressed as n (%). Quantitative variables are expressed as mean (SD).
These patients had also a higher incidence of in-hospital complications such as atrial fibrillation, atrioventricular block, contrast-induced nephropathy and infectious complications requiring antibiotics. Bleeding was significantly more common in patients with anemia regardless of the definition used. In-hospital mortality was almost three times higher in patients with anemia.
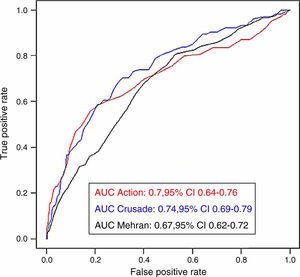
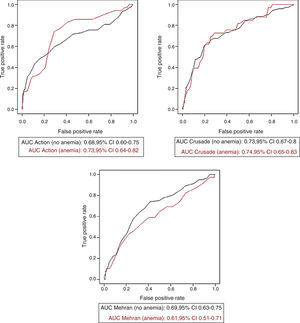
Bleeding risk prediction according to anemia statusThe three bleeding risk scores adequately predicted major bleeding according to their own definitions in the whole cohort (Figure 1). No significant differences were observed regarding the predictive ability of the bleeding risk scores in patients with and without anemia. While the predictive ability of the CRUSADE score was almost the same as for the whole cohort, the AUC of the ACTION score was slightly greater and that of the Mehran score was slightly smaller, without reaching statistical significance. Figure 2 shows the AUCs of each of the three bleeding risk scores in patients with and without anemia.
The assessment of patients with severe anemia showed different findings. The predictive ability of both the CRUSADE and ACTION scores decreased slightly in patients with severe anemia, but without significant difference. In contrast, the predictive ability of the Mehran score showed a significant decrease in patients with severe anemia. Table 3 shows the AUC values of each of the bleeding risk scores in patients without anemia, with anemia according to the WHO criteria, and with severe anemia.
Predictive ability of each of the bleeding risk scores according to different anemia categories.
| Bleeding risk score | No anemia, AUC (95% CI) (n=1705) | Anemia by WHO criteria, AUC (95% CI) (n=550) | Severe anemia,a AUC (95% CI) (n=173) | No anemia vs. anemia by WHO criteria, p | No anemia vs. severe anemia,a p |
|---|---|---|---|---|---|
| CRUSADE | 0.73 (0.67-0.80) | 0.74 (0.65-0.83) | 0.62 (0.49-0.76) | 0.913 | 0.552 |
| Mehran | 0.69 (0.63-0.75) | 0.61 (0.51-0.71) | 0.51 (0.38-0.65) | 0.210 | 0.044 |
| ACTION | 0.68 (0.60-0.75) | 0.73 (0.64-0.82) | 0.61 (0.50-0.72) | 0.353 | 0.181 |
AUC: area under the curve; CI: confidence interval; WHO: World Health Organization.
The main findings from our study are: (a) a high prevalence of anemia was found in a series of unselected ACS patients from routine clinical practice; (b) no significant differences were observed regarding the predictive ability of the three bleeding risk scores in patients with or without anemia according to the WHO criteria; and (c) only the Mehran risk score showed a significantly poorer predictive ability in patients with severe anemia.
Anemia is a common comorbidity in ACS, and is strongly associated with higher mortality and morbidity in this setting.15,21,22 The reasons for this association have not been clearly elucidated. Anemia worsens myocardial ischemia by reducing oxygen delivery to the injured myocardium as well as by increasing myocardial oxygen demands due to a larger stroke volume and higher heart rate. At the same time, anemia potentially reflects occult disease, such as malignancy or kidney disease, and can have unfavorable effects on the clinical course of noncardiac disease. Recent data suggest that causes of mortality in patients with ACS and anemia may differ according to age subgroups.23 As reported above, patients with anemia from our series were significantly older, with a higher incidence of comorbidities and higher in-hospital mortality.
