Several mechanisms contribute to myocardial hypertrophy and fibrosis in aortic stenosis (AS). MicroRNAs are post-transcriptional modulators of such processes. We hypothesized that their expression in myocardial biopsies from patients with AS could be linked with the degree of left ventricular (LV) hypertrophy and remodeling and to plasma levels of important biomarkers of extracellular matrix turnover.
MethodsWe performed myocardial biopsies in eleven patients with isolated severe AS undergoing aortic valve replacement. Echocardiographic exams and biomarker quantification were also performed. Five explanted hearts were used as controls for microRNA expression.
ResultsOverexpression of microRNA-101-3p was found in AS, which correlated with higher levels of preoperative valvuloarterial impedance, angiotensin II receptor and angiotensin-converting enzyme, and LV mass regression after surgery. Although not differently expressed in AS compared to controls, both upregulation of miR-4268 and downregulation of microRNA-125-5p were associated with higher LV mass. MicroRNA-125b-5p correlated negatively with LV mass and with relative wall thickness at six-month follow-up. MicroRNA-4268 correlated positively with LV mass regression and was associated with higher plasma angiotensin II receptor levels.
ConclusionsMicroRNA-101-3p and microRNA-4268 have potential new roles in the modulation of the hypertrophic response to AS via the renin-angiotensin-aldosterone system and as predictors of reverse remodeling after aortic valve replacement. Our results open new avenues in the understanding of myocardial response to pressure overload and of reverse remodeling after unloading. They also support the possibility of medical therapy to modulate the renin-angiotensin-aldosterone system in hypertrophic hearts.
Numerosos mecanismos contribuem para hipertrofia e fibrose miocárdica em doentes com estenose aórtica (EA), incluindo modulação pós-transcripcional por microARNs. Levantamos a hipótese da sua expressão em biópsias miocárdicas de doentes com EA relacionar-se com o grau de hipertrofia ventricular esquerda e processos de remodelagem ventricular, bem como com níveis plasmáticos de biomarcadores importantes de turnover da matriz extracelular.
MétodosRealizamos biópsias miocárdicas em 11 doentes com EA grave isolada referenciados para substituição valvular. Executamos também ecocardiografia e quantificação de biomarcadores. Cinco corações explantados foram usados como controlo da expressão dos microARNs.
ResultadosDescobrimos uma sobre-expressão do microARN-101-3p em doentes com EA que se correlaciona com níveis mais elevados pré-operatórios de impedância valvuloarterial, recetor tipo 2 da angiotensina (AT2), enzima conversora da angiotensina e regressão da massa ventricular esquerda (MVE) pós-cirurgia. Apesar de não significativamente diferentes entre grupos, descobrimos que sobrerregulação do microARN-4268 e sub-regulação do microARN-125b-5p estão associadas a maior MVE pré-operatória. O microARN-125-5p correlaciona-se negativamente com a MVE indexada e com a espessura relativa da parede aos seis meses de seguimento. O microRNA-4268 correlaciona-se positivamente com a regressão da MVE e associa-se a níveis mais elevados do recetor AT2.
ConclusõesOs microARN-101-3p e microARN-4268, via sistema renina-angiotensina-aldosterona, têm potencial novo papel na modulação hipertrófica na EA e como preditores de remodelagem reversa pós-cirurgia. Estes resultados auxiliam na compreensão da resposta miocárdica à sobrecarga de pressão e da remodelagem reversa após diminuição da carga. Suportam também a hipótese de modulação farmacológica do sistema renina-angiotensina-aldosterona em corações hipertróficos.
