Infective endocarditis (IE) is associated with high morbidity and mortality. It is important to determine which factors increase the risk of poor outcome in order to enable early detection and aggressive treatment, including surgery. The aim of our study was to identify factors predicting complications and in-hospital mortality in patients with IE and to analyze conditions predisposing to surgery and its outcome.
MethodsWe performed a retrospective study including patients with IE who underwent transesophageal echocardiography in a tertiary hospital center (2006-2014).
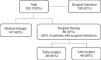
ResultsA total of 233 patients were analyzed (69.1% male; mean age 63.4±15.2 years; mean follow-up 28.4±30.7 months). The complication rate was 56.6% and in-hospital mortality was 16.3%. Independent predictors of mortality were chronic obstructive pulmonary disease (OR 4.89; CI 1.36-17.63; p=0.015), clinical course complicated by cerebral embolism (OR 9.38; CI 3.26-26.96; p<0.001), and IE due to Staphylococcus spp. (OR 3.78; CI 1.32-10.85; p=0.014) and non-HACEK Gram-negative bacilli (OR 12.85; CI 2.61-63.23; p=0.002). Surgery was performed in 36.9%. This group had higher percentages of males, younger patients, aortic valve IE, large vegetations, perivalvular extension, severe valvular regurgitation and heart failure. In patients with surgical indication (n=133), those who underwent surgery had lower in-hospital mortality (15.5% vs. 32.6%, p=0.028) and better long-term survival (log-rank p=0.029).
ConclusionThe results of this study may help to identify IE patients who are at increased risk of worse outcome, offering the opportunity to change the course of the disease and to improve prognosis with earlier and more aggressive intervention.
A endocardite infeciosa (EI) está a associada a elevada morbi-mortalidade. Torna-se importante definir os fatores que aumentam o risco de um desfecho desfavorável de modo a tratar de uma forma precoce e agressiva, incluindo tratamento cirúrgico. O objetivo deste estudo foi identificar fatores preditivos de complicações e mortalidade intra-hospitalar em doentes com EI e analisar as condições predisponentes para cirurgia e o seu resultado.
MétodosEstudo retrospetivo que incluiu doentes com EI que fizeram ecocardiograma transesofágico num centro hospitalar terciário (2006-2014).
ResultadosForam avaliados 233 pacientes (69,1% homens; idade média 63,4±15,2 anos; follow-up médio 28,4±30,7 meses). A taxa de complicações foi 56,6% e mortalidade intra-hospitalar 16,3%. Preditores independentes de mortalidade foram doença pulmonar obstrutiva crónica (OR 4,89; CI 1,36-17,63; p=0,015), embolia cerebral (OR 9,38; CI 3,26-26,96; p<0,001) e EI por Staphylococcus spp. (OR 3,78; CI 1,32-10,85; p=0,014) e bacilos Gram-negativos não HACEK (OR 12,85; CI 2,61-63,23; p=0,002). Fez-se cirurgia em 36,9%. Esse grupo apresentava maior percentagem de homens, jovens, EI da válvula aórtica, vegetações grandes, extensão perivalvular, regurgitação valvular grave e insuficiência cardíaca. No subgrupo de doentes com indicação cirúrgica (133), aqueles submetidos a cirurgia tiveram uma mortalidade intra-hospitalar menor (15,5% versus 32,6%, p=0,028) e melhor sobrevida em longo prazo (log-rank p=0,029).
ConclusãoOs resultados deste estudo podem ajudar a identificar doentes com EI que apresentam maior risco de um desfecho desfavorável, permitem alterar o curso da doença e melhorar o prognóstico através de uma intervenção mais precoce e agressiva.
The challenges associated with infective endocarditis (IE) are greater than ever. The patients affected are older and sicker than in the past, and often have many comorbidities.1
The role of surgery in active IE has expanded progressively, and nowadays almost half of patients with IE undergo surgery.2 There are three main indications: valve dysfunction leading to heart failure, uncontrolled infection, and for prevention of embolism.
Despite improvements in management, IE is still associated with high mortality and severe complications. Prognosis is influenced by many factors and early identification of high-risk patients may change the course of the disease and improve outcome.
