We present the case of a previously healthy 42-year-old man who attended the emergency department due to a sudden onset of rapid and regular palpitations. The ECG showed 190 bpm, wide QRS with left bundle branch block tachycardia. He was started on amiodarone with progression to 230 bpm, wide QRS tachycardia with multiple morphologies, followed by spontaneous conversion to sinus rhythm, normal PR interval and rS pattern in LIII. The echocardiogram was negative for structural heart disease. The electrophysiological study demonstrated the presence of an accessory pathway with anterograde decremental conduction and no retrograde conduction. Both episodes of clinical tachycardia were induced. A diagnosis of Mahaim fiber-mediated antidromic atrioventricular reentrant tachycardia and pre-excited atrial fibrillation was made. Mapping was performed with detection of an M potential (His-like) at the lateral region of the tricuspid ring followed by radiofrequency ablation with immediate success criteria. Post-ablation there was a change to a qR pattern in LIII. At 12-months follow-up there was no recurrence of the tachycardia.
É apresentado um caso de um doente de 42 anos, previamente saudável, com episódio de palpitações de início súbito, rápidas e regulares que motivaram ida ao serviço de urgência. O ECG mostrou taquicardia regular, 190 bpm, QRS alargados e padrão de bloqueio de ramo esquerdo. Foi instituída perfusão de amiodarona com progressão para taquicardia irregular, 230 bpm com QRS alargados e de diferentes morfologias seguida de conversão espontânea a ritmo sinusal, com intervalo PR de duração normal e padrão rS em DIII. O ecocardiograma não mostrava cardiopatia estrutural. O estudo eletrofisiológico demonstrou a presença de via acessória sem capacidade de condução retrógrada e com condução anterógrada com propriedades decrementais; e indução de ambas as taquidisritmias clínicas. Foi feito o diagnóstico de taquicardia de reentrada auriculoventricular antidrômica e de fibrilação auricular pré-excitada mediadas por via acessória do tipo Mahaim. Foi efetuado mapeamento com detecção de potencial M (His-like) no nível do anel tricúspide lateral e feita ablação com radiofrequência com critérios de sucesso imediato. Após ablação verificou-se alteração do padrão em DIII para qR. Após 12 meses de seguimento não se verificou recorrência da taquidisritmia.
Mahaim accessory pathways (MAP) are uncommon accessory pathways (APs) with specific characteristics. In general, they are located in the lateral region of the tricuspid annulus, establish a direct connection between the atrial tissue and the infra-His conduction tissue (most commonly the right branch of the bundle of His), and are characterized by (AV-node-like) anterograde decremental conduction and a lack of retrograde conduction. MAPs cause various types of dysrhythmias. They are associated with other APs in 40% of cases and have been associated with Ebstein's anomaly. The treatment of choice is radiofrequency ablation.
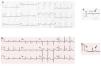
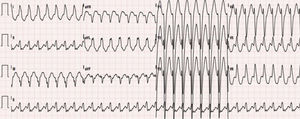
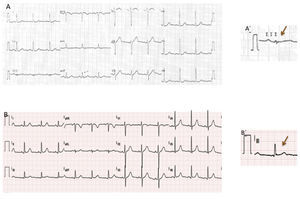
Case reportWe present the case of a healthy 42-year-old male who presented at the emergency department due to a one-hour history of sudden-onset rapid and regular palpitations. The patient was awake, oriented, eupneic, and had a systemic blood pressure of 130/80 mmHg and a heart rate of 190 bpm. He had no congestive or low cardiac output symptoms. The 12-lead electrocardiogram (ECG) showed regular tachycardia, 190 bpm, with widened QRS complexes (131 ms), left bundle branch block (LBBB) morphology, axis -30°, with monophasic R in L1, rS in V1, and RS transition in V5 (Figure 1). Amiodarone infusion was initiated with progression to irregular tachycardia, 230 bpm (occasional RR intervals <200 ms), widened QRS complexes of varying morphologies (Figure 2) and preserved hemodynamic stability. After two minutes, spontaneous conversion to sinus rhythm, 70 bpm, a normal PR interval and an rS pattern in L3 were confirmed (Figure 3A).
