We aimed in this study to assess the role of longitudinal left ventricular (LV) systolic function in heart failure with preserved ejection fraction (HFpEF) in delayed intra- and interatrial conduction time.
MethodsIn 85 consecutive patients with HFpEF (age 60±11 years, ejection fraction [EF] ≥45%), a complete M-mode echocardiographic and tissue Doppler imaging (TDI) study was performed. The times from the onset of the P wave on the ECG to the beginning of the A′ wave (PA) from the lateral and septal mitral and tricuspid annuli on TDI were recorded. The difference between these intervals gave the intra- and interatrial dyssynchrony. Based on mitral annular plane systolic excursion (MAPSE), patients were classified as having HFpEF with impaired (MAPSE ≤1.2 cm) or normal (MAPSE >1.2 cm) longitudinal systolic function.
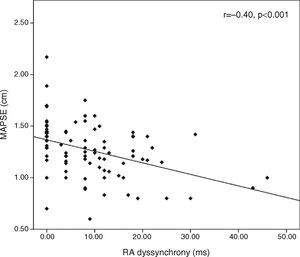
ResultsPatients with impaired MAPSE were older (p<0.001), had higher LV mass index (p<0.001), greater left atrial (LA) minimum volume (p=0.007), reduced left atrial EF (p<0.001), higher E/e′ ratio (p=0.002), reduced lateral and septal e′ wave (p=0.005 and p=0.006, respectively), prolonged tricuspid PA′ (p=0.03) and significantly increased right atrial (RA) dyssynchrony (p=0.001) compared with normal MAPSE. MAPSE correlated with RA dyssynchrony (r=−0.40, p<0.001) but not with interatrial and LA dyssynchrony.
ConclusionIn patients with HFpEF and impaired MAPSE, RA dyssynchrony is increased, compared to those with normal MAPSE. As patients with RA dyssynchrony are at higher risk for arrhythmia, assessment of this dyssynchrony may help to improve treatment, as well as to predict outcome in these patients.
Com este estudo pretendemos avaliar o papel da função sistólica longitudinal ventricular esquerda (VE) na insuficiência cardíaca com fração de ejeção preservada (ICFEp) no atraso da condução intra e interauricular.
MétodosEm 85 doentes consecutivos com ICFEp (60 ± 11 anos, FE ≥ 45%) foi realizado estudo ecocardiográfico completo modo-M e Doppler tecidular. O intervalo desde o início da onda P no ECG, até ao início da onda A no anel mitral lateral e septal e no anel tricúspide direito (em Doppler tissular) foi registado. A diferença entre estes intervalos forneceu a dessincronia intra e interauricular. Baseado na excursão sistólica de pico do anel mitral (ESPAM), os doentes foram divididos em ICFEp com disfunção (ESPAM ≤ 1,2 cm) e função sistólica normal longitudinal (ESPAM > 1,2 cm).
ResultadosOs doentes com ESPAM baixa eram mais velhos (p < 0,001), tinham índice de massa VE mais elevado (p < 0,001), volume auricular esquerdo (VAE) superior (p = 0,007), FE auricular esquerda (AE) mais baixa (p < 0,001), rácio E/e’ mais elevado (p = 0,002), onda e’ lateral e septal reduzidas (p = 0,005 e p = 0,006), onda tricúspide PA prolongada (p = 0,03) e aumento significativo da dessincronia da aurícula direita (AD) (p = 0,001), quando comparados com doentes com ESPAM normal. A ESPAM estava correlacionada com a dessincronia AD (r = -0,40, p < 0,001), mas não com a dessincronia interauricular e da AE.
ConclusãoNos doentes com ICFEp e ESPAM baixa, existe dessincronia AD, quando comparada com os doentes com ESPAM normal. Como os doentes com dessincronia AD têm risco mais elevado de arritmia, a avaliação desta dessincronia pode ajudar a melhorar o tratamento, bem como a auxiliar na previsão prognóstica destes doentes.