Fibrosis can be defined as a non-physiological scarring process that is characterized by disproportionate deposition of extracellular matrix (ECM). This process leads to loss of tissue architecture, which impairs organ function, eventually resulting in organ failure.1
In patients with aortic stenosis (AS), left ventricular (LV) hypertrophy (LVH) is an adaptive mechanism that attempts to compensate pressure overload.2 This myocardial remodeling process includes hypertrophy of cardiomyocytes and abnormal synthesis of ECM. Myocardial fibrosis is one of the earliest modifications in AS patients, playing a key role in the progression to LV systolic and diastolic dysfunction.3
Myocardial fibrosis is the result of increased production of ECM (mainly collagen types I and III) and decreased or unchanged ECM destruction.4 Collagen turnover is regulated by a highly complex system of proteolytic and anti-proteolytic enzymes. Several studies have shown that matrix metalloproteinases and their tissue inhibitors (TIMPs) are important enzymes that regulate cardiac tissue remodeling.5,6 In LVH associated with AS, myocardial biopsies have higher expression of collagen and up-regulation of TIMP1 and TIMP2 messenger RNA (mRNA), thus favoring inhibition of collagen degradation.7 Cytokines, including transforming growth factor beta 1 (TGF-β1), tumor necrosis factor alpha (TNF-α) and connective tissue growth factor (CTGF), and the renin-angiotensin-aldosterone system (RAAS) are among numerous stimuli that contribute to myocardial fibrosis.8
MicroRNAs (miRs) are short noncoding RNAs that interfere in post-transcriptional gene regulation by targeting mRNA.9 MiRs typically act as intracellular mediators and are involved in pathophysiological processes, including cardiovascular disease.10 Cardiomyocytes, endothelial cells and fibroblasts are sources of miRs, which are released to varying degrees according to the specific cardiovascular disease.11 MiRs have recently been implicated in myocardial fibrosis, regulating enzymes and signaling pathways involved in ECM remodeling.12
In this study, we hypothesized that miR expression in myocardial biopsies from patients with severe AS could be linked to the degree of LVH and myocardial remodeling at baseline and six months after aortic valve replacement (AVR). In addition, we explored their relationship with plasma levels of important biomarkers of ECM turnover.
MethodsPatient selectionAmong a cohort of 141 consecutive patients aged over 18 years with severe symptomatic AS (aortic valve area <1 cm2 or mean transaortic gradient >40 mmHg) referred for AVR at Centro Hospitalar Universitário São João, Porto, Portugal, 56 consented to a myocardial biopsy.13 All biopsies underwent histological examination, which revealed that only 11 patients had sufficient material for miR analysis, and these were included in the present study. Patients were selected between January 2006 and December 2009 and were in sinus rhythm at the time of inclusion. Exclusion criteria were aortic regurgitation >II/IV or other significant (more than mild) valve disease, significant coronary artery disease (defined as >50% lesions on coronary angiography) or previous cardiac surgery. Explanted hearts were used as controls for myocardial biopsies.
The diagnosis of hypertension was made on the basis of clinical records. The use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) was recorded. Estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 by the Cockcroft-Gault formula was used to determine the presence of chronic kidney disease (CKD). This investigation is in accordance with the Declaration of Helsinki and was approved by the relevant institutional review board (Centro Hospitalar Universitário São João ethics committee), and each study participant provided written informed consent before enrollment.
Surgical techniqueAll surgeries were performed following standard procedures for AVR. Myocardial biopsy from the LV interventricular septum was performed at the time of surgery. Excised myocardium was immediately snap-frozen in liquid nitrogen and stored at -80°C.
Echocardiographic examinationsAll patients underwent echocardiographic assessment before and six months after surgery. Echocardiographic studies were conducted by a qualified cardiologist and digitally recorded. All records were examined in an independent accredited laboratory (Hospital Clínico San Carlos, Madrid, Spain) by an experienced echocardiographer who was blinded to patients’ data. Examinations were performed using Phillips IE-33 equipment with an S5-1 transducer and M-mode, two-dimensional, pulsed, continuous, color flow and tissue Doppler capabilities. All measurements were performed according to the recommendations of the European Association of Echocardiography (EAE)/American Society of Echocardiography (ASE).14
LV mass (LVM) was estimated according to the joint EAE/ASE recommendations,14 using Devereux's formula for measurements in diastole: LV mass=0.8×(1.04×([LV internal dimension+posterior wall thickness+interventricular septal thickness]3-[LV internal dimension]3)+0.6 g. LVH was defined as LV mass index (LVMI) >115 g/m2 in men and >95 g/m2 in women. Relative wall thickness (RWT) was calculated for the assessment of LV geometry, using the formula 2×posterior wall thickness/LV diastolic diameter. RWT was considered to be increased when this ratio was more than 0.4214. Valvuloarterial impedance (Zva) was calculated as a measure of global LV load, using the formula Zva=(SBP+MG)/SVI, where SBP is systolic blood pressure, MG is mean transvalvular pressure gradient and SVI is stroke volume index. Blood pressure was measured prior to echocardiography with patients in the supine position, and the mean of three measurements was used. All indexed values were obtained by dividing by body surface area according to the Mosteller formula.15
Measurement of plasma biomarkers of extracellular matrix remodelingBlood samples were collected from AS patients before AVR surgery in ethylenediaminetetraacetic acid tubes. All samples were centrifuged at 5000 rpm for 15 min at 4°C and plasma was separated and frozen at -80°C until analysis. Endogenous plasma levels of atrial natriuretic peptide (ANP, Biomatik), brain natriuretic peptide (BNP, Biomatik), CTGF (USCN), angiotensin II (Ang II) (Biomatik) receptor and angiotensin-converting enzyme (ACE) (Biomatik) were quantified using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. All samples were analyzed in duplicate. Absorbance was recorded at 450 nm using an ELISA plate reader (Perkin-Elmer, Wellesley, MA).
