The use of mechanical circulatory support is increasing in cases of cardiogenic shock (CS) and high-risk percutaneous coronary intervention (HR-PCI). The Impella® is a percutaneous ventricular assist device that unloads the left ventricle by ejecting blood to the ascending aorta. We report our center's experience with the use of the Impella® device in these two clinical settings.
MethodsWe performed a single-center retrospective study including all consecutive patients implanted with the Impella® between 2007 and 2019 for CS treatment or prophylactic support of HR-PCI. Data on clinical and safety endpoints were collected and analyzed.
ResultsTwenty-two patients were included: 12 were treated for CS and 10 underwent an HR-PCI procedure. In the CS-treated population, the main cause of CS was acute myocardial infarction (five patients); hemolysis was the most frequent device-related complication (63.7%). In-hospital, cumulative 30-day and one-year mortality were 58.3%, 66.6% and 83.3%, respectively. In the HR-PCI group, all patients had multivessel disease (mean baseline SYNTAX I score: 44.1±13.7). In-hospital, 30-day and one-year mortality were 10.0%, 10.0% and 20.0%, respectively. There were no device- or procedure-related deaths in either group.
ConclusionThe short- and long-term results of Impella®-supported HR-PCI were comparable to those in the literature. In the CS group, in-hospital and short-term outcomes were poor, with high mortality and non-negligible complication rates.
O uso de suporte mecânico no choque cardiogénico (CS) e intervenção coronária percutânea de alto risco (HR-PCI) tem aumentado. O Impella® é um sistema de suporte ventricular percutâneo que ejeta sangue do ventrículo esquerdo para a aorta ascendente. Reportamos a experiência do nosso centro com o Impella® nestes dois cenários clínicos.
MétodosEstudo retrospetivo unicêntrico incluindo todos os doentes consecutivos submetidos a implantação de Impella® entre 2007 e 2019, para tratamento de CS ou suporte profilático para HR-PCI. Dados sobre endpoints clínicos e de segurança foram analisados.
ResultadosForam incluídos 22 doentes: 12 tratados por CS e 10 submetidos a HR-PCI. Na população de CS, a principal causa de choque foi o enfarte agudo do miocárdio (5 doentes); a hemólise foi a complicação relacionada com o dispositivo mais frequente (63,7%); a mortalidade intra-hospitalar, a 30 dias e um ano, foi, respetivamente, 58,3%, 66,6% e 83,3%. No grupo da HR-PCI, todos os doentes apresentavam doença multivaso (SYNTAX I score médio: 44,1±13,7); a mortalidade intra-hospitalar, a 30 dias e um ano, foi, respetivamente, 10,0%, 10,0% e 20,0%. Não houve mortes relacionadas com o dispositivo ou procedimento em ambos os grupos.
ConclusãoOs resultados em curto e longo prazo da HR-PCI protegida por Impella® foram comparáveis aos da literatura disponível. No grupo de CS, os resultados intra-hospitalares e em curto prazo foram desanimadores, com elevada mortalidade e taxas de complicações apreciáveis.
Short-term mechanical circulatory support (MCS) devices aim to provide hemodynamic support and maintain coronary and systemic perfusion. Currently, among the main indications for MCS are cardiogenic shock (CS) and high-risk percutaneous coronary intervention (HR-PCI).1–3
The intra-aortic balloon pump (IABP) has been the most used device for decades. However, the IABP-SHOCK II trial showed no survival benefit with the IABP over best medical treatment.4 The European Society of Cardiology (ESC) accordingly downgraded its recommendation for its routine use in CS to class III in 2017.5
Despite the lack of evidence from randomized controlled trials (RCT), the use of MCS in CS patients is increasing.6 Supportive medical therapies have historically failed to improve outcomes in this setting4 and CS mortality remains unacceptably high.2
As coronary lesions formerly considered unsuitable for percutaneous coronary interventions (PCI) are increasingly being treated percutaneously in high-risk patients, prophylactic implantation of percutaneous ventricular assist devices (pVAD) for protected PCI is also increasing. Although there is no universal definition of HR-PCI, certain patient- (advanced age, left ventricular dysfunction, comorbidities) and lesion-related (multivessel disease, unprotected left main disease, last remaining vessel) features3 make the revascularization procedure undeniably challenging.
