Device closure of interatrial communications has become a well-established technique to treat left-to-right shunt associated with atrial septal defect (ASD) and to prevent paradoxical embolism in patients with patent foramen ovale (PFO). Guidance by transesophageal echocardiography (TEE) is the standard practice but intracardiac echocardiography (ICE) is a feasible and safe alternative for monitoring these procedures.
ObjectivesTo report our experience in the percutaneous closure of ASD and PFO guided by ICE.
MethodsWe retrospectively reviewed all patients with ASD or PFO who underwent percutaneous closure guided exclusively by ICE between January 2008 and December 2010. All patients were followed clinically with regular echocardiographic evaluation (at discharge, one month, three, six and twelve months) to exclude residual shunt and device malposition.
ResultsA total of 127 patients (mean age 46.6±12.2years; 71% female) underwent transcatheter device closure of ASD or PFO during the study period. Device deployment with ICE monitoring was 100% successful, with a low rate of complications and eliminating the need for additional imaging techniques.
ConclusionsICE provides anatomical detail of ASD/PFO and cardiac structures, facilitating congenital cardiac interventional procedures. It eliminates the major drawbacks associated with TEE and enables the interventional cardiologist to control all aspects of the procedure without relying on additional echocardiographic support.
O encerramento percutâneo (EP) de defeitos do septo interauricular (SIA) é uma técnica comprovadamente eficaz para o tratamento da comunicação interauricular (CIA) e prevenção de embolia paradoxal em doentes com foramen ovale patente (FOP). A monitorização com ecocardiograma transesofágico (ETE) é o método habitualmente utilizado mas o ecocardiograma intracardíaco (EIC) constitui uma alternativa prática e segura para o controlo imagiológico destes procedimentos.
ObjetivoApresentar a experiência de um centro no EP de CIA e FOP guiado exclusivamente por EIC.
MétodosAnalisámos retrospetivamente uma série de doentes consecutivos com CIA ou FOP submetidos a EP sob controlo por EIC no período de janeiro de 2008 a dezembro de 2010. Foram todos avaliados clínica, eletro e ecocardiograficamente à data da alta e após o 1.°, 3.°, 6.° e 12.° mês para excluir shunt residual (SR) e malaposição do dispositivo.
ResultadosUm total de 127 doentes (46,6±12,2 anos; 71% de mulheres) foi submetido a EP de CIA ou FOP durante o referido período. A utilização do EIC durante o EP permitiu a colocação eficaz dos dispositivos em 100% dos casos, com uma reduzida taxa de complicações, dispensando a utilização adicional de outras técnicas de imagem durante os procedimentos.
ConclusãoO EIC fornece informação anatómica detalhada da CIA/FOP e estruturas cardíacas adjacentes, facilitando assim os procedimentos de intervenção. Elimina os inconvenientes do ETE e possibilita ao cardiologista de intervenção controlar os vários aspetos do procedimento sem necessitar de apoio ecocardiográfico adicional.
Transcatheter closure of atrial septal defect (ASD) and patent foramen ovale (PFO) has become an effective and safe alternative to surgical treatment. Current devices and advances in implantation techniques mean that 80% of ASDs can now be closed percutaneously.1 However, accurate measurement of the orifice and rims is essential for effective closure without complications. The most commonly used methods for device selection and implantation are angiographic assessment with balloon measurement2,3 and transesophageal echocardiographic monitoring (TEE).4,5 While an effective monitoring technique, TEE usually involves general anesthesia (with or without endotracheal intubation) in order to avoid the discomfort associated with the presence of the probe during the procedure.6 There have been a few reports of aspiration and obstruction of the airway, esophageal perforation and damage to vocal cords.7,8
The development of intracardiac echocardiography (ICE) has extended the range of imaging techniques available to the cardiologist.9 With the transducer positioned in the right atrium, ICE enables direct visualization of atrial septal morphology and can assess the size of the defect and exclude the presence of additional defects. Current ICE transducers provide high-quality images, with acquisition of multiple planes through a four-way steerable catheter and complete Doppler study.
The aim of this work is to report the experience of a single center in the percutaneous closure of ASD and PFO guided by ICE.