Analysis of microRNAs in myocardial biopsiesAll experiments were conducted at Exiqon Services, Denmark. Total RNAs were isolated using TriPure reagent (Roche) according to the manufacturer's instructions and further purified using an RNeasy Mini Kit (Qiagen). The quality of total RNA was verified by an Agilent 2100 bioanalyzer profile. In brief, 500 ng total RNA from both sample and reference was labeled with Hy3™ and Hy5™ fluorescent labels, respectively, using the miRCURY LNA™ microRNA Hi-Power Labeling Kit, Hy3™/Hy5™ (Exiqon, Denmark) following the procedure described by the manufacturer. The Hy3™-labeled samples and a Hy5™-labeled reference RNA sample were mixed pair-wise and hybridized to the miRCURY LNA™ microRNA Array 7th gen (Exiqon, Denmark), which contains capture probes targeting all miRs for human, mouse or rat registered in miRBASE 18.0. Hybridization was performed according to the miRCURY LNA™ microRNA Array instruction manual using a Tecan HS4800™ hybridization station (Tecan, Austria). After hybridization, the microarray slides were scanned and stored in an ozone-free environment (ozone level <2.0 ppb) in order to prevent bleaching of the fluorescent dyes. The miRCURY LNA™ microRNA Array slides were scanned using the Agilent G2565BA Microarray Scanner System (Agilent Technologies, Inc., USA) and image analysis was carried out using ImaGene® 9 (miRCURY LNA™ microRNA Array Analysis Software, Exiqon, Denmark). The quantified signals were background corrected (Normexp with offset value 10)16 and normalized using the global Lowess (LOcally WEighted Scatterplot Smoothing) regression algorithm.
An explorative analysis of the results obtained was performed, and polymerase chain reaction (PCR) was then used to validate the 40 most dysregulated miRs in AS patients compared with controls. Real-time PCR for quantitative miR expression was performed, as described previously.17 In summary, first-strand complementary DNA was synthesized with the miScript II Rt Kit (Qiagen) following the manufacturer's instructions. The PCR reaction was then performed in a 7300 qPCR system (Applied Biosystems) in accordance with the supplier's protocols.
Statistical analysisCategorical variables were expressed as percentages and continuous variables as mean ± standard deviation or median and interquartile range, according to their distribution. An independent-sample t test was used to compare miR expression between biopsies of AS patients and of healthy controls. Spearman's rank correlation was used to determine correlations between miR expression and echocardiographic parameters and biomarkers. Logarithmic transformation of miR and biomarker expression was used to plot graphs. All probability values are two-tailed, and p<0.05 was considered statistically significant. The statistical analysis was performed with IBM® SPSS® Statistics version 25.0 (IBM SPSS Corporation, USA). Graphs were plotted using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA).