Heart failure (HF) with preserved ejection fraction (HFpEF) is a major public health problem worldwide, with increasing prevalence and morbidity.1 Although current therapeutic approaches have improved quality of life, mortality remains high.2,3 Many studies have reported comorbidity of atrial fibrillation (AF) in HFpEF as a result of electrical and structural remodeling of the atria.4,5 Atrial remodeling in these patients may cause delayed intra- and interatrial conduction time, as well as atrial dyssynchrony,6,7 which is among the factors responsible for the initiation of reentry and the development of AF.8,9
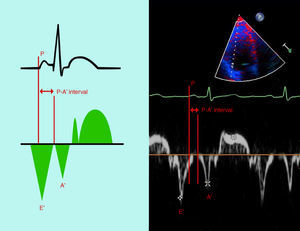
The time from the onset of the P wave on the ECG to the beginning of the A′ wave on tissue Doppler imaging (TDI) (PA′-TDI interval) is a simple and noninvasive method for assessing atrial conduction time10,11 which correlates strongly with total atrial conduction time in invasive procedures.12
The presence of atrial dyssynchrony and prolonged total intra- and interatrial activation time in HF patients has been previously reported.13,14 Several biochemical and echocardiographic parameters have been shown to correlate with atrial dyssynchrony,15,16 such as left ventricular ejection fraction (LVEF) and diastolic dysfunction.17,18 However, the relationship of longitudinal left ventricular (LV) systolic function, assessed by mitral annular plane systolic excursion (MAPSE), with delayed atrial conduction time has not been tested. We aimed in this study to investigate the relationship between LV longitudinal systolic function and atrial dyssynchrony in HFpEF patients.
MethodsStudy populationWe studied 85 patients (mean age 60±11 years, 75% female) with HFpEF (LVEF ≥45%) and New York Heart Association (NYHA) functional class I–III. Based on the mean normal value of MAPSE derived from previous studies, patients were classified as having HFpEF with impaired MAPSE (≤1.2 cm, n=42), or with normal MAPSE (>1.2 cm, n=43).19,20
Patients were referred to the Service of Cardiology, Internal Medicine Clinic, University Clinical Centre of Kosovo, between February 2013 and November 2013. At the time of the study all patients were on conventional medical treatment, optimized at least two weeks prior to enrollment, based on symptoms and renal function: 76% were receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 58% beta-blockers, 34% diuretics, 66% aspirin and 20% calcium channel blockers. Patients with clinical evidence of cardiac decompensation, limited physical activity due to factors other than cardiac symptoms (e.g. arthritis), stage >2 chronic renal failure (glomerular filtration rate ≤89 ml/min), chronic obstructive pulmonary disease (COPD) or recent acute coronary syndrome, stroke or anemia, were excluded. Patients gave written informed consent to participate in the study, which was approved by the local Ethics Committee.
Data collectionDetailed history and clinical assessment were obtained in all patients, and routine biochemical tests were also performed including lipid profile, blood glucose level, and kidney function tests. Body mass index was calculated from weight and height measurements. Waist and hip measurements were also recorded and waist/hip ratio calculated.
Echocardiographic examinationA single operator performed all echocardiographic examinations using a Philips iE33 system with a multi-frequency transducer, and harmonic imaging as appropriate. Images were obtained with the patient in left lateral decubitus position and during quiet expiration. LV end-systolic and end-diastolic dimension (LVESD and LVEDD) were measured in left parasternal long-axis view with the M-mode cursor positioned by the tips of the mitral valve leaflets. LV volumes and ejection fraction (EF) were calculated from apical 2- and 4-chamber views using the modified Simpson's method. MAPSE and tricuspid annular plane systolic excursion (TAPSE) were assessed by placing the M-mode cursor at the lateral and septal angles of the mitral annulus and the lateral angle of the tricuspid annulus.
