A new drug with prognostic impact on heart failure, sacubitril/valsartan, has been introduced in current guidelines. However, randomized trial results can be compromised by lack of representativeness. We aimed to assess the representativeness of the PARADIGM-HF trial in a real-world population of patients with heart failure.
MethodsWe reviewed the records of 196 outpatients followed in a heart failure clinic between January 2013 and December 2014. After exclusion of 44 patients with preserved ejection fraction, the inclusion and exclusion criteria of the trial were applied.
ResultsOf the 152 patients with systolic heart failure, 106 lacked one or more inclusion criteria and 45 had at least one exclusion criterion. Considering only patients with ejection fraction ≤35% (HFrEF) (n=88), 43 patients lacked at least one inclusion criterion and 25 patients had at least one exclusion criterion. Combining the inclusion and exclusion criteria, 24.3% of patients with systolic HF (ejection fraction ≤50%) and 42% of patients with HFrEF would be eligible for the PARADIGM-HF trial.
ConclusionOne in four patients with systolic HF followed in a heart failure outpatient clinic would fulfill the reference study criteria for treatment with the new drug, sacubitril/valsartan.
Um novo medicamento com impacto prognóstico em doentes com insuficiência cardíaca foi introduzido nas guidelines mais recentes. Contudo, os resultados de estudos aleatorizados podem ser prejudicados pela falta de representatividade. Os autores ambicionam avaliar a representatividade do estudo PARADIGM-HF numa população do mundo real de doentes com insuficiência cardíaca.
MétodosForam revistos os registos de 196 pacientes seguidos em consulta dedicada a insuficiência cardíaca de um hospital terciário entre janeiro de 2016 e dezembro de 2014. Após exclusão de 44 doentes com fração de ejeção preservada, os critérios de inclusão e exclusão foram aplicados.
ResultadosDos 152 doentes com insuficiência cardíaca com disfunção sistólica, 106 não preenchiam um ou mais critérios de inclusão e tinham pelo menos um critério de exclusão. Considerando apenas os doentes com fração de ejeção ≤ 35% (N = 88), 43 doentes não preenchiam pelo menos um critério de inclusão e 25 tinham pelo menos um critério de exclusão. Combinando os critérios de inclusão e exclusão, 24,3% dos doentes com fração de ejeção < 50% e 42% dos doentes com fração de ejeção ventricular esquerda reduzida seriam elegíveis para o estudo PARADIGM-HF.
ConclusãoUm em cada quatro doentes com insuficiência cardíaca sistólica, seguidos em ambulatório na consulta de insuficiência cardíaca, cumpririam os critérios do estudo de referência que levou à aprovação do novo fármaco inibidor dos recetores de angiotensina e da neprilisina.
The mainstay of the management of chronic systolic heart failure (HF) is neurohormonal blockade specifically targeting the sympathetic nervous system and the renin-angiotensin-aldosterone system.1–3 Yet, despite the use of beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), and aldosterone receptor antagonists in optimized doses, mortality and morbidity remain high in these patients.1
Several randomized controlled trials over a period of more than a decade exploring other potential therapeutic targets, such as endothelin, vasopressin and tumor necrosis factor alpha, failed to demonstrate further reductions in mortality.4–7 This period of consecutive negative study outcomes ended in 2014, when the PARADIGM-HF trial results were reported. In PARADIGM-HF the combination of a neprilysin inhibitor (sacubitril) and an ARB (valsartan) was superior to enalapril in reducing the risk of death from cardiovascular causes and hospitalization for heart failure in patients with chronic systolic HF on optimized medical therapy.8
After its efficacy is proven, a new drug has to show effectiveness under real-life conditions.9,10 In a real-world setting, the representativeness of randomized clinical trials findings may be limited, since these studies are conducted under idealized and rigorously controlled conditions that may compromise their external validity. Ineligibility rates in cardiology trials show that as many as 25-67% of the general disease population are excluded from these trials.11,12 Therefore, we aimed to assess the representativeness of PARADIGM-HF in a real-world population of patients with systolic HF.