ResultsCharacteristics of the study cohortOur study group consisted of 11 patients with severe symptomatic AS referred for AVR (45.5% men, mean age 70.4±18.9 years). The demographic, clinical, and echocardiographic (before and six months after AVR) characteristics of the study cohort are shown in Tables 1 and 2. The characteristics of the control group for miR expression in biopsies are not presented, since explanted hearts were used as controls. At baseline, 63.6% were in New York Heart Association (NYHA) functional class I-II. Hypertension was diagnosed in 63.6%, however only 33.3% were under ACEI/ARB therapy. Before surgery, patients had a mean LVMI of 132.7 g/m2 (119.8-146.4 g/m2) and 90.9% had gender-specific criteria for LVH. Six months after AVR, median LVMI was 112.3 g/m2 (88.0-127.0 g/m2) with a median LV mass variation (ΔLVM) of 65.3 g/m2 (28.5-89.0 g/m2). The expression of plasma ECM turnover biomarkers was also measured at baseline in this subgroup of patients. These findings are summarized in Table 3. There were no differences regarding miR expression when stratified according to gender, age group, NYHA classification, hypertension, use of ACEI/ARB or CKD.
Clinical characteristics of patients with aortic stenosis (n=11).
| Age, years | 70.4±18.9 |
| Male gender | 5 (45.5%) |
| BSA, m2 | 1.7±0.2 |
| Hypertension, n (%) | 7 (63.6%) |
| ARB/ACEI, n (%)a | 3 (33.3%) |
| Diabetes, n (%) | 1 (9.1%) |
| CKD, n (%) | 6 (54.5%) |
| eGFR, ml/min | 62.7±15.4 |
| NYHA ≥III, n (%) | 4 (36.4%) |
| LVH at baseline, n (%) | 10 (90.9%) |
n=9.
Values are mean ± standard deviation unless otherwise indicated.
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BSA: body surface area; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; LVH: left ventricular hypertrophy; NYHA: New York Heart Association functional class.
Echocardiographic measures in patients with aortic stenosis.
| Baseline | Six months | |
|---|---|---|
| Interventricular septum, cm | 1.3 (1.2-1.5) | 1.30 (1.10-1.50) |
| Posterior wall, cm | 1.00 (0.98-1.20) | 1.00 (0.83-1.20) |
| RWT | 0.45 (0.42-0.48) | 0.44 (0.38-0.50) |
| LVMI, g/m2 | 132.7 (119.8-146.4) | 112.3 (88.0-127.0) |
| LVED volume index, ml/m2 | 50.6 (38.8-59.5) | 44.2 (37.1-56.9)a |
| Zva, mmHg/ml/m2 | 6.1 (5.4-7.6) | - |
| ΔLVM, g/m2 | - | 65.3 (28.5-89.0) |
| LA volume index, ml/m2 | 38.8 (32.4-50.0)1 | 34.2 (27.3-44.2)b |
| AV area index, cm2/m2 | 0.45 (0.34-0.48) | 0.77 (0.67-0.83)c |
n=6.
Values are median (interquartile range) unless otherwise indicated.
ΔLVM: left ventricular mass variation; AV: aortic valve; LA: left atrial; LV: left ventricular; LVED: left ventricular end-diastolic; LVMI: left ventricular mass index; RWT: relative wall thickness; Zva: valvuloarterial impedance.
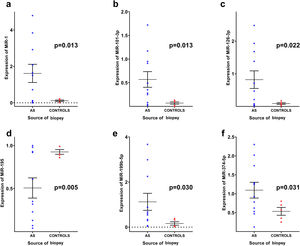
Out of the 40 miRs investigated (Supplementary Table 1), miR-1 (1.62±1.65 vs. 0.11±0.09; p=0.013), miR-101-3p (0.93±0.55 vs. 0.07±0.05; p=0.013), miR-126-3p (0.82±0.89 vs. 0.09±0.06; p=0.022), miR-199b-5p (1.12±1.25 vs. 0.16±0.17; p=0.030) and miR-374a-5p (1.09±0.69 vs. 0.53±0.23; p=0.031) were significantly upregulated in myocardial biopsies of AS patients compared to controls, while only miR-195 (0.51±0.39 vs. 0.93±0.06; p=0.005) was significantly downregulated (Figure 1). Expression of all other miRs did not differ significantly between groups, as shown in Supplementary Table 2.