In addition, anemia is a well-known predictor of bleeding24 in patients with ACS. However, although currently available bleeding risk scores have been successfully validated in different subsets,25–27 there is little information on bleeding risk stratification in patients with anemia. Our group previously described13 a poorer performance of the three bleeding risk scores in predicting major in-hospital bleeding in patients with ACS aged 75 years or older. One possible explanation for these findings is that certain aging-related variables such as frailty, disability, or comorbidities, which are rarely assessed in trials and registries in the cardiovascular area, might hamper bleeding risk stratification in this clinical scenario. In addition, most registries show a strong association between anemia and other comorbidities in different clinical settings. In the previously mentioned paper,13 elderly patients with ACS had a significantly higher prevalence of anemia compared to younger patients. Therefore, poorer predictive ability could also be expected in patients with anemia. However, data from our series showed no significant differences regarding the predictive ability of the bleeding risk scores in patients with and without anemia.
An important point when analyzing our findings is the role of hematocrit status in the composition of the different bleeding risk scores. Hemoglobin level is part of the CRUSADE,9 Mehran10 and ACTION11 risk scores, but the contribution of hemoglobin values to these scores varies. While in CRUSADE and Mehran hemoglobin levels account for around 10% of the overall points, in ACTION they represent almost 20% of the score. Assessment of the predictive ability of these bleeding risk scores stratified by one of their components might in itself lead to a reduction in the AUC. In our opinion, the non-significant reduction in AUC values in patients with anemia may at least in part be due to this fact, especially for the ACTION score, given the greater role of hemoglobin status in the overall composition of the score.
The differences in therapeutic management according to anemia status in our series also deserves particular mention. Patients with anemia underwent more conservative antithrombotic management, thus possibly leading to a reduction in the rate of bleeding and potentially affecting the performance of bleeding risk scores. However, this management was performed according to current recommendations, since patients with anemia are usually considered at higher bleeding risk and a more conservative antithrombotic approach is encouraged in this scenario. In our opinion, one of the strengths of this work is its assessment of the performance of the current bleeding risk scores in patients from our routine clinical practice managed according to the clinical judgment of the attending physician, taking into account current recommendations and patients’ profile in terms of ischemic and bleeding risk.
The different characteristics of the populations from whom these scores were derived also deserve special comment. The CRUSADE and ACTION scores were based on large American registries (89134 patients for CRUSADE and 90273 for ACTION) with baseline characteristics that were probably similar to patients included in our study. In contrast, the Mehran score was based on 17421 patients included in two clinical trials (ACUITY and HORIZONS AMI). Populations from clinical trials usually have a low prevalence of comorbidities and patients at higher risk are usually under-represented. The prevalence of anemia in Mehran et al.’s series was clearly lower (14.5%) than in ours. The poorer predictive ability of the Mehran score in patients with severe anemia may be related to this fact. However, these data must be interpreted cautiously due to the small sample size of this subgroup.
Our study has several limitations. This is a single-center study and therefore our conclusions should be applied only to similar populations undergoing similar clinical management. Since this is an observational study we cannot rule out the possibility of selection bias and residual confounding. The cutoff point of <11.0 g/dl for defining severe anemia was selected by investigators after assessing the distribution of hemoglobin in our series. The number of bleeding events was relatively small. In addition, the use of novel antithrombotic drugs like prasugrel or ticagrelor was uncommon. On the other hand, excluding patients with missing bleeding scores might have led to a certain bias. However, analysis of the baseline characteristics of these patients showed no significant differences from the other patients. Finally, the Mehran bleeding risk score was designed to predict bleeding during the first 30 days and not only during hospitalization as in our study.
In spite of these limitations, we believe that our findings show reasonably acceptable predictive ability in the main available bleeding risk scores in a series of consecutive ACS patients with and without anemia from routine clinical practice.
ConclusionsThe prevalence of anemia was high in our series of unselected ACS patients from routine clinical practice. The ability of the most important currently available bleeding risk scores to predict in-hospital major bleeding was acceptable in patients with anemia, especially in patients with mild anemia. The progressive aging of the population makes it particularly important to improve risk stratification in patients with comorbidities.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.