MethodsStudy populationA total of 127 consecutive patients (mean age 46.6±12.2years; 71% female) were referred to our center for percutaneous closure of defects of the atrial septum between January 2008 and December 2010. In accordance with the department's protocol, all patients were assessed by TEE prior to the procedure. Inclusion criteria for ASD closure were QP/QS >1.5, right chamber dilatation, and previous cryptogenic stroke or transient ischemic attack (TIA). Indications for PFO closure were a history suggestive of paradoxical embolism in patients with imaging evidence of multiple ischemic foci or previous cryptogenic stroke or TIA.
All patients with cryptogenic stroke or TIA and suspicion of paradoxical embolism underwent prior neurological assessment.
Echocardiographic studyTEE was performed on a GE Vivid 7 scanner (General Electric Corp., Norfolk, VA, USA) with the microbubble test and Valsalva maneuver under local anesthesia. A diagnosis of PFO was established if microbubbles were observed passing into the left chambers in the three cardiac cycles following opacification of the right chambers. The following anatomical criteria indicating high risk for paradoxical embolism were also assessed: atrial septal aneurysm (defined as excursion of at least 10mm beyond the septal plane), prominent Eustachian valve, wide or long tunnel (length≥12mm) and the presence of spontaneous shunt.
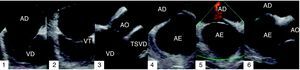
ICE studies were performed using an 8F AcuNav catheter (manufactured by Siemens Medical Solutions and distributed by Biosense Webster). This has a 64-element transducer (5.5–10MHz) with tissue penetration of 1–15cm and all Doppler functions (tissue, continuous, pulsed and color). ICE study began by advancing the catheter under fluoroscopic guidance up to the mid right atrium with a 15–30° clockwise rotation (termed ‘home view’), which enables visualization of the tricuspid valve, atrium and right ventricle (Figure 1.1). The Eustachian valve can be observed by slightly withdrawing the catheter to the inferior right atrium (Figure 1.2). In the home view, and with the catheter directed towards the tricuspid valve, a 30–45° clockwise rotation enables visualization of the right ventricular outflow tract and the aortic valve (Figure 1.3). The anterior septum can be observed in this view with a further slight clockwise rotation (Figure 1.4). The transducer is directed towards the interatrial septum (IAS) by tilting the catheter posteriorly (Figure 1.5), in order to visualize the septum, coronary sinus and pulmonary veins, depending on the precise location of the transducer (septal view). After locking the distal tip of the catheter in this position, other views are possible by anterior-posterior and left-right rotation. The superior vena cava and its relation to the IAS can be visualized by advancing the catheter superiorly and the superior and inferior extension of the IAS can be observed, corresponding to long-axis view in TEE. The catheter is locked in this position, and then rotated clockwise until it is located proximally to the tricuspid annulus and inferior to the aortic valve. In this view, tilting the transducer to the right enables visualization of the aortic valve in short-axis view and the anterior IAS (Figure 1.6).
ASD diameter, IAS length and rim dimensions were measured in various planes. Rims were defined as deficient when measuring less than 5mm (for the aortic rim) and less than 7mm for the remainder.
Closure protocol and device selectionProphylactic antibiotic therapy (cefuroxime 750mg) was administered intravenously 1hour before the procedure and unfractionated heparin (70U/kg) during the intervention. All patients began dual antiplatelet therapy (aspirin 100mg and clopidogrel 75mg) the evening before, clopidogrel being continued for one month and aspirin for six months in patients with ASD. In the case of PFO, aspirin was prescribed according to the neurologist's recommendation (usually for an indefinite period).
ICE was the only imaging technique used to guide device positioning and implantation. The procedure began with puncture of both femoral veins, an 8F introducer being inserted in the right femoral vein for right catheterization, replaced when necessary by a 10F or 12F introducer for device implantation. A 24-cm 8F introducer was inserted in the left femoral vein for the ICE transducer.