Differences in microRNA expression in myocardial biopsies between aortic stenosis patients and controls. Graphs represent mean ± standard error of the mean. The following values are shown as mean ± standard deviation: (a) miR-1 (1.62±1.65 vs. 0.11±0.09; p=0.013); (b) miR-101-3p (0.93±0.55 vs. 0.07±0.05; p=0.013); (c) miR-126-3p (0.82±0.89 vs. 0.09±0.06; p=0.022); (d) miR-195 (0.51±0.39 vs. 0.93±0.06; p=0.005) (e) miR-199b-5p (1.12±1.25 vs. 0.16±0.17; p=0.030); (f) miR-374a-5p (1.09±0.69 vs. 0.53±0.23; p=0.031). Data were analyzed using the independent-samples t test. AS: aortic stenosis.
To determine whether differentially expressed miRs were possible mediators of LV remodeling in AS, we analyzed the correlation between miR expression and echocardiographic parameters (LVMI, RWT and Zva at baseline, and RWT, LVMI and ΔLVM at six-month follow-up), and biomarkers of ECM remodeling (ANP, BNP, ACE, CTGF and Ang II receptor) (Table 4).
Correlations between microRNAs associated with aortic stenosis and echocardiographic variables and biomarkers.
| miR-1 | miR-101-3p | miR-126-3p | miR-195 | miR-199b-5p | miR-374a-5p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | rs | p | rs | p | rs | p | ||
| Echocardiographic variables | |||||||||||||
| Baseline | |||||||||||||
| RWTa | -0.151 | 0.658 | 0.274 | 0.415 | -0.251 | 0.456 | 0.151 | 0.658 | 0.137 | 0.688 | -0.137 | 0.688 | |
| LVMIa | -0.118 | 0.729 | 0.373 | 0.259 | -0.555 | 0.077 | 0.118 | 0.729 | -0.364 | 0.272 | -0.036 | 0.915 | |
| Zvab | 0.006 | 0.987 | 0.770 | 0.009 | -0.285 | 0.425 | -0.006 | 0.987 | 0.079 | 0.829 | -0.285 | 0.425 | |
| 6-months | |||||||||||||
| RWTa | -0.378 | 0.252 | 0.392 | 0.233 | -0.515 | 0.105 | 0.378 | 0.252 | -0.123 | 0.719 | -0.073 | 0.831 | |
| LVMIa | -0.378 | 0.252 | 0.196 | 0.564 | -0.706 | 0.015 | 0.378 | 0.252 | -0.378 | 0.252 | -0.228 | 0.501 | |
| ΔLVMa | -0.173 | 0.612 | 0.727 | 0.011 | -0.073 | 0.832 | 0.173 | 0.612 | 0.300 | 0.370 | 0.073 | 0.832 | |
| Biomarkers | |||||||||||||
| ANPc | 0.107 | 0.819 | 0.679 | 0.094 | -0.500 | 0.253 | -0.107 | 0.819 | -0.500 | 0.253 | -0.143 | 0.760 | |
| BNPd | -0.333 | 0.381 | 0.300 | 0.433 | -0.367 | 0.332 | 0.333 | 0.381 | -0.217 | 0.576 | -0.017 | 0.966 | |
| AT2 receptore | -0.200 | 0.704 | 0.829 | 0.042 | -0.714 | 0.111 | 0.200 | 0.704 | -0.714 | 0.111 | -0.943 | 0.005 | |
| ACEe | -0.371 | 0.468 | 0.829 | 0.042 | -0.371 | 0.468 | 0.371 | 0.468 | -0.371 | 0.468 | -0.771 | 0.072 | |
| CTGFe | 0.029 | 0.957 | 0.371 | 0.468 | -0.829 | 0.042 | -0.029 | 0.957 | -0.829 | 0.042 | -0.600 | 0.208 | |
n=6.
Spearman's rank correlation was used. Significant correlations are in bold.
ΔLVM: left ventricular mass variation; ACE: angiotensin-converting enzyme; Ang II: angiotensin II; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; CTGF: connective tissue growth factor; LVMI: left ventricular mass index; RWT: relative wall thickness; Zva: valvuloarterial impedance.
Among the variables studied, miR-101-3p was positively correlated with Zva (rs=0.77, p=0.009), ΔLVM at six-month follow-up (rs=0.727, p=0.011), and Ang II receptor (rs=0.829, p=0.042) and ACE (rs=0.829, p=0.042) plasma levels. Expression of miR-126-3p was negatively correlated with LVMI at six-month follow-up (rs=-0.706, p=0.015) and CTGF (rs=-0.829, p=0.042). Concerning miR-199b-5p, only a negative correlation with plasma CTGF level (rs=-0.829, p=0.042) was identified. Regarding miR-374-5p, only a negative correlation with Ang II receptor expression (rs=-0.943, p=0.005) was found. Expression of miR-1 and mir-195 was not correlated with any echocardiographic measures or plasma ECM biomarkers. No other relevant correlations between miR expression and echocardiographic variables and biomarkers were found.