The aim of this study was to identify factors predicting in-hospital mortality and complications and to analyze conditions predisposing to surgical therapy and its outcome in a single tertiary hospital with facilities for surgical therapy.
MethodsStudy populationAll patients who underwent transesophageal echocardiography in a tertiary hospital center with cardiac surgery facilities due to suspicion of IE and diagnosed with IE between 2006 and 2014 were recorded in a uniform database.
The study included cases from our hospital, as well as from community and referral hospitals.
Criteria and definitionsPatients were included if they met criteria for definite or possible IE (if considered and treated as endocarditis) by the modified Duke criteria.3 Patients with cardiac device-related IE were excluded.
In-hospital mortality was defined as all in-hospital deaths, including patients transferred to other acute care facilities.
The complications analyzed were heart failure, perivalvular extension, and cerebral and peripheral embolism. Perivalvular extension was defined by the presence of abscesses, pseudoaneurysms or fistulae.
Regarding timing, prosthetic valve endocarditis was defined as early if it occurred within 12 months of previous surgery. Surgical therapy was defined as early if the intervention was performed within seven days of diagnosis, and late if performed thereafter.
Indications for surgery were in accordance with the current European Society of Cardiology guidelines.4 Follow-up data were obtained by review of medical records, outpatient clinical visits, and telephone contact.
Ethical and hospital permissions were obtained from the appropriate local authorities.
Statistical analysisDescriptive results are expressed as means ± standard deviation or medians (interquartile range). Categorical variables are expressed as absolute numbers and proportions. Inferential statistics using parametric or nonparametric tests were used as appropriate for the type of data. The chi-square test or Fisher's exact test were used to analyze all categorical variables. The Student's t test was used to analyze continuous variables with a normal distribution.
Statistical significance was established at a two-tailed level of <5%. Factors associated with mortality on univariate analysis were entered into a multivariate stepwise logistic regression model. The cumulative mortality rate was calculated using the Kaplan-Meier method. All analyses were performed with SPSS for Windows, version 22.0 (IBM SPSS, Inc., Chicago, IL, USA).
ResultsIn total, 233 patients were diagnosed with IE during the study period, of whom 108 (46.4%) cases were considered as definite and 125 (53.6%) as possible IE according to the modified Duke criteria. The mean age was 63.4±15.2 years (range: 13-89) and 161 were male.
Baseline characteristics, comorbidities, echocardiographic findings and isolated microorganisms are shown in Table 1. A substantial proportion of the population were affected by comorbidities, the most frequent being chronic heart failure and diabetes (32.2% and 26.1%, respectively), while the most common predisposing condition was the presence of a prosthetic valve, with 22.7% being considered early prosthetic valve IE.
Baseline clinical characteristics of the patients (n=233).
| Mean age, years | 63.4±15.2 |
| Male gender | 161 (69.1%) |
| Comorbidities, n (%) | |
| Diabetes | 61 (26.1%) |
| Chronic renal failure | 37 (15.8%) |
| COPD | 22 (9.4%) |
| Chronic hepatic failure | 15 (6.4%) |
| Chronic heart failure | 75 (32.2%) |
| Anemia | 50 (21.4%) |
| Colon cancer | 17 (7.3%) |
| HIV positive | 6 (2.6%) |
| Congenital cardiomyopathy | 15 (6.4%) |
| Previous native valvulopathy | 39 (16.7%) |
| Previous IE | 25 (10.7%) |
| IV drug user | 18 (7.7%) |
| Prosthetic valve IE | 92 (39.4%) |
| Affected valve, n (%) | |
| Aortic | 130 (55.7%) |
| Mitral | 89 (38.2%) |
| Tricuspid | 18 (7.7%) |
| Pulmonary | 2 (0.8%) |
| Echocardiographic findings, n (%) | |
| Vegetation >10 mm | 113 (48.5%) |
| Abscess | 30 (12.9%) |
| Pseudoaneurysm | 16 (6.9%) |
| Fistula | 4 (1.7%) |
| Severe valvular regurgitation | 50 (21.5%) |
| Isolated microorganisms, n (%) | |
| Staphylococcus spp. | 65 (27.9%) |
| Streptococcus spp. | 38 (16.3%) |
| Non-HACEK Gram negative | 14 (6.0%) |
| Negative | 85 (36.4%) |
COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; IE: infective endocarditis; IV: intravenous.