MethodsPopulation and designThe records of all outpatients (n=196) followed in the heart failure clinic of a tertiary university-affiliated hospital between January 2013 and December 2014 were reviewed. Standard of care includes a regular clinical assessment every 3-6 months, drug titration, follow-up inquiry and serial N-terminal pro-brain natriuretic peptide (NT-proBNP) measurement. All data are included in a prospective registry. Patients with preserved left ventricular ejection fraction (LVEF), defined as LVEF ≥50%, were excluded (n=44). The inclusion and exclusion criteria of the PARADIGM-HF trial were subsequently applied to the remaining population.
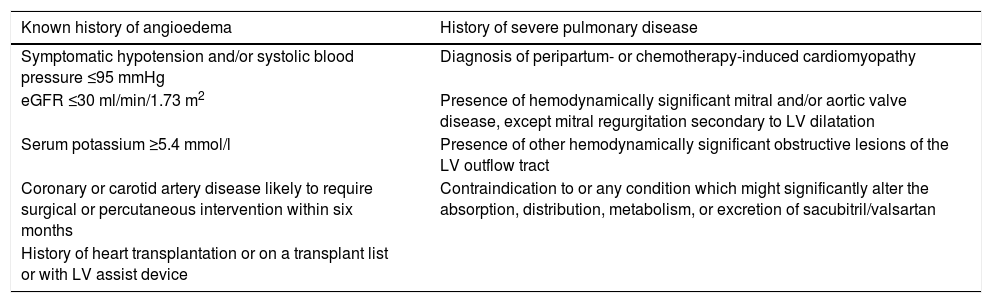
Patients were considered eligible for treatment with sacubitril/valsartan if they fulfilled all of the following criteria: New York Heart Association (NYHA) functional class II-IV; LVEF ≤35%; NT-proBNP ≥600 pg/ml. Furthermore, eligible patients had to be taking enalapril 10mg twice daily (used as an entry criterion in PARADIGM-HF) or equivalent (defined by target dosage in the current guidelines) as part of their optimal medical therapy.1 All LVEF measurements were obtained by two-dimensional transthoracic echocardiography. Patients were considered ineligible for treatment with sacubitril/valsartan if they presented any of the conditions listed in Table 1.
Criteria of ineligibility for treatment with sacubitril/valsartan.
| Known history of angioedema | History of severe pulmonary disease |
|---|---|
| Symptomatic hypotension and/or systolic blood pressure ≤95 mmHg | Diagnosis of peripartum- or chemotherapy-induced cardiomyopathy |
| eGFR ≤30 ml/min/1.73 m2 | Presence of hemodynamically significant mitral and/or aortic valve disease, except mitral regurgitation secondary to LV dilatation |
| Serum potassium ≥5.4 mmol/l | Presence of other hemodynamically significant obstructive lesions of the LV outflow tract |
| Coronary or carotid artery disease likely to require surgical or percutaneous intervention within six months | Contraindication to or any condition which might significantly alter the absorption, distribution, metabolism, or excretion of sacubitril/valsartan |
| History of heart transplantation or on a transplant list or with LV assist device |
eGFR:estimated glomerular filtration rate; LV: left ventricular.
Continuous variables with a normal distribution are expressed as means ± standard deviation. Discrete variables are expressed as frequencies and percentages. When appropriate, 95% confidence intervals (CI) or percentiles were calculated. The statistical analysis was performed using SPSS software, version 21.0 (IBM SPSS Inc., Chicago, IL, USA).