Total amplitude of long-axis motion (MAPSE or TAPSE) was measured as previously described21 from peak inward to peak outward points. LV and right ventricular (RV) long-axis myocardial velocities were also assessed using Doppler myocardial imaging. From apical 4-chamber view, longitudinal velocities were recorded with the pulsed wave Doppler sample volume placed at the basal part of the LV lateral and septal segments as well as the RV free wall. Systolic (s′) and early and late (e′ and a′) diastolic myocardial velocities were measured with the gain optimally adjusted. Mean lateral and septal LV velocities were calculated.
LV and RV diastolic function was assessed from filling velocities using spectral pulsed wave Doppler with the sample volume positioned at the tips of the mitral and tricuspid valve leaflets, respectively, during short breath-hold. Peak LV and RV early (E wave) and late (A wave) diastolic velocities were measured and E/A ratios were calculated. The E/e′ ratio was calculated as the ratio between the transmitral E wave and mean lateral and septal e′ wave velocities. Isovolumic relaxation time was also measured from aortic valve closure to mitral valve opening on pulsed wave Doppler recording. The LV filling pattern was considered restrictive when the E/A ratio was >2.0, E wave deceleration time <140 ms and the left atrium dilated more than 40 mm in transverse diameter.22
Mitral regurgitation severity was assessed by color and continuous wave Doppler and was graded as mild, moderate, or severe according to the jet area relative to that of the left atrium as well as the flow velocity profile, in line with the recommendations of the American Society of Echocardiography (ASE).23 Likewise, tricuspid regurgitation was assessed by color Doppler and continuous-wave Doppler. A retrograde transtricuspid pressure drop of >35 mmHg was taken as evidence of pulmonary hypertension.24 All M-mode and Doppler recordings were performed at a high speed of 100 mm/s with superimposed ECG (lead II).
LA diameter was measured from aortic root recordings with the M-mode cursor positioned at the level of the aortic valve leaflets. LA volumes were measured using the area-length method from apical 4- and 2-chamber views, according to the ASE guidelines.25 Left atrial maximum volume (LAVmax) was measured at LV end-systole, just before the opening of the mitral valve, LA minimum volume (LAVmin) was measured at end-diastole, immediately after closure of the mitral valve, and left atrial ejection fraction (LAEF) was calculated automatically.25,26
Measurements of intra- and interatrial electromechanical delayThe time from the onset of the P wave on the ECG to the beginning of the A′ wave on TDI (PA′-TDI interval) was calculated from the lateral (PA′ lateral) and septal (PA′ septal) mitral annuli as well as the lateral tricuspid annulus (PA′ tricuspid) (Figure 1).10,11 Values for the PA′-TDI interval were averaged over three consecutive beats. The difference between PA′ lateral and PA′ septal was taken as the left atrial (LA) dyssynchrony. The difference between PA′ septal and PA′ tricuspid was taken as right atrial (RA) dyssynchrony and the difference between PA′ lateral and PA′ tricuspid as interatrial dyssynchrony.12,27
Statistical analysisData are presented as mean ± SD or proportions (percentage of patients). Continuous data were compared with the two-tailed unpaired Student's t test and discrete data with the chi-square test. Correlations were tested with Pearson coefficients. A significant difference was defined as p<0.05 (two-tailed).
Patients were divided according to MAPSE into those with HFpEF and impaired MAPSE (≤1.2 cm), and those with HFpEF and normal MAPSE (>1.2 cm) were compared using the unpaired Student's t test.
ResultsPatients’ mean age was 60±11 years, and 75% were female (Table 1).