The aortic valve was most often affected, in 55.7% of cases, followed by the mitral valve, with 7.7% of patients having both aortic and mitral valve involvement. Though right-sided IE was more frequent in intravenous drug users (p=0.017), left-sided IE was still more prevalent in this group (77.8% vs. 22.2%). All patients underwent transthoracic and transesophageal echocardiography and more than 90% had evidence of vegetations, which were larger than 10 mm in 48.5%. Abscess was the most common paravalvular complication, followed by pseudoaneurysm and fistula. Regarding isolated microorganisms, Staphylococcus spp. was the most frequent etiological agent (27.9%), followed by Streptococcus spp. Blood culture-negative IE was present in more than a third of the patients.
Mortality and complicationsThirty-eight patients died during hospital stay (16.3%) and 30-day mortality was 22.7%. The results of univariate and multivariate analysis for in-hospital and 30-day mortality are presented in Table 2 and Supplementary Table 1 (Appendix). In multivariate analysis, independent predictors of in-hospital mortality were chronic obstructive pulmonary disease (COPD), clinical course complicated by cerebral embolism and IE due to Staphylococcus spp. and non-HACEK Gram-negative bacilli, the latter being the strongest predictor.
Univariate and multivariate analysis of in-hospital mortality.
| Variable | In-hospital mortality | |||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| OR (CI) | p | OR (CI) | p | |
| Age | 1.003 (0.980-1.025) | 0.826 | ||
| Male gender | 1.199 (0.574-2.504) | 0.630 | ||
| Comorbidities | ||||
| Diabetes | 0.804 (0.342-1.890 | 0.617 | ||
| Chronic renal failure | 2.077 (0.875-4.929) | 0.097 | ||
| COPD | 3.852 (1.460-0.160) | 0.006 | 4.876 (1.354-17.561) | 0.002 |
| Chronic hepatic failure | 0.859 (0.184-4.016) | 0.846 | ||
| Chronic heart failure | 1.996 (0.955-4.172) | 0.066 | ||
| Colon cancer | 2.522 (0.819-7.762) | 0.107 | ||
| HIV positive | 1.309 (0.142-2.072) | 0.812 | ||
| Previous valvulopathy | 2.836 (1.202-6.693) | 0.017 | 2.401 (0.773-7.459) | 0.132 |
| IV drug user | 0.940 (0.198-4.456) | 0.938 | ||
| Prosthetic IE | 1.746 (0.868-3.516) | 0.118 | ||
| Vegetation >10 mm | 1.523 (0.660-3.516) | 0.324 | ||
| Isolated microorganisms | ||||
| Staphylococcus spp. | 3.046 (1.436-6.460) | 0.004 | 3.760 (1.310-10.797) | 0.014 |
| Streptococcus spp. | 0.121 (0.016-0.918) | 0.041 | ||
| Enterococcus spp. | 1.325 (0.412-4.262) | 0.637 | ||
| Non-HACEK Gram negative | 3.990 (1.185-3.431) | 0.025 | 12.759 (2.592-62.806) | 0.002 |
| Fungi | 4.941 (0.302-0.948) | 0.263 | ||
| Negative | 0.315 (0.124-0.802) | 0.150 | ||
| Complications | ||||
| Heart failure | 1.714 (0.771-3.811) | 0.186 | ||
| Perivalvular extension | 2.078 (0.925-4.664) | 0.076 | ||
| Cerebral embolism | 4.898 (2.125-1.292) | <0.001 | 9.321 (3.241-26.808) | <0.001 |
| Peripheral embolism | 1.986 (0.713-5.529) | 0.189 | ||
| Therapy | ||||
| Surgery (vs. no surgery) | 0.938 (0.450-1.958) | 0.865 | ||
| Early surgery (vs. late) | 2.343 (0.694-7.906) | 0.170 | ||
CI: confidence interval; COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; IE: infective endocarditis; OR: odds ratio; IV: intravenous.