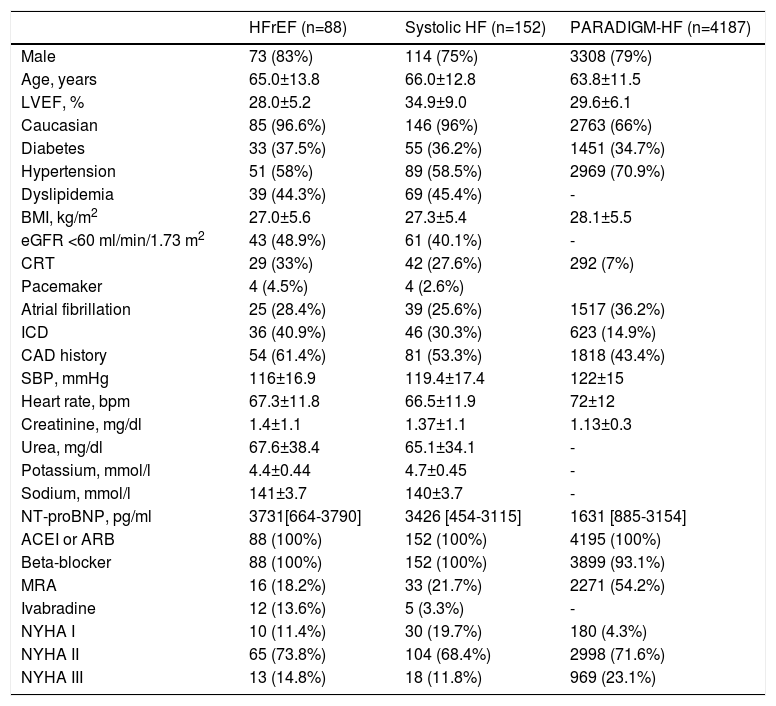
ResultsOf the 196 HF patients initially screened, 44 were excluded due to preserved LVEF. Of the remaining 152 patients with LVEF <50% (systolic HF), 75% were male, mean age was 66±12.8 years, and mean LVEF was 34.9±9.0%. Other baseline characteristics are displayed in Table 2.
Baseline characteristics of the study population.
| HFrEF (n=88) | Systolic HF (n=152) | PARADIGM-HF (n=4187) | |
|---|---|---|---|
| Male | 73 (83%) | 114 (75%) | 3308 (79%) |
| Age, years | 65.0±13.8 | 66.0±12.8 | 63.8±11.5 |
| LVEF, % | 28.0±5.2 | 34.9±9.0 | 29.6±6.1 |
| Caucasian | 85 (96.6%) | 146 (96%) | 2763 (66%) |
| Diabetes | 33 (37.5%) | 55 (36.2%) | 1451 (34.7%) |
| Hypertension | 51 (58%) | 89 (58.5%) | 2969 (70.9%) |
| Dyslipidemia | 39 (44.3%) | 69 (45.4%) | - |
| BMI, kg/m2 | 27.0±5.6 | 27.3±5.4 | 28.1±5.5 |
| eGFR <60 ml/min/1.73 m2 | 43 (48.9%) | 61 (40.1%) | - |
| CRT | 29 (33%) | 42 (27.6%) | 292 (7%) |
| Pacemaker | 4 (4.5%) | 4 (2.6%) | |
| Atrial fibrillation | 25 (28.4%) | 39 (25.6%) | 1517 (36.2%) |
| ICD | 36 (40.9%) | 46 (30.3%) | 623 (14.9%) |
| CAD history | 54 (61.4%) | 81 (53.3%) | 1818 (43.4%) |
| SBP, mmHg | 116±16.9 | 119.4±17.4 | 122±15 |
| Heart rate, bpm | 67.3±11.8 | 66.5±11.9 | 72±12 |
| Creatinine, mg/dl | 1.4±1.1 | 1.37±1.1 | 1.13±0.3 |
| Urea, mg/dl | 67.6±38.4 | 65.1±34.1 | - |
| Potassium, mmol/l | 4.4±0.44 | 4.7±0.45 | - |
| Sodium, mmol/l | 141±3.7 | 140±3.7 | - |
| NT-proBNP, pg/ml | 3731[664-3790] | 3426 [454-3115] | 1631 [885-3154] |
| ACEI or ARB | 88 (100%) | 152 (100%) | 4195 (100%) |
| Beta-blocker | 88 (100%) | 152 (100%) | 3899 (93.1%) |
| MRA | 16 (18.2%) | 33 (21.7%) | 2271 (54.2%) |
| Ivabradine | 12 (13.6%) | 5 (3.3%) | - |
| NYHA I | 10 (11.4%) | 30 (19.7%) | 180 (4.3%) |
| NYHA II | 65 (73.8%) | 104 (68.4%) | 2998 (71.6%) |
| NYHA III | 13 (14.8%) | 18 (11.8%) | 969 (23.1%) |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor antagonist; BMI: body mass index; CAD: coronary artery disease; CRT: cardiac resynchronization therapy; eGFR: estimated glomerular filtration rate; HFrEF: heart failure with reduced ejection fraction (LVEF ≤35%); ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; NYHA: New York Heart Association class; SBP: systolic blood pressure; Systolic HF: heart failure with LVEF ≤50%.
