Patent ductus arteriosus (PDA) is one of the most common congenital heart defects, and causes left ventricular volume overload. However, it is a rare finding among adults due to the lack of symptoms related to its small size, and it is not usually associated with other congenital lesions.
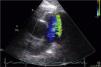
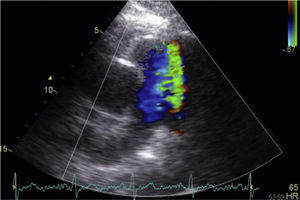
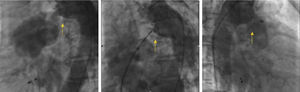
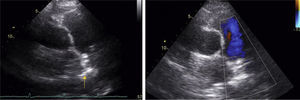
A 53-year-old woman was referred for percutaneous closure of a PDA. She had undergone a secundum atrial septal defect (ASD) closure in 2007, detected after a cryptogenic stroke. After this procedure, her clinical history was remarkable for fatigue, dyspnea and orthopnea with New York Heart Association (NYHA) functional class III, and a continuous murmur was heard over the left sternal border. A routine transthoracic echocardiogram performed in 2013 revealed a continuous flow from the aorta towards the pulmonary artery, with left-sided chamber dilation (ejection fraction 50%) and normal pulmonary artery pressure. The device previously implanted at the atrial septum was correctly positioned and without residual shunt (Figure 1). The PDA was closed by placing an Amplatzer™ Duct Occluder guided by fluoroscopy and intracardiac echocardiography (Figure 2). The procedure was uneventful. The 12-month follow-up showed significant clinical improvement (NYHA functional class II), no residual shunt, and normal left chamber dimensions (Figure 3).
Fluoroscopy during percutaneous patent ductus arteriosus closure: left, a continuous flow can be seen from the aorta to the pulmonary artery (yellow arrow); center, during device placement (yellow arrow); right, after the procedure the device is properly positioned with no residual shunt (yellow arrow).
In this case, the association with an ASD may have delayed the clinical manifestations of the PDA, as the previous left-to-right shunt probably compensated for the left ventricular volume overload. It is crucial to perform a complete high-quality echocardiogram in such patients with congenital heart disease, in order to screen for associated anomalies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.