Overall, the complication rate was 56.6% and the most frequent complications were (in descending order): heart failure (37.3%); perivalvular extension (25.8%); cerebral embolism (23.2%); and peripheral embolism (13.0%). In multivariate analysis, the variables significantly associated with each complication were: for heart failure, previous history of COPD (odds ratio [OR] 3.86; confidence interval [CI] 1.16-12.81; p=0.028) and presence of valvular regurgitation (OR 2.74; CI 1.26-5.96; p=0.011); for perivalvular extension, IE affecting the aortic valve (OR 6.63; CI 2.34-18.76; p<0.001) and prosthetic valve endocarditis (OR 2.83; IC 1.21-6.61; p=0.016); for cerebral embolism, IE affecting the mitral valve (OR 2.38; IC 1.12-5.04; p=0.024); and for peripheral embolism, Staphylococcus spp. (OR 3.22; CI 1.21-8.59; p=0.019) and age under 65 years (OR 3.34; CI 1.25-8.94; p=0.017).
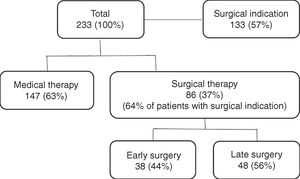
Surgical management of infective endocarditisValve surgery was performed in 86 patients (36.9%), early in 44.2% of cases (Figure 1). The main indications for surgery were heart failure (50.0%) and uncontrolled infection (46.8%), followed by prevention of embolism (15.6%).
Comparing patients who underwent surgical treatment with those who received only medical therapy (Table 3), the former were more likely to be male, younger and intravenous drug users. Aortic valve IE was more frequent in the surgical group, but there were no differences regarding prosthetic valve endocarditis. Surgical therapy was associated with the presence of perivalvular abscesses, vegetations >10 mm and severe valvular regurgitation. Heart failure and perivalvular extension were more common in the surgical group, without difference in embolic complications.
Clinical characteristics of patients treated with surgery vs. no surgery.
| Variable | Surgery (n=86) | No surgery (n=147) | p |
|---|---|---|---|
| Mean age, years | 59.4±15.3 | 65.4±15.1 | 0.001 |
| Male gender | 78.2% | 64.4% | 0.027 |
| Comorbidities | |||
| Diabetes | 25.6% | 26.9% | 0.833 |
| Chronic renal failure | 11.6% | 19.4% | 0.128 |
| COPD | 12.8% | 7.5% | 0.189 |
| Chronic hepatic failure | 1.2% | 9.7% | 0.011 |
| Chronic heart failure | 38.4% | 28.4% | 0.121 |
| Anemia | 18.6% | 23.1% | 0.424 |
| Colon cancer | 3.4% | 9.6% | 0.082 |
| HIV positive | 2.3% | 3.0% | 0.769 |
| Congenital cardiomyopathy | 8.0% | 5.5% | 0.440 |
| Previous native valvulopathy | 21.7% | 13.4% | 0.112 |
| Previous IE | 10.4% | 11.3% | 0.842 |
| IV drug user | 14.1% | 3.6% | 0.010 |
| Prosthetic valve IE | 42.5% | 37.6% | 0.453 |
| Affected valve | |||
| Aortic | 64.4% | 51.0% | 0.046 |
| Mitral | 31.0% | 42.3% | 0.086 |
| Tricuspid | 9.2% | 6.7% | 0.488 |
| Pulmonary | 1.1% | 0.7% | 0.699 |
| Echocardiographic findings | |||
| Vegetation >10 mm | 62.3% | 40.7% | 0.004 |
| Abscess | 19.5% | 9.4% | 0.026 |
| Severe valvular regurgitation | 44.4% | 7.9% | <0.001 |
| Isolated microorganisms | |||
| Staphylococcus spp. | 32.5% | 24.6% | 0.217 |
| Streptococcus spp. | 15.0% | 17.5% | 0.643 |
| Non-HACEK Gram negative | 7.5% | 4.8% | 0.413 |
| Negative | 32.9% | 39.2% | 0.364 |
| Complications | |||
| Heart failure | 60.8% | 22.1% | <0.001 |
| Perivalvular extension | 39.2% | 18.0% | 0.001 |
| Cerebral embolism | 20.8% | 23.7% | 0.651 |
| Peripheral embolism | 11.3% | 15.0% | 0.586 |
COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; IE: infective endocarditis; IV: intravenous.