Baseline patient data.
| Female (%) | 75 |
| Age (years) | 60±11 |
| Smoking (%) | 29 |
| BMI (kg/m2) | 29±3.8 |
| BSA (m2) | 1.06±0.21 |
| Waist/hip ratio | 0.94±0.08 |
| NYHA class | 1.29±0.74 |
| DD grade | 1.17±0.73 |
| SBP (mmHg) | 139±21 |
| DBP (mmHg) | 85±11 |
| HR (bpm) | 79±13 |
| Fasting glucose (mmol/l) | 6.02±1.98 |
| Total cholesterol (mmol/l) | 5.30±1.28 |
| Triglycerides (mmol/l) | 1.70±0.97 |
| Urea (mmol/l) | 6.94±4.41 |
| Creatinine (mmol/l) | 81.26±23 |
| Leukocytes (103/mm3) | 7.43±2.8 |
| Erythrocyte count (106/mm3) | 4.32±0.4 |
| Hemoglobin (g/dl) | 12.5±1.9 |
BMI: body mass index; BSA: body surface area; bpm: beats per minute; DBP: diastolic blood pressure; DD: diastolic dysfunction; HR: heart rate; NYHA: New York Heart Association; SBP: systolic blood pressure.
Forty-three patients had normal longitudinal LV systolic function and the other 42 had reduced longitudinal function. Patients with reduced longitudinal LV systolic function (MAPSE ≤1.2 cm) were older (p<0.001) and had higher body surface area (BSA) (p=0.004). All other clinical and biochemical data did not differ between the groups (Table 2).
Comparison of clinical and biochemical data between patient groups.
| Variable | HFpEF patients | HFpEF patients | p |
|---|---|---|---|
| (MAPSE >1.2 cm) | (MAPSE <1.2 cm) | ||
| n=43 | n=42 | ||
| Age (years) | 55±10 | 64±8.7 | <0.001 |
| BMI (kg/m2) | 30±3.8 | 28±4.1 | 0.20 |
| BSA (m2) | 1.14±0.1 | 1.0±0.2 | 0.004 |
| Waist/hip ratio | 0.9±0.1 | 0.9±0.5 | 0.26 |
| SBP (mmHg) | 137±17 | 141±24 | 0.48 |
| DBP (mmHg) | 83±10 | 86±12 | 0.25 |
| NYHA class | 1.1±0.8 | 1.4±0.7 | 0.14 |
| DD grade | 1.0±0.73 | 1.2±0.73 | 0.27 |
| HR (bpm) | 81±14 | 78±12 | 0.37 |
| Fasting glucose (mmol/l) | 6.3±2.3 | 5.9±1.8 | 0.52 |
| Total cholesterol (mmol/l) | 5.5±1.5 | 5.2±1.2 | 0.49 |
| Triglycerides (mmol/l) | 1.6±0.8 | 1.7±0.9 | 0.76 |
| Urea (mmol/l) | 7.4±5.7 | 6.7±3.5 | 0.65 |
| Creatinine (mmol/l) | 87±30 | 78±17 | 0.21 |
| Leukocytes (103/mm3) | 7.5±3.7 | 7.4±2.3 | 0.94 |
| Erythrocyte count (106/mm3) | 4.4±0.6 | 4.2±0.4 | 0.38 |
| Hemoglobin (g/dl) | 13±2.3 | 12±1.6 | 0.72 |
BMI: body mass index; BSA: body surface area; bpm: beats per minute; DBP: diastolic blood pressure; DD: diastolic dysfunction; HFpEF: heart failure with preserved ejection fraction; HR: heart rate; NYHA: New York Heart Association; SBP: systolic blood pressure.
Patients with HFpEF and reduced MAPSE had higher LV mass index (p<0.001), thicker interventricular septum (p=0.003), larger LAVmin (p=0.007) and reduced LAEF (p<0.001) compared with normal MAPSE, but LA diameters and LV dimensions and volumes did not differ between groups. Patients with reduced MAPSE also had higher E/e′ ratio (p=0.002), reduced lateral and septal e′ (p=0.005 and p=0.006, respectively), and reduced TAPSE (r=0.40, p<0.001) and right e′ and tricuspid E (p=0.02 and p=0.03, respectively) compared to patients with normal MAPSE (Table 3).