Based on ICE findings, the operators selected one of the following: an Amplatzer PFO occluder, Cribriform occluder, or ASD occluder (AGA Medical Corporation), Occlutech Figulla PFO and ASD occluder, Solysafe septal occluder (Swissimplant AG), or Premere Closure System (St. Jude Medical). After passing a 0.35-in.×260-cm guidewire and positioning a 6F multipurpose catheter at the ASD, it was replaced by an Amplatzer Super Stiff guidewire, which was introduced up to the left superior pulmonary vein. The selected device's delivery sheath was then positioned and the guidewire withdrawn. After advancing the device up to the ASD, the distal disc was released in the left atrium and the sheath withdrawn. Under simultaneous fluoroscopic and ICE guidance, the entire device was positioned by releasing the proximal disc in the right atrium. Once good apposition and absence of residual shunt or additional defects had been confirmed, the device was finally deployed. Any devices seen to be malpositioned were withdrawn and repositioned.
Procedure and fluoroscopy times and length of hospital stay were analyzed.
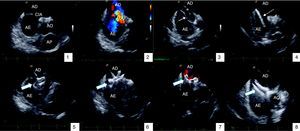
Figure 2 shows a case of ASD closure with a 22-mm Amplatzer ASD® device.
Atrial septal defect closure guided by intracardiac echocardiography: (1) visualization of defect; (2) color Doppler showing left-to-right shunt at the level of the defect; (3) balloon measurement estimating diameter of 19mm; (4) device delivery sheath with guidewire in the left superior pulmonary vein; (5) initial positioning of the device and release of the distal disc in the left atrium; (6) release of the proximal disc in the right atrium; (7) color Doppler showing absence of residual shunt; (8) deployment of device. AE: left atrium; AD: right atrium; AO: aorta; AP: pulmonary artery; CIA: atrial septal defect; VPSE: left superior pulmonary vein. Filled arrow: Amplatzer® device; unfilled arrow: measuring balloon; dashed arrow: device delivery sheath.
All patients underwent clinical, electrocardiographic and transthoracic echocardiographic reevaluation at discharge, one month, three, six and twelve months after implantation to exclude residual shunt (defined as very mild if the color Doppler jet width was <1mm, mild if 1–2mm, moderate if 2–4mm and severe if >4mm),10 device malposition or other complications. TEE was also performed in patients with residual shunt or large ASDs (>25mm).
ResultsThe demographic and clinical characteristics of the study population are shown in Tables 1 and 2, respectively. Of a total of 127 patients undergoing percutaneous closure procedures, 71 (56%) had PFO and 56 (44%) had ASD, 19 of which were multifenestrated (34%). The mean age of those with ASD was 44.5±14.4years, and 48.2±10.1years in those with PFO. There was a predominance of women in both groups.
Clinical characteristics.
| PFO | 71 | ASD | 56 |
| Previous stroke/TIA, n | 62 (88%) | Right chamber dilatation or QP/QS >1.5 | 42 (75%) |
| Single event | 51 | Previous stroke/TIA | 14 (25%) |
| Recurrent events | 11 | ||
| Positive MRI or CT (without previous stroke) | 9 (12%) | ||
ASD: atrial septal defect; CT: computed tomography; MRI: magnetic resonance imaging; PFO: patent foramen ovale; TIA: transient ischemic attack.
Anatomical features and procedural characteristics are shown in Tables 3 and 4, respectively. Among patients with ASD, 34 (60%) had floppy rims and 21 (38%) had at least one deficient rim. Mean ASD diameter was 22.6±6.8mm. Of the 71 patients with PFO, 62 (87%) had at least one high-risk anatomical criterion: 34 (48%) with atrial septal aneurysm, 28 (39%) with prominent Eustachian valve, and 38 (54%) with spontaneous shunt. Fifty-three patients (49%) presented long-tunnel type morphology and four patients had all of the risk criteria.
Anatomical features.
| ASD | PFO | ||
| Total (n) | 56 | Total (n) | 71 |
| Mean diameter (mm) | 22.6±6.8 | ASA (%) | 48 |
| Size >25mm, n (%) | 14 (25) | Prominent Eustachian valve (%) | 39 |
| Multifenestrated, n (%) | 19 (34) | Spontaneous shunt (%) | 54 |
| Floppy rims, n (%) | 34 (60) | Long tunnel (%) | 49 |
| Deficient rims, n (%) | |||
| 1 | 15 (27) | ||
| 2 | 4 (7) | ||
| 3 | 2 (4) | ||
ASA: atrial septal aneurysm; ASD: atrial septal defect; PFO: patent foramen ovale.