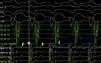
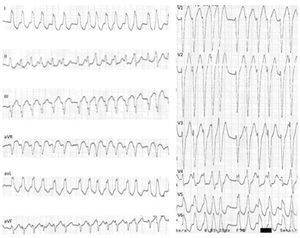
The patient was admitted to the Cardiology Department with a diagnosis of wide complex tachycardia with LBBB morphology of undetermined etiology. Laboratory tests were normal. Transthoracic echocardiography showed no structural heart disease. An electrophysiology study (EPS) revealed the following: 1) Retrograde ventriculoatrial conduction via nodal pathway, with no evidence of retrograde conduction through accessory pathway (VA interval with progressive prolongation during incremental ventricular pacing) – Supplemental Figure 1A; 2) Under incremental atrial pacing, the AV interval increased (increase in AH interval, reduction in HV) and progressive QRS widening (evidence of ventricular pre-excitation via AP with decremental anterograde properties) – Supplemental Figures 1B, 1C and Supplemental Figure 2; 3) Induction of antidromic atrioventricular reentrant tachycardia (AVRT) with LBBB morphology, similar to the documented tachycardia – Supplemental Figures 3A and 3B; 4) Progression to pre-excited atrial fibrillation, mimicking clinical arrhythmia – Supplemental Figures 4A and 4B. These findings led to the diagnosis of MAP-mediated antidromic AVRT and pre-excited atrial fibrillation. Mapping detected (His-like) Mahaim (M) potential at the lateral tricuspid annulus and radiofrequency ablation was immediately successful (Figure 4) – elimination of the M potential and inability to induce AVRT. In the post-ablation ECG, there was a change of the pattern in L3 to qR, suggesting “masked” pre-excitation in the previous tests in sinus rhythm (Figure 3B). At 12 months of follow up, there was no recurrence of tachycardia and ECG showed no pre-excitation.
DiscussionThe differential diagnosis of regular wide complex tachycardias with LBBB pattern – QRS >130 ms, QS or rS in V1, monophasic R (without Q) in V6 and notching in ≥2 leads of V1, V2, V5, V6, L1, or aVL1 – and left axis deviation is limited to three conditions: 1) Supraventricular tachycardia (SVT) with typical LBBB aberrancy (fixed or functional); 2) MAP-mediated SVT; 3) Intramyocardial ventricular tachycardia (i.e., region adjacent to the lateral tricuspid annulus), or bundle branch reentrant ventricular tachycardia.2
MAPs are associated with two different types of SVT: 1) SVT in which the MAP is part of the tachycardia circuit; 2) SVT in which the MAP is not part of said circuit, but contributes to ventricular activation. Antidromic AVRT falls under the first SVT group. It involves anterograde conduction through the MAP and retrograde conduction through the AV node, or alternatively through another AP (present in up to 40% of MAP cases). Since in most cases MAPs are inserted in the distal portion of the right branch of the bundle of His, ventricular activation resulting from the anterograde conduction through the MAP shows an LBBB pattern in the ECG. MAPs are characterized by the absence of retrograde conduction, so there is no possibility of orthodromic AVRT (anterograde conduction through the AV node and retrograde conduction through the MAP). Atrial tachycardia, atrial flutter, atrial fibrillation, and atrioventricular nodal reentrant tachycardia (AVNRT) are in the second SVT group, and the MAP is not part of the tachycardia circuit but may be a channel for ventricular activation. Moreover, in said group, AV conduction may occur via the MAP or the AV node, conferring varying morphologies to the tachycardia.
In regular wide complex tachycardia with an LBBB pattern, a non-invasive differential diagnosis should be considered based on a previous ECG, ECG during tachycardia and transthoracic echocardiography. Regarding the findings in the previous ECG, the following should be noted: 1) Presence or absence of typical LBBB in the patient's baseline rhythm. The presence of typical LBBB with characteristics similar to those of tachycardia is suggestive of SVT with fixed typical LBBB aberrancy. Nevertheless, the pattern of typical LBBB during tachycardia may result from tachycardia-dependent (functional) conduction delay in the left branch of the bundle of His, so the absence of typical LBBB on the previous ECG does not rule out SVT with functional typical LBBB aberrancy. 2) Presence or absence of signs of ventricular pre-excitation (short PR and delta wave). However, such signs are rare in MAPs. The ECG during tachycardia may be suggestive of antidromic AVRT associated with an MAP. Bardy GH et al.3 suggest that, in regular wide complex tachycardia with a typical LBBB pattern, meeting two of the following criteria are suggestive of antidromic AVRT associated with an MAP: heart rate between 130 bpm and 270 bpm, QRS axis between 0° and -75°, QRS duration <150 ms, R in L1, rS in V1 and precordial transition in V4, V5 or V6. Moreover, the transthoracic echocardiography is useful in significant structural heart disease, since it makes the diagnostic hypothesis of intra-myocardial ventricular tachycardia or bundle branch reentrant ventricular tachycardia more likely. No previous ECG was available for this patient; the wide complex tachycardia with an LBBB pattern was fully suggestive of MAP-mediated antidromic AVRT and the echocardiography did not show any structural heart disease. It should be noted that in the event of diagnostic doubt, any wide complex tachycardia should be treated in the emergency department assuming a diagnosis of ventricular tachycardia.