Comparison of echocardiographic data between patient groups.
| Variable | HFpEF patients | HFpEF patients | p |
|---|---|---|---|
| (MAPSE >1.2 cm) | (MAPSE <1.2 cm) | ||
| n=43 | n=42 | ||
| LV dimensions and mass | |||
| LV mass index (g/m2.7) | 42±11 | 59±20 | <0.001 |
| LVEDD (cm) | 4.9±0.5 | 5.0±0.5 | 0.19 |
| LVESD (cm) | 3.1±0.4 | 3.2±0.5 | 0.27 |
| IVSd (cm) | 1.1±0.1 | 1.2±0.2 | 0.003 |
| LVPWd (cm) | 0.97±0.1 | 1.0±0.1 | 0.42 |
| EDV (ml) | 113±25 | 121±32 | 0.18 |
| ESV (ml) | 41±12 | 47±15 | 0.24 |
| LV systolic function | |||
| LVEF (%) | 64±8.2 | 63±8.0 | 0.76 |
| LV shortening fraction (%) | 35±6.2 | 35±6.0 | 0.95 |
| Lateral s′ (cm) | 5.9±1.5 | 5.6±1.6 | 0.31 |
| Septal s′ (cm/s) | 5.3±1.7 | 4.9±1.0 | 0.16 |
| E/e′ ratio | 7.9±2.1 | 9.8±4.1 | 0.008 |
| LV diastolic function | |||
| E-wave velocity (cm/s) | 59±15 | 58±19 | 0.73 |
| A-wave velocity (cm/s) | 69±17 | 74±18 | 0.17 |
| E/A ratio | 0.9±0.3 | 0.8±0.4 | 0.27 |
| E-wave DT (ms) | 194±42 | 202±44 | 0.47 |
| Lateral e′ (cm/s) | 8.8±3.1 | 7.0±2.9 | 0.005 |
| Lateral a′ (cm/s) | 9.5±2.7 | 8.5±2.7 | 0.09 |
| Septal e′ (cm/s) | 6.8±2.7 | 5.4±1.9 | 0.006 |
| Septal a′ (cm/s) | 8.6±2.6 | 7.8±1.9 | 0.11 |
| RV function | |||
| Tricuspid E (cm/s) | 50±11 | 44±13 | 0.03 |
| Tricuspid A (cm/s) | 48±12 | 51±16 | 0.26 |
| Right e′ (cm/s) | 11±3.6 | 9.7±3.4 | 0.02 |
| Right a′ (cm/s) | 13±4.9 | 13±5.1 | 0.94 |
| Right s′ (cm/s) | 9.7±2.7 | 8.8±3.1 | 0.15 |
| TAPSE (cm) | 2.6±0.4 | 2.3±0.4 | 0.001 |
| LA dimensions and function | |||
| LA diameter (cm) | 3.9±0.4 | 4.0±0.6 | 0.33 |
| LA transverse diameter (cm) | 3.8±0.6 | 3.9±0.6 | 0.40 |
| LA longitudinal diameter (cm) | 5.4±0.7 | 5.6±0.8 | 0.30 |
| LAVmax (ml) | 50±15 | 50±19 | 0.96 |
| LAVmin (ml) | 16±6.3 | 21±9.9 | 0.007 |
| LAEF (%) | 65±10 | 57±9.8 | <0.001 |
A: atrial diastolic velocity; a′: late diastolic myocardial velocity; DT: deceleration time; E: early diastolic filling velocity; e′: early diastolic myocardial velocity; EDV: end-diastolic volume; ESV: end-systolic volume; HFpEF: heart failure with preserved ejection fraction; IVSd: interventricular septum in diastole; LA: left atrial; LAEF: left atrial ejection fraction; LAVmax: left atrial maximum volume; LAVmin: left atrial minimum volume; LV: left ventricular; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic dimension; LVPWd: left ventricular posterior wall in diastole; s′: systolic myocardial velocity; TAPSE: tricuspid annular plane systolic excursion.