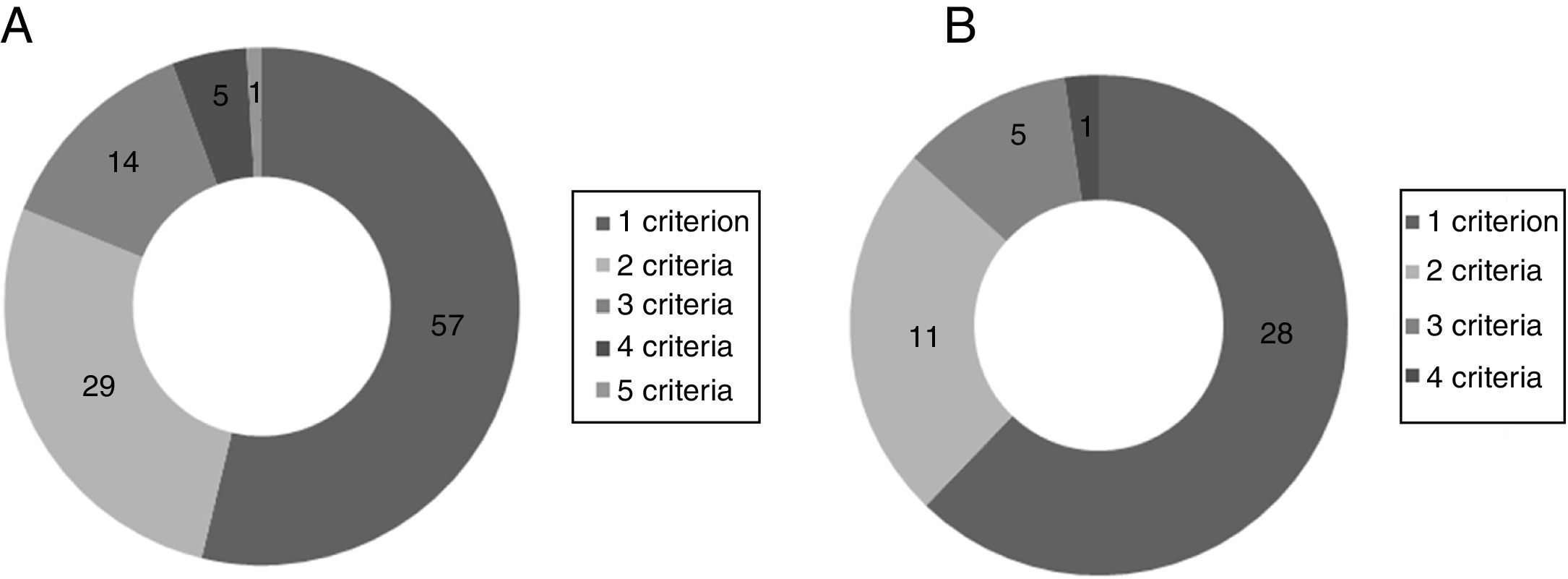
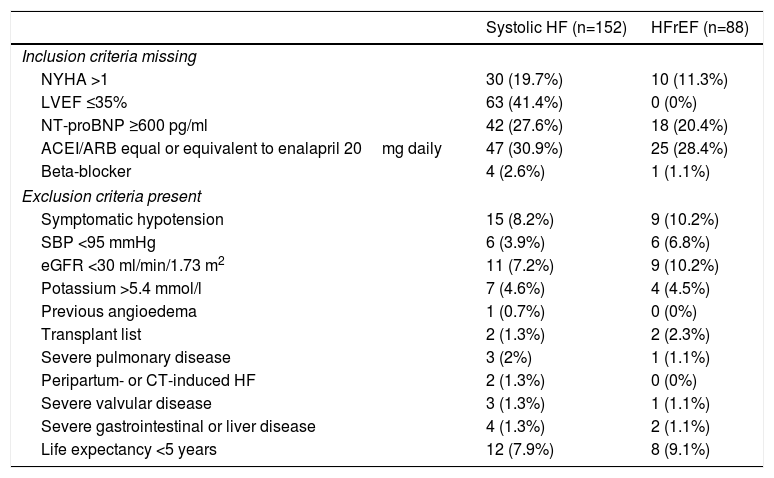
Of the 152 patients with systolic HF 106 (69.7%) failed to meet at least one inclusion criterion and 45 (29.6%) had at least one exclusion criterion. Considering only patients with HFrEF (LVEF ≤35%) (n=88), 43 (48.9%) lacked at least one inclusion criterion and 25 (28.4%) had at least one exclusion criterion. Combining inclusion and exclusion criteria, 24.3% (n=37) of patients with systolic HF and 42% (n=37) of patients with HFrEF would have been eligible for the PARADIGM-HF trial (Table 3).