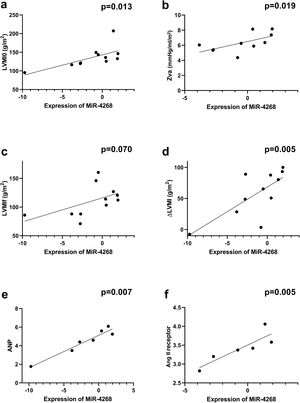
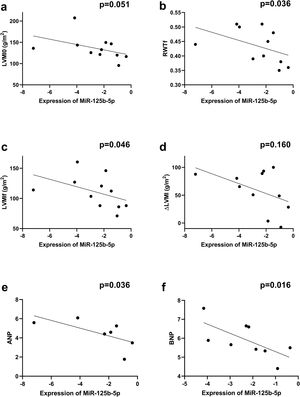
Correlations between overall microRNA expression in aortic stenosis and ventricular remodelingTo further elucidate the role of miRs in ventricular remodeling, correlations were determined between all the studied miRs and the echocardiographic parameters and biomarkers of ECM remodeling described above (Supplementary Table 3). We found that miR-4268 was positively correlated with LVMI at baseline (rs=0.718, p=0.013, Figure 2a), Zva (rs=0.721, p=0.019, Figure 2b), ΔLVM at six-month follow-up (rs=0.773, p=0.005, Figure 2d), ANP (rs=0.893, p=0.007, Figure 2e) and Ang II receptor (rs=0.943, p=0.005, Figure 2f). Regarding miR-125b-5p, a negative correlation was found with LVMI at six-month follow-up (rs=-0.610, p=0.046, Figure 3c), RWT (rs=-0.633, p=0.036, Figure 3b), ANP (rs=-0.786, p=0.036, Figure 3e) and BNP (rs=-0.767, p=0.016, Figure 3f).
Correlations between miR-4268 expression and echocardiographic variables and biomarkers. Spearman's rank correlation was used. ΔLVM: left ventricular mass variation; Ang II: angiotensin II; ANP: atrial natriuretic peptide; LVMI0: left ventricular mass index at baseline; LVMIf: left ventricular mass index at six-month follow-up; Zva: valvuloarterial impedance. MiR-4268 was positively correlated with (a) LVMI0 (rs=0.718, p=0.013, n=11), (b) Zva (rs=0.721, p=0.019, n=10), (d) ΔLVM (rs=0.773, p=0.005, n=11), (e) ANP (rs=0.893, p=0.007, n=7) and (f) Ang II receptor (rs=0.943, p=0.005, n=6).
Correlations between miR-125b-5p expression and echocardiographic variables and biomarkers. Spearman's rank correlation was used. ΔLVM: left ventricular mass variation; ANP: atrial natriuretic peptide; LVMI0: left ventricular mass index at baseline; LVMIf: left ventricular mass index at six-month follow-up; RWTf: relative wall thickness at six-month follow-up. MiR-125b-5p was negatively correlated with (b) RWT (rs=-0.633, p=0.036, n=11), (c) LVMIf (rs=-0.610, p=0.046, n=11), (e) ANP (rs=-0.786, p=0.036, n=7) and (f) BNP (rs=-0.767, p=0.016, n=9).
In this study, we analyzed differences in expression of miRs in myocardial biopsies of patients with severe symptomatic AS and compared it with that of explanted hearts. We also investigated the potential impact of miR expression on LV mass and reverse remodeling and plasma levels of ECM biomarkers. Compared with controls, we found an overexpression of miR-101-3p in AS. Interestingly, this was associated with higher plasma levels of Ang II receptor and ACE, suggesting it may be involved in the response of the RAAS to increased load. Additionally, it had a positive correlation with LV mass regression after surgery, implying that patients with higher levels of miR-101-3p before surgery could have a more favorable response to AVR.