Furthermore, tricuspid annulus electromechanical time (PA′ tricuspid) was longer (p=0.03), and RA dyssynchrony was increased (p=0.001), compared with patients with normal MAPSE. In contrast, LA dyssynchrony and interatrial asynchrony did not differ between groups (Table 4).
Comparison of intra- and interatrial electromechanical delay between patient groups.
| Variable | HFpEF | HFpEF | p |
|---|---|---|---|
| (MAPSE >1.2 cm) | (MAPSE <1.2 cm) | ||
| n=43 | n=42 | ||
| PA′ lateral (ms) | 75±14 | 81±17 | 0.08 |
| PA′ septal (ms) | 71±13 | 74±17 | 0.40 |
| PA′ tricuspid (ms) | 70±15 | 77±15 | 0.03 |
| LA dyssynchrony (ms) | 7.9±9.1 | 11±9.6 | 0.83 |
| RA dyssynchrony (ms) | 5.9±7.6 | 13±10 | 0.001 |
| Interatrial dyssynchrony (ms) | 7.7±7.9 | 11±10 | 0.06 |
HFpEF: heart failure with preserved ejection fraction; LA: left atrial; MAPSE: mitral annular plane systolic excursion; PA: time from onset of the P wave on the ECG to the beginning of the A′ wave on tissue Doppler imaging; RA: right atrial.
Of the biochemical and clinical findings only age (r=−0.34, p=0.003), NYHA class (r=−0.35, p=0.004), waist/hip ratio (r=−0.24, p=0.05) and BSA (r=0.33, p=0.004) correlated with MAPSE (Table 5).
Correlations of clinical and echocardiographic variables with mitral annular plane systolic excursion.
| Variable | R | p |
|---|---|---|
| Clinical correlates | ||
| Age | −0.34 | 0.003 |
| NYHA class | −0.35 | 0.004 |
| BSA (m2) | 0.33 | 0.004 |
| Waist/hip ratio | −0.24 | 0.05 |
| Echocardiographic correlates | ||
| RA dyssynchrony (ms) | −0.40 | <0.0001 |
| LV mass (g) | −0.34 | 0.01 |
| LV mass index (g/m2.7) | −0.56 | <0.001 |
| LVEDD (cm) | −0.22 | 0.04 |
| LVESD (cm) | −0.27 | 0.01 |
| IVSd (cm) | −0.33 | 0.02 |
| E/e′ ratio | −0.27 | 0.01 |
| Lateral e′ (cm/s) | 0.26 | 0.01 |
| Lateral a′ (cm/s) | 0.24 | 0.02 |
| Septal e′ (cm/s) | 0.26 | 0.01 |
| Septal a′ (cm/s) | 0.22 | 0.03 |
| Tricuspid E (cm/s) | 0.28 | 0.009 |
| Right e′ (cm/s) | 0.22 | 0.03 |
| TAPSE (cm) | 0.40 | <0.001 |
| LAVmin (ml) | −0.38 | <0.001 |
| LAEF (%) | 0.44 | <0.001 |
A: atrial diastolic velocity; a′: late diastolic myocardial velocity; BSA: body surface area; e′: early diastolic myocardial velocity; EDV: end-diastolic volume; ESV: end-systolic volume; HFpEF: heart failure with preserved ejection fraction; IVSd: interventricular septum in diastole; LA: left atrial; LAEF: left atrial ejection fraction; LAVmin: left atrial minimum volume; LV: left ventricular; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic dimension; NYHA: New York Heart Association; RA: right atrial; s′: systolic myocardial velocity; TAPSE: tricuspid annular plane systolic excursion.