PARADIGM-HF inclusion and exclusion criteria in the study population.
| Systolic HF (n=152) | HFrEF (n=88) | |
|---|---|---|
| Inclusion criteria missing | ||
| NYHA >1 | 30 (19.7%) | 10 (11.3%) |
| LVEF ≤35% | 63 (41.4%) | 0 (0%) |
| NT-proBNP ≥600 pg/ml | 42 (27.6%) | 18 (20.4%) |
| ACEI/ARB equal or equivalent to enalapril 20mg daily | 47 (30.9%) | 25 (28.4%) |
| Beta-blocker | 4 (2.6%) | 1 (1.1%) |
| Exclusion criteria present | ||
| Symptomatic hypotension | 15 (8.2%) | 9 (10.2%) |
| SBP <95 mmHg | 6 (3.9%) | 6 (6.8%) |
| eGFR <30 ml/min/1.73 m2 | 11 (7.2%) | 9 (10.2%) |
| Potassium >5.4 mmol/l | 7 (4.6%) | 4 (4.5%) |
| Previous angioedema | 1 (0.7%) | 0 (0%) |
| Transplant list | 2 (1.3%) | 2 (2.3%) |
| Severe pulmonary disease | 3 (2%) | 1 (1.1%) |
| Peripartum- or CT-induced HF | 2 (1.3%) | 0 (0%) |
| Severe valvular disease | 3 (1.3%) | 1 (1.1%) |
| Severe gastrointestinal or liver disease | 4 (1.3%) | 2 (1.1%) |
| Life expectancy <5 years | 12 (7.9%) | 8 (9.1%) |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CT: chemotherapy; eGFR: estimated glomerular filtration rate; HF: heart failure; HFrEF: heart failure with reduced ejection fraction (LVEF ≤35%);LVEF: left ventricular ejection fraction; NYHA: New York Heart Association class; SBP: systolic blood pressure; Systolic HF: heart failure with LVEF ≤50%.
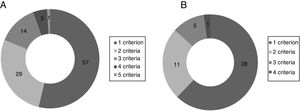
The inclusion criterion most often missing in the population with systolic HF was LVEF ≤35% (41.4%), followed by ACEI/ARB dose equal or equivalent to 20mg enalapril daily (30.9%). The most frequent exclusion criteria in this group were hypotension (8.2%) and estimated life expectancy <5 years (7.9%). Considering only patients with HFrEF, the most frequent missing inclusion criteria were ACEI/ARB dose equal or equivalent to 20mg enalapril daily (28.4%) and NT-proBNP ≥600 pg/ml (20.4%). The exclusion criteria more often present were hypotension (10.2%) and estimated glomerular filtration rate <30ml/min/1.73 m2 (10.2%). The distribution of patients according to the number of unfulfilled eligibility criteria and number of ineligibility criteria present is shown in Figure 1.
DiscussionOur analysis shows that, if the PARADIGM-HF study criteria were applied to a real-world population of HF patients, only two in five patients with systolic HF would be deemed eligible for treatment with the novel angiotensin receptor-neprilysin inhibitor.