Procedural characteristics.
| ASD | PFO | ||
| Total devices (n) | 57 | Total devices (n) | 71 |
| Device implanted | Device implanted | ||
| Amplatzer, n (%) | 53 (93) | Amplatzer, n (%) | 29 (41) |
| ASD | 34 | Cribriform | 25 |
| Cribriform | 19 | PFO | 4 |
| Occlutech ASD, n (%) | 3 (5) | Premere, n (%) | 36 (51) |
| Solysafe, n (%) | 1 (2) | Solysafe, n (%) | 5 (7) |
| Occlutech PFO, n (%) | 1 (1) | ||
ASD: atrial septal defect; PFO: patent foramen ovale.
The procedure was successful in all patients, including one in whom implantation of two devices was required (18-mm and 25-mm Amplatzer). The type or size of device had to be changed in three cases, due to residual shunt around the device.
Eighty-two patients received Amplatzer septal occluders, 36 Premere devices, six Solysafe occluders, and the other four Occlutech devices. Mean procedure time was 43.2±10.1min and mean fluoroscopy time was 7.6±3.3min; mean hospital stay was 2.2±0.5days.
Follow-upThere was one case of right femoral hematoma in the immediate postprocedural period, resolved by compression and monitoring, which prolonged hospital stay by 24hours.
During a mean follow-up of 16±10months, three patients presented mild residual shunt (confirmed by TEE). There was one case of transient cardiac rhythm disturbance in a 28-year-old woman who developed junctional rhythm in the third month following the procedure; subsequent 24hour Holter monitoring showed no recurrence of arrhythmias and the patient remains asymptomatic. Another patient had a stroke six months after PFO closure, but there was no evidence of residual shunt, confirmed by TEE after injection of agitated saline. There was no case of device embolization, aortic erosion or device thrombosis.
DiscussionEchocardiographic monitoring is a routine practice during percutaneous ASD or PFO closure, TEE being the most commonly used technique. However, it has drawbacks, particularly the need for general anesthesia and the risk of complications related to airway manipulation in the dorsal decubitus position.3 Several small studies have demonstrated that ICE using an AcuNav® catheter is a feasible and precise alternative to guide ASD or PFO closure.11–14 While the aims of the present study did not include a cost-effectiveness analysis, since it is difficult to calculate direct and indirect costs of TTE with general anesthesia in a public hospital, some studies have suggested that use of ICE also has economic benefits in terms of the overall costs of the procedure.15,16
Accurate assessment of the atrial septum, the right atrium and their anatomical relations has been shown to be essential for safe and effective ASD closure.17 ICE provides precise measurements of the fossa ovalis and the ASD rims, facilitating the selection of an appropriately-sized device.18 The technique is equally valuable for measuring the septal defect and for monitoring procedures with complex anatomy.12,18
In our study population, appropriate patient selection and accurate assessment of ASD anatomy resulted in a high success rate of ASD closure, with a low percentage of complications. There were only three cases of residual shunt during follow-up, all of them mild with no clinical repercussions. The prognostic importance of residual shunt following closure appears to be directly related to its extent. Moderate and severe residual shunts are associated with a fourfold increase in risk of recurrent paradoxical embolic events, while mild shunts do not appear to have long-term clinical significance.19,20 Only one patient had rhythm disturbances—transient junctional rhythm—which was asymptomatic. Although indications for percutaneous ASD closure are outside the scope of this article, the case of recurrent stroke in our series highlights the need for appropriate selection of patients for this type of procedure.
Finally, while TEE was not used during the intervention, it is essential for correct diagnosis and for assessing suitability for percutaneous closure. ICE during the procedure provided important additional information for correct device implantation.
ConclusionsIn our series, ICE provided excellent anatomical detail of ASD and PFO, particularly their location and size, type of rims and relations to adjacent structures. It was efficient in guiding correct device implantation, with a low rate of complications, and eliminated the major drawbacks associated with TEE such as the need for general anesthesia and airway manipulation. Lastly, it enabled the interventional cardiologist to control all aspects of the procedure without relying on additional echocardiographic support.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Seca, L, et al., Encerramento percutâneo de defeitos do septo interauricular guiado por ecocardiograma intracardíaco—A experiência de um centro. Rev Port Cardiol 2012. http://dx.doi.org/10.1016/j.repc.2011.11.009