MAPSE correlated with RA dyssynchrony (r=−0.40, p<0.0001, Figure 2), LV mass index (r=−0.56, <0.001), LVEDD (r=−0.22, p=0.04) and LVESD (r=−0.27, p=0.01), as well as with LAVmin (r=−0.22, p<0.001) and LAEF (r=0.44, p<0.001), but not with LA or interatrial dyssynchrony.
MAPSE also showed significant correlation with TDI indices such as a′ wave (lateral a′, r=0.24, p=0.02 and septal a′, r=0.22, p=0.03, respectively) and e′ wave (lateral e′ and septal e′, r=0.26, p=0.01 for both). Among right ventricular echocardiographic indices, TAPSE (r=0.40, p<0.001), right e′ (r=0.22, p=0.03) and tricuspid E (r=0.28, p=0.009) also correlated with MAPSE (Table 5).
DiscussionOur study shows that patients with HFpEF and impaired LV longitudinal systolic function, in addition to impaired LV filling and LAEF, have increased RA dyssynchrony, compared to HFpEF patients with preserved LV longitudinal systolic function. The other finding of our study is that LV longitudinal systolic function in these patients has a strong relationship with RA dyssynchrony and LAEF.
Delayed intra- and interatrial electromechanical conduction in HF patients is a result of structural remodeling, as well as wall tension changes. The RA dyssynchrony observed parallels that seen in several studies assessing electromechanical conduction characteristics in patients with HF, in whom a number of echocardiographic parameters have been shown to correlate with atrial dyssynchrony, including reduced EF and diastolic dysfunction.17,18 In our study, LAEF was significantly decreased in HFpEF with reduced longitudinal LV systolic function, despite no changes in LA diameters. These findings suggest that impaired longitudinal systolic function in HFpEF affects LA function before changing its dimensions. In addition, we found that in this group of patients PA′ tricuspid was prolonged and RA dyssynchrony was increased. Furthermore, in our study, MAPSE correlated significantly with RA dyssynchrony. The mechanism of RA dyssynchrony in these patients is unclear. However, we believe that it is due to RA structural remodeling in these patients. The effect of RA structural remodeling on RA dyssynchrony in HF patients has been previously reported.6,28,29 This increased RA dyssynchrony has been described as a result of prolonged conduction time in the right atrium, especially its lateral wall, due to the loss of functional parts of atrial myocardium caused by interstitial fibrosis.
The absence of LA and interatrial dyssynchrony is explained by LA activation occurring independently of prolonged RA activation.13,30 This may be due to the biatrial propagation of the impulse through Bachmann's bundle and the coronary sinus, which may lead to early activation of the upper part of the left atrium without continuous propagation from right to left atrium.31
Abnormal longitudinal LV function may also contribute to abnormalities of mechanical loading in wall tension of the heart. Altered wall tension may lead to stretched and more anisotropic atrial walls,32 suggesting a greater impact in the interatrial septum as a result of more complex and heterogeneous myoarchitecture.33 Finally, anisotropic conduction leads to increased conduction velocity34 and also increases the difference between PA′ tricuspid and PA′ septal, as well as RA dyssynchrony.
In addition to the known relationship between EF and intra- and interatrial dyssynchrony, our findings showed that impaired longitudinal LV systolic function has an important relationship with RA dyssynchrony. RA dyssynchrony is known to be a predictor of atrial arrhythmias, including atrial fibrillation. Our findings suggest that early and better treatment of HF could prevent prolonged RA dyssynchrony, thus preventing atrial arrhythmias including atrial fibrillation.
LimitationsThe small sample size, as well as the known heterogeneity of this syndrome, are limitations. We did not assess LA and RA intrinsic myocardial function in this study using strain and strain rate measurements.
ConclusionIn patients with HFpEF and impaired longitudinal LV systolic function, RA dyssynchrony is increased compared to those with normal longitudinal LV systolic function. As patients with RA dyssynchrony are at higher risk for arrhythmia, assessment of this dyssynchrony may help to improve treatment, as well as to predict outcome in these patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.