As pointed out by Wieringa et al.,13 Maggioni et al.14 and Niederseer et al.,15 real-world heart failure patients are different from the populations of randomized trials. Our population differs from PARADIGM-HF patients in several aspects: LVEF ≤35% and ACEI/ARB dosage of at least enalapril 20mg daily or equivalent (the minimum required dosage) were the most frequently unmet eligibility criteria, while symptomatic hypotension and severe chronic kidney disease were the most prevalent ineligibility criteria (Table 3).
Patient selection for randomized trials may influence the effectiveness and safety of a new drug in the real world, due to different comorbid conditions, differing age groups, race, gender and ethnic variances, and different concomitant drugs, disease severity and compliance.11,12,16 Heart failure patients are often very complex, increasingly with multiple comorbidities, partly due to prolonged life expectancy. This heterogeneity has the potential to preclude a wider generalization of trial results (obtained using a strict protocol) to the unselected population encountered in daily clinical practice.11,13–15
The higher incidences of hypotension and severe chronic disease suggest that, as expected in tertiary reference centers, a sicker group of patients are being managed, who may be more susceptible to drugs with blood pressure-lowering effects. These features possibly contributed to the difficulty of ACEI or ARB titration up to the dose of 20mg enalapril equivalent daily required in PARADIGM-HF.
The requirement for severely compromised LVEF, baseline NT-proBNP ≥600 pg/ml (BNP ≥150 pg/ml) and persistent HF symptoms despite optimal medical therapy, as applied in PARADIGM-HF, identifies higher-risk patients, facilitating the demonstration of a positive effect on HF prognosis.
In PARADIGM-HF, a protocol amendment changed the initial maximum LVEF permitted by the study protocol from 40% to 35% in order to select higher-risk patients.8,17 However, the exclusion of patients with mild or moderate LV systolic dysfunction from the PARADIGM-HF trial does not necessarily mean that patients with LVEF between 35% and 50% will not derive benefit. As all patients with reduced LVEF share the same neurohormonal changes, the significant benefits observed in PARADIGM-HF can be expected to apply to all of them, although not necessarily to the same extent. Some previous heart failure trials that led to the approval of various drugs with survival advantage suggested that the benefit was less in patients with higher LVEF, while others found no difference.18–22 A recent analysis by Solomon et al. demonstrated that LVEF does not influence the impact of sacubitril/valsartan on outcomes.23 An ongoing study (PARAGON-HF) is assessing the combination of sacubitril and valsartan in HF patients with LVEF ≥45%.
Optimal medical management has been shown to reduce natriuretic peptide levels and to render HF patients asymptomatic, despite underlying severe LV systolic dysfunction.24–27 Although not represented in PARADIGM-HF, it is not unreasonable to anticipate a positive effect of the sacubitril/valsartan combination in delaying disease progression in asymptomatic patients.28
Another pertinent feature in all trials is safety. Many exclusion criteria such as hypotension, hyperkalemia or severe chronic kidney disease relate to common concerns in the daily management of HF patients. Sicker patients have a higher incidence of adverse effects and are frequently excluded from HF trials like PARADIGM-HF, limiting the applicability of a new drug for many patients and reflecting the challenging nature of HF outpatient management.14,15
The exclusion of patients who cannot tolerate enalapril 20mg daily could restrict the use of the new drug in such patients, due to a possible greater risk of a collateral event, requiring closer monitoring in this subgroup. Our population was older, had worse renal function, higher NT-proBNP levels and more implantable devices, possibly representing a sicker group of patients, which would have contributed to the exclusion of some.
Once efficacy has been proved, novel treatments should be assessed for their effectiveness.29 Our study provides an indication of which patient characteristics may influence the effectiveness of sacubitril/valsartan in a real-world population.
This study presents certain limitations, some of them inherent to a single-center registry. Also, optimal follow-up in the HF clinic could have contributed to the higher proportion of NYHA class I patients. Lastly, we cannot be sure whether a number, albeit probably small, of patients with HF symptoms were followed in general cardiology consultations and were thus not included in this analysis.
ConclusionDespite the strictness of our standard protocol, about two in five of our real-world patients with systolic HF would be considered eligible for PARADIGM-HF, a significant number. This finding puts into perspective the applicability of PARADIGM-HF to the real-world population of HF patients. A larger registry with outcome analysis is needed to validate a wider use of the new drug.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. This study was investigator-driven and independent of any commercial funding.