Although they were not differently expressed in AS compared with controls, we found that miR-4268 and miR-125b-5p may play a pivotal role in ventricular remodeling. Both upregulation of miR-4268 and downregulation of miR-125-5p were strongly correlated with LVMI. Although we were unable to find correlations between ECM biomarkers and miR-125b-5p that could explain its relationship with LVH, we found that it correlates negatively with both LVMI and RWT at six-month follow-up.
We also found that, although miR-4268 is not upregulated in AS patients, it correlates positively with LV mass regression and is associated with higher plasma Ang II receptor levels. This correlation might explain the regression of hypertrophy after surgery, since this receptor has antihypertrophic and antifibrotic properties. All these effects warrant further study in order to understand the underlying processes.
Importance of microRNAs in ventricular remodeling in aortic stenosisMiR expression in cardiac biopsies differs between AS and non-AS patients, and this variation could explain different ventricular remodeling responses before and after AVR. Our study found six miRs that may be involved in this process and could provide further knowledge in this area.
MiR-101-3p, which was found to be upregulated in our AS patients, had only been identified as a biomarker for acute rejection in heart transplantation, but no other association with cardiac disease or with the RAAS has yet been described.18 This miR has also been associated with several oncogenic pathways, however we are the first to report its effects in AS. We found that upregulation of miR-101-3p is associated with preoperative Zva, a measure of global LV load, and that its overexpression at the time of surgery correlates with regression of LVH, an important goal after AVR.13 This could be explained by the decrease in load after surgery. This MiR-101-3p overexpression is correlated with Ang II receptor and ACE levels, suggesting that Ang II receptor and ACE expression may be a counter-regulatory mechanism in response to increased load. After surgery, downregulation of miR-101-3p may facilitate the protective effects of Ang II receptor stimulation. Previous studies reported that the RAAS plays a pivotal role in hypertrophy and fibrosis, since mechanical stretch stimulates local production of Ang II, inducing the release of multiple growth factors and cytokines from cardiac fibroblasts and leading to the progression of cardiac hypertrophy and remodeling.19,20 Ang II receptors are expressed at low levels in the heart. However, research shows that in pathological conditions such as hypertension and myocardial infarction, it plays a significant antifibrotic and antihypertrophic role.21 Decreases in load may thus reverse RAAS activation and contribute to LV reverse remodeling.
A previous study in nude rats with ischemic cardiomyopathy found that high plasma miR-126-3p levels were associated with endothelial function, regulating proangiogenic and anti-inflammatory cytokine expression, and could enhance cardiac function by decreasing infarction size, increasing angiogenesis, and inhibiting inflammation in the heart.22 However, this miR has never been associated with the RAAS or CTGF. In our study, we found this miR to be overexpressed in AS patients. It was negatively correlated with CTGF and with LVMI at six-month follow-up, suggesting that over-expression of this miR may be a protective response of cardiac tissue to the deleterious effect of chronic pressure overload in these patients.
Regarding miR-199b-5p, its effects in the heart have been studied mainly in LV remodeling after myocardial infarction, and its upregulation is associated with a poor outcome in this subset of patients.23 This miR has also been identified as a potent regulator of pathological cardiac hypertrophy by activating calcineurin/nuclear factor of activated T-cell (CnA/NFAT) signaling.24 There are no reports in the literature of associations between this miR and the RAAS. We found that this miR is also upregulated in patients with AS, but no correlation was found with echocardiographic data to suggest a role in hypertrophy. Since we did not study the CnA/NFAT signaling pathway, we cannot provide further data regarding the effects of this miR in AS patients.
MiR-374a-5p has never been associated with cardiac disease or the RAAS in the literature, however this miR has several associations including with oncogenic pathways, and has an anti-inflammatory effect in patients with obesity.25 In our study, miR-374a-5p was found to be upregulated in AS compared to controls and was negatively correlated with plasma Ang II receptor levels, suggesting that this miR might be a potential additional regulator against the negative effect of the RAAS in the heart.