The Impella® system (Abiomed Inc., Danvers, MA, USA) is a pVAD that directly unloads the left ventricle by ejecting blood forward to the ascending aorta.7,8 The device is a catheter-based non-pulsatile axial flow pump, usually inserted via the femoral artery under fluoroscopic guidance and placed across the aortic valve in the left ventricular (LV) cavity. The Impella® improves forward blood flow and cardiac output, increasing coronary perfusion pressure and end-organ perfusion. In addition, it decreases LV myocardial oxygen consumption and may reduce infarct size.9 End-diastolic compliance and end-diastolic wall stress and pulmonary capillary wedge pressure are also reduced.10 Several versions for LV support are currently available (Impella® 2.5, CP, and 5.0).
Evidence on clinical outcomes with the use of the Impella® is scarce and limited to small clinical trials or registries, leading to controversy regarding its widespread use. Nevertheless, previous studies showed that its implantation is safe and feasible in the setting of CS7,9,11 and HR-PCI.8,12–15
We report the experience and clinical outcomes with the Impella® device in a single Portuguese tertiary center, in the settings of CS and HR-PCI. To the best of our knowledge, this is the largest series of patients treated with the Impella® ever reported in the Portuguese population.
MethodsStudy populationThis is a single-center retrospective observational cohort study including all consecutive patients treated with the Impella® device in our center between April 2007 and October 2019.
Data regarding demographics, comorbidities, procedural aspects, supportive therapy, complications and immediate, 30-day and one-year outcomes were entered into a database. All available angiographic and echocardiographic data were recorded. Glomerular filtration rate (GFR) was calculated using the Cockcroft-Gault formula.
Patients were divided into two groups: CS and HR-PCI. For simplification, patients under extracorporeal membrane oxygenation (ECMO) support who subsequently received an Impella® for venting of the left ventricle were included in the CS group.
In the HR-PCI group, the Society of Thoracic Surgeons (STS) score,16 EUROSCORE II17 and the British Columbia PCI risk score18 were computed to assess the predicted morbidity and mortality risks. The baseline SYNTAX score19 was used to assess the complexity of coronary disease, with the exception of patients with previous bypass grafts. Online calculators were used to estimate the scores and repeated by more than one investigator to ensure correct calculation.
DeviceDevice implantation was performed by an experienced interventional cardiologist under fluoroscopic guidance in the catheterization laboratory (Figure 1). The femoral route was used in all cases. PCI was performed according to the center's standard practice and the need for prophylactic pVAD in the setting of HR-PCI was based on the clinical judgment of the operator and overall risk assessment.
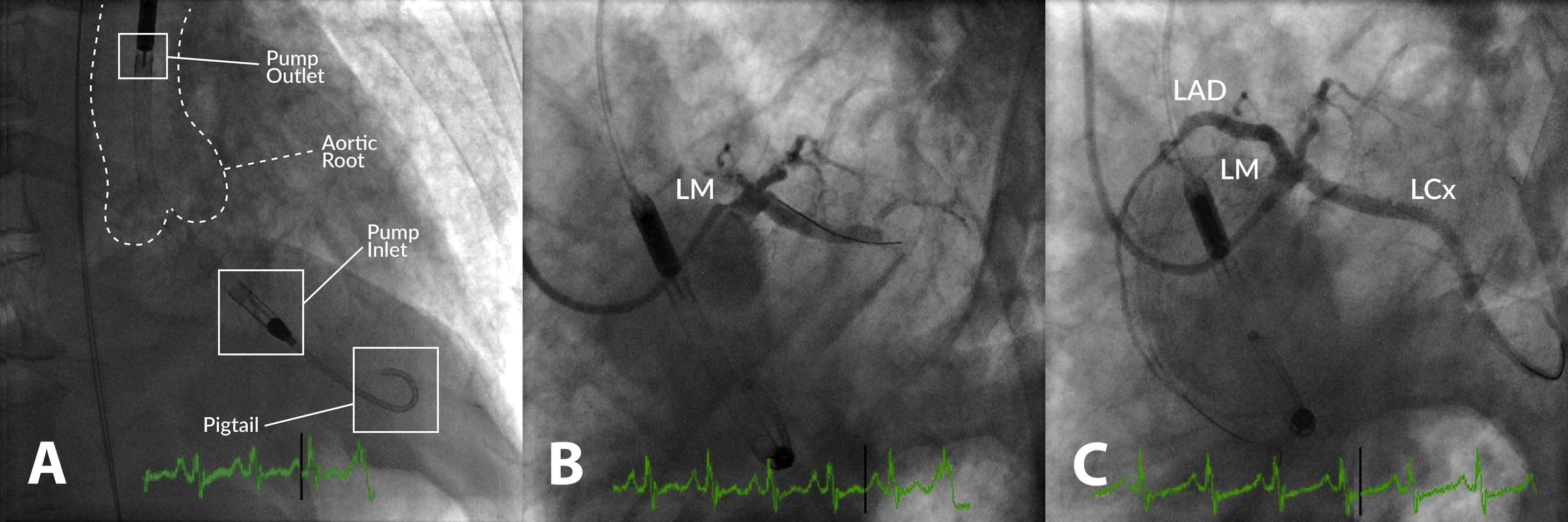
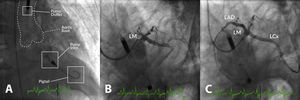
Example of high-risk percutaneous coronary intervention (PCI) supported with an Impella® CP in a patient with multivessel disease presenting with acute myocardial infarction, severe left ventricular (LV) dysfunction and shock. (A) Impella® CP device placed in the LV cavity and ascending aorta; the main radiographic components are depicted; (B) coronary angiogram showing occlusion of the proximal left anterior descending (LAD) and mid-left circumflex (LCx) coronary arteries; (C) coronary angiogram following PCI of the LAD and LCx. LM: left main coronary artery.