The cause of regular wide complex tachycardia with a typical LBBB pattern and left axis deviation is definitively diagnosed by means of EPS. In particular, for MAP, diagnosis is made based on the presence of ventricular pre-excitation mediated by accessory pathway with anterograde decremental conduction and the absence of retrograde conduction. Inducing clinical tachycardia confirms the diagnosis.
Anatomically, most MAPs are located at the lateral tricuspid annulus. However, their location at the tricuspid annulus can vary, especially in patients with Ebstein's anomaly. Mapping of atrial and ventricular/fascicular insertions of the MAP is controversial and problematic. On one hand, since MAPs do not entail retrograde conduction, mapping the atrial insertion by means of ventricular pacing is not an option. On the other hand, the ventricular insertion is generally quite long and branches out along the myocardium and right branch of the bundle of His, which makes ablation a lengthy process and makes a complete ablation practically impossible. In addition, some patients with MAPs who have undergone ventricular/fascicular insertion ablation developed an ablation-induced proarrhythmia due to delayed conduction of the right branch of the bundle of His and consequent facilitation of antidromic AVRT, which may become incessant.
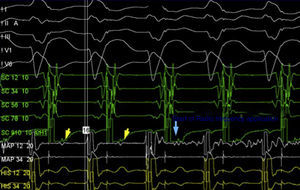
Currently, the most effective mapping and ablation technique involves detecting the M potential in sinus rhythm.4 This potential is located between the atrial and ventricular electrograms and can have a His-like morphology or can be narrow and of low amplitude (Figure 4). The M potential is recorded at the atrial insertion site of the MAP and, therefore, is considered a good predictor of a suitable ablation site. The success rate of M potential-guided ablation is 90-100%. Given the intracardiac location of this type of pathway, trauma-induced conduction loss associated with manipulation of the catheter is common. This conduction loss may last for minutes to hours and may compromise mapping and subsequent MAP ablation. Therefore, it is preferable to perform ablation during atrial pacing and to use long sheaths in order to stabilize the ablation catheter, or alternatively to use electroanatomical mapping systems that enable the surgeon to return to the previous location of the catheter. Mahaim automatic tachycardia (MAT) generally occurs during radiofrequency ablation and results from increased heat-induced automaticity in AV node-like tissue (similar to the junctional rhythm seen during ablation of the slow pathway in AVNRT). Other less effective techniques in MAP mapping involve detecting the site with the shortest interval between pacing on the atrial side of the tricuspid annulus and the earliest pre-excited QRS; and the introduction of atrial extra-stimulus during antidromic AVRT to detect the site where the latest extra-stimulus induces a “reset” of the tachycardia. Finally, if the two methods above are unsuccessful, electroanatomical activation mapping can be performed. The earliest annular and ventricular activation can be determined and ablation of the site can be performed.
Post-ablation ECG seems to be essential in confirming the success of the procedure.5 The resting ECG of patients with MAPs is normal due to preferential ventricular activation via the AV node at normal heart rates. However, 60% of patients with MAPs have an rS pattern in L3, which occurs in only 6% of the healthy young population. After MAP ablation, there is a change in the pattern in L3 to a qR- or R-type morphology. This finding suggests that, in this group of patients, the rS pattern in L3 may be a marker of “masked” ventricular pre-excitation.
In the case described in this paper, MAP mapping was performed by detecting the M potential, followed by radiofrequency ablation with immediate success (elimination of the M potential and inability to induce AVRT). There was also a change in the pattern in L3 from rS pre-ablation to qR post-ablation. After 12 months of follow up, there was no recurrence of the tachycardia.
ConclusionMAPs exhibit AV-node-like properties and are associated with two types of tachycardia: SVT in which the MAP is part of the tachycardia circuit and SVT in which the MAP is not part of the circuit, but contributes to ventricular activation. In general, MAPs are located in the lateral tricuspid annulus. Multiple techniques can be used to achieve MAP mapping. Mapping and ablation of the site with the M potential in the tricuspid annulus has the greatest success rate. The occurrence of MAT is considered a predictor of success. Post-ablation ECG is important in confirming the success of the procedure.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Lima da Silva G, Cortez-Dias N, Bernardes A, de Sousa J. Taquicardia mediada por via Mahaim. Rev Port Cardiol. 2018;37:265.e1–265.e5.