When the overall cohort was analyzed, surgery was not associated with decreased in-hospital mortality (15.5% in the surgical group vs. 16.3% in the medical group, p=0.865). However, considering only the subgroup of patients with surgical indication according to the guidelines (133 patients), surgical treatment was associated with a significant reduction in in-hospital mortality (15.5% vs. 32.6%, p=0.028).
The mean follow-up was 28.4±30.7 months and was achieved in 95.3% of the population. Seventy patients died during the follow-up period, with one-year mortality of 33.0% and five-year mortality of 42.9%.
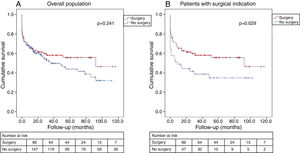
There were no differences between the groups regarding all-cause mortality in the overall cohort (log-rank p=0.241) (Figure 2A). In patients with surgical indication, surgical treatment continued to have a beneficial effect, with better long-term survival (log-rank p=0.029) (Figure 2B).
Kaplan-Meier estimate of all-cause mortality. (A) In the overall cohort, the probability of all-cause mortality did not differ significantly between patients who underwent surgery and those treated with medical therapy only; (B) in patients with surgical indication, surgical treatment led to better long-term survival.
Regarding timing of the procedure, early surgery was not associated with increased mortality compared with later surgery (OR 2.34, CI 0.69-7.91, p=0.176).
DiscussionThe findings of the present study can be summarized as follows: (i) in-hospital mortality from IE was >15% and more than half of the patients had a complicated clinical course; (ii) COPD, cerebral embolism and IE due to Staphylococcus spp. and non-HACEK Gram-negative bacilli were independent predictors of worse in-hospital outcomes; and (iii) surgery was associated with lower in-hospital and long-term mortality in patients with surgical indication.
The characteristics of our population were similar to those described in other studies, including those in Portuguese cohorts.5 In our study the patients’ mean age was 63.4 years, older than that of initial endocarditis descriptions,6 but in agreement with more recent series.1 Overall, the most commonly observed comorbidities in our cohort were chronic heart failure and diabetes (32.2% and 26.1%, respectively). In a large multinational observational study, diabetes was the main underlying condition (16.2%).1 The higher prevalence of comorbidities observed in our study, including chronic heart failure, could be due to the fact that some patients had been referred to our surgical hospital, and so the percentage of sicker patients may be overestimated.
In agreement with recent studies,1,7,8 there was a predominance of Staphylococcus spp. over Streptococcus spp., which used to be the most prevalent pathogen.6 The higher incidence of culture-negative endocarditis could be explained by frequent previous antibiotic administration due to the suspicion of other localized infections, although IE due to fastidious and intracellular microorganisms may also have contributed to this finding.9
The reported in-hospital mortality of patients with IE varies from 12% to 30%.1,10–12 In our study, in-hospital mortality of 16.3% was observed, suggesting that our population is similar to other registries.
Prognosis in IE is influenced by four main factors: patient characteristics, the presence or absence of cardiac and non-cardiac complications, the infecting organism and the echocardiographic findings.4 Multivariate analysis in our study identified COPD, Staphylococcus spp., non-HACEK Gram-negative bacilli and cerebral embolism as independent predictors of in-hospital mortality. Of these four factors, non-HACEK Gram-negative bacilli had the highest odds ratio. Other studies have confirmed the association of these factors with worse prognosis.1,13,14 A possible explanation for the dismal prognosis associated with non-HACEK Gram-negative bacilli endocarditis could that these microorganisms are healthcare-related agents.15 Increasing age,16 diabetes,17 prosthetic valve involvement,18 heart failure and paravalvular complications13 have been identified in other studies as risk factors for higher mortality.
In our study, clinical course was complicated in more than half of the patients. Heart failure is the most frequent complication of IE19,20 and is associated with severe acute valvular regurgitation,19 which was also observed in our study. Uncontrolled infection, the next most likely reason for surgical intervention,20–22 is frequently due to perivalvular extension, which is more common in aortic valve and prosthetic valve endocarditis,22 as also demonstrated in our study.