We also found miR-1 and miR-195 to be significantly altered in AS patients. MiR-1 is a well-studied mediator in patients with heart failure and is a key regulator of cardiac hypertrophy, since high levels of this miR are associated with improvement of cardiac function.26 In our study, no relationship was found between this miR and echocardiographic measures or plasma ECM biomarkers. A previous study found that downregulation of miR-195 is associated with valvular calcification via targeting Mothers against decapentaplegic homolog 7 (SMAD7), which promotes the development of fibrosis and ECM remodeling.27 In our study, we found that this miR is downregulated in AS, which is consistent with the literature, but, as we did not have data on valvular calcification, we were unable to confirm this mechanism. Moreover, both miRs have been linked to the RAAS in the literature. A previous study aimed to determine the effects of chronic losartan treatment on cardiac ischemia and reperfusion injury in rats found that it upregulates the angiotensin I receptor and significantly increases several miRs, including miR-1, leading to increased cardiac vulnerability to ischemia and reperfusion injury.28 Enhancement of MiR-195 expression in an Ang II-induced cardiac hypertrophy mouse model suppressed the effects of TGF-β, a known profibrotic and prohypertrophic mediator.29
Unlike previous studies assessing miR expression in AS patients, we did not obtain significantly different results regarding the miR-29, miR-21 and miR-133 family.30,31 One potential explanation for this discrepancy could be that those studies were performed using serum miRs, while we used cardiac tissue to assess miR expression.
Role of other microRNAs in ventricular remodelingTo the best of our knowledge, this is the first report in the literature of miR-4268 as a possible marker of cardiac disease or in RAAS regulation. In our study, we found this miR to be strongly correlated with LVMI at baseline and with ΔLVM. It may thus be a new marker of cardiac hypertrophy in chronic pressure overload and a predictor of success in AVR surgery, since patients with high levels of miR-4268 had a better reverse remodeling response. We also found this miR to be positively correlated with Zva, meaning that it is overexpressed in response to increased LV load. This could explain the correlation with LV mass measures in echocardiographic exams. Furthermore, we were able to correlate this miR positively with ANP, a marker of atrial pressure overload, which occurs in the presence of elevated LV filling pressures in LVH. In similar fashion to miR-101-3p, we found that the prohypertrophic and profibrotic effects of miR-4268 are possibly mediated through RAAS modulation in response to increased load.
Previous studies found that miR-125b-5p is downregulated in heart failure and a murine knockdown model of miR-125b suppressed Ang II-induced cardiac fibrosis by regulating fibroblast proliferation.17,32 In our study, we found that low levels of this miR are correlated with higher LV mass and RWT at six-month follow-up. These findings might help explain the effects of this specific miR in the progression of concentric remodeling to heart failure in patients with hypertrophy and AS. We also found this miR to be negatively correlated with BNP, which is consistent with its role in heart failure.
LimitationsOur study was limited by the small sample size and the fact that we only measured plasma biomarkers in some patients. However, this limitation is common in similar studies, especially those using myocardial biopsies. Additionally, clinical and echocardiographic information was not available on patients whose biopsies were used as controls. This information might have helped in further understanding the clinical variables that affect miR expression.
One of the major issues regarding this work is the impossibility of determining the influence of therapeutic RAAS inhibition on miR expression due to lack of a significant number of patients under this therapy: only three of the nine patients with data regarding medical therapy were prescribed RAAS inhibitors, thus limiting our ability to observe differences.
ConclusionsOur results, showing the role of miR-101-3p and miR-4268 in the hypertrophic response in AS, shed light on possible new mediators involved in myocardial remodeling. Their association with the RAAS probably reflects their role as regulators of this pathway and supports the concept of RAAS modulation in hypertrophic hearts. In the future, there may be a role for assessment of miR-101-3p and miR-4268 levels as markers for LV remodeling after AVR, since higher levels of these miRs are related to higher levels of LV mass regression. We also found other new cardiac miRs that may play significant roles in AS. Further studies are needed regarding this matter to provide more detailed information.
In view of our findings, it is tempting to use RAAS antagonists in AS patients, and some specific miRs could help to identify suitable candidates for this treatment. Nevertheless, more information and large-scale randomized clinical trials are required in order to establish the role of RAAS blockade after AVR.
Funding sourcesThis work was supported by the Portuguese Foundation for Science and Technology (POCI/SAU-PIC/IC/82943/2007).
Conflicts of interestThe authors have no conflicts of interest to declare.
These authors are co-authors of this article and both contributed equally in the making of this work.