Embolic events are a life-threatening complication. Predictors of cerebral embolism have been identified in previous studies and include size and mobility of vegetations, causal microorganism and the affected valve.23–25 Our study identified mitral valve IE as an independent predictor of embolism. Large vegetations on the mitral valve are usually associated with a higher incidence of embolism,25 and this is probably the reason for our finding. Regarding peripheral embolism, older patients in the present study had lower rates of peripheral embolism, as reported previously,16 possibly simply due to being underdiagnosed because of mild clinical signs and symptoms.
Surgery is performed in 23-53% of patients with IE in most series,2,10,26 including Portuguese cohorts,5 and our study was consistent with this prevalence (36.9%). Factors associated with the need for surgery were demographics (male and younger patients more often underwent surgery) and the affected valve (the aortic valve was more commonly treated in the surgical group, probably due to a higher incidence of perivalvular extension of the infection, as we also demonstrated). Although intravenous drug users usually have a predominance of right-sided IE caused by Staphylococcus spp., only rarely needing surgery because they are usually responsive to medical therapy alone,27 in our study population intravenous drug use was associated with a higher rate of surgical therapy. This probably results from the fact that we are a surgical center and most cases that are indicated for surgery are referred to us. Also, there has recently been an increase in left-sided IE in intravenous drug users.28
Some echocardiographic findings and complications such as acute severe valvular regurgitation and perivalvular extension were also associated with surgical treatment. However, embolism was not more prevalent in the surgical group. Embolism is usually more frequent in the first two weeks of therapy and less so thereafter.29 Although surgery is not contraindicated in IE complicated by embolism, including ischemic stroke unless the neurological prognosis is judged too poor,29–31 the optimal timing remains controversial because of the risk of recurrent embolism and the hemorrhagic risk associated with surgery.29
Some studies appear to question the importance of surgery in IE patients, as they found no survival benefit. However, in the International Collaboration on Endocarditis-Prospective Cohort Study of 1552 patients with native valve IE,32 surgery during the index hospitalization was associated with a significant reduction in in-hospital mortality (12.1% vs. 20.7%, p<0.001). After propensity matching and adjustment for survival bias, the survival benefit of surgery remained (absolute risk reduction of 5.9%), especially in patients with complications.32 Furthermore, a meta-analysis by Head et al.33 demonstrated a highly significant benefit of surgery (p=0.0008). In our cohort we found no significant difference in mortality between patients who had valve surgery and those who did not in the overall population, but in patients with surgical indication according to the guidelines, mortality was significantly lower in those who underwent surgery (15.5% vs. 32.6%, p=0.028). This shows that surgery by itself is not associated with higher mortality, and the fact that overall mortality was better appears to indicate a prognostic benefit of surgery when performed in selected patients.
Regarding surgical timing, we also found no increase in mortality in patients who underwent early vs. late surgery. In a prospective randomized study by Kang et al. on patients with left-sided IE, severe valve disease and large vegetations, there was no difference in survival at six months between those who underwent early surgery (within 48 hours after randomization) or after, but those operated early had significantly lower rates of the composite endpoint of death from any cause, embolic events, or recurrence of IE at six months (hazard ratio 0.08, CI 0.01-0.65; p=0.02).34
Study limitationsOur study has some limitations. First, it was a retrospective observational study, and therefore information was limited to medical records and important data may have been incomplete. This study reported findings of a single tertiary referral center, which limits generalization of the results. Finally, since some cases came from other hospital centers, the percentage of complicated cases may be overestimated, and some patients (4.7%) were lost to follow-up because of transfer to their hospitals of origin.
However, this study involves an analysis of a significant number of patients and enables some interesting conclusions to be drawn.
ConclusionThe results of the present study help to identify IE patients who are at increased risk of worse outcome. We identified COPD, clinical course complicated by cerebral embolism and IE due to Staphylococcus spp. and non-HACEK Gram-negative bacilli as independent predictors of in-hospital mortality. More than half of the patients presented complicated IE, and predictive factors for each complication were analyzed. Surgery was performed in more than one third of patients and in patients with surgical indication it was associated with improved long-term survival.
Rapid identification of patients with IE at higher risk of mortality may offer the opportunity to change the course of the disease and improve prognosis.
Conflicts of interestThe authors have no conflicts of interest to declare.