The Impella® 2.5 (12-F pump, maximum flow rate 2.5 l/min) was used until 2011 and was thereafter replaced by the Impella® CP (14-F pump, maximum flow rate up to 4.0 l/min).
All patients were subsequently admitted to the cardiac intensive care unit (CICU) immediately post-procedure. Parenteral anticoagulation with unfractionated heparin was used in all cases with a target activated clotting time of >200 s.
Study endpointsClinical endpoints included intraprocedural and in-hospital mortality, 30-day mortality, 30-day major adverse cardiovascular events (MACE) and one-year mortality. MACE were defined as death, myocardial infarction (MI), stroke or rehospitalization at 30 days.
Safety endpoints comprised vascular complications according to the standardized definitions of the Bleeding Academic Research Consortium20 (BARC), acute renal failure (defined as an increase in serum creatinine from baseline ≥0.3 mg/dl), need for renal replacement therapy, hemolysis and need for transfusion.
Statistical analysisDescriptive data were summarized using the appropriate statistical tools, given the nature of the variables involved – mean ± standard deviation or median ± interquartile range or P25-P75 for continuous variables and number and percentage for categorical variables. The Kolmogorov-Smirnov test was used to assess the normal distribution of continuous data. The Student's t test or its non-parametric equivalent (Mann-Whitney U test or Wilcoxon signed-rank test) were used to compare the distribution of continuous variables, and Pearson's chi-square test was used to test the association between categorical variables. Kaplan-Meier survival analysis was performed to assess outcomes and Cox regression was carried out to identify predictors of mortality. A p-value of less than 0.05 was considered significant. The statistical analysis was performed using IBM SPSS version 26 (IBM SPSS, Armonk, New York, USA).
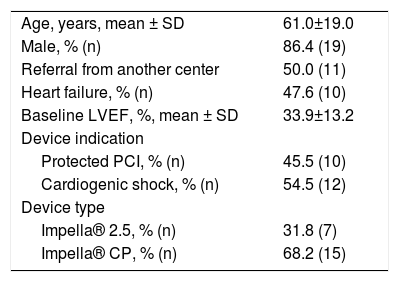
ResultsStudy populationWe retrospectively reviewed 22 patients treated in our center (Table 1). Half of the patients were referred from another hospital. The mean age was 61±19 years and 86.4% of the patients were male. Most patients (68.2%) were treated with the Impella® CP device. The indication for MCS was CS in 12 (54.5%) of the patients and HR-PCI in the other 10 (45.5%). The mean baseline left ventricular ejection fraction (LVEF) was 33.9±13.2% and 62.5% of patients had LVEF≤35%. Median (P25-P75) duration of hospital stay was 5.0 (3.5-11.5) days.
General cohort data (n=22).
| Age, years, mean ± SD | 61.0±19.0 |
| Male, % (n) | 86.4 (19) |
| Referral from another center | 50.0 (11) |
| Heart failure, % (n) | 47.6 (10) |
| Baseline LVEF, %, mean ± SD | 33.9±13.2 |
| Device indication | |
| Protected PCI, % (n) | 45.5 (10) |
| Cardiogenic shock, % (n) | 54.5 (12) |
| Device type | |
| Impella® 2.5, % (n) | 31.8 (7) |
| Impella® CP, % (n) | 68.2 (15) |
IQR: interquartile range; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; SD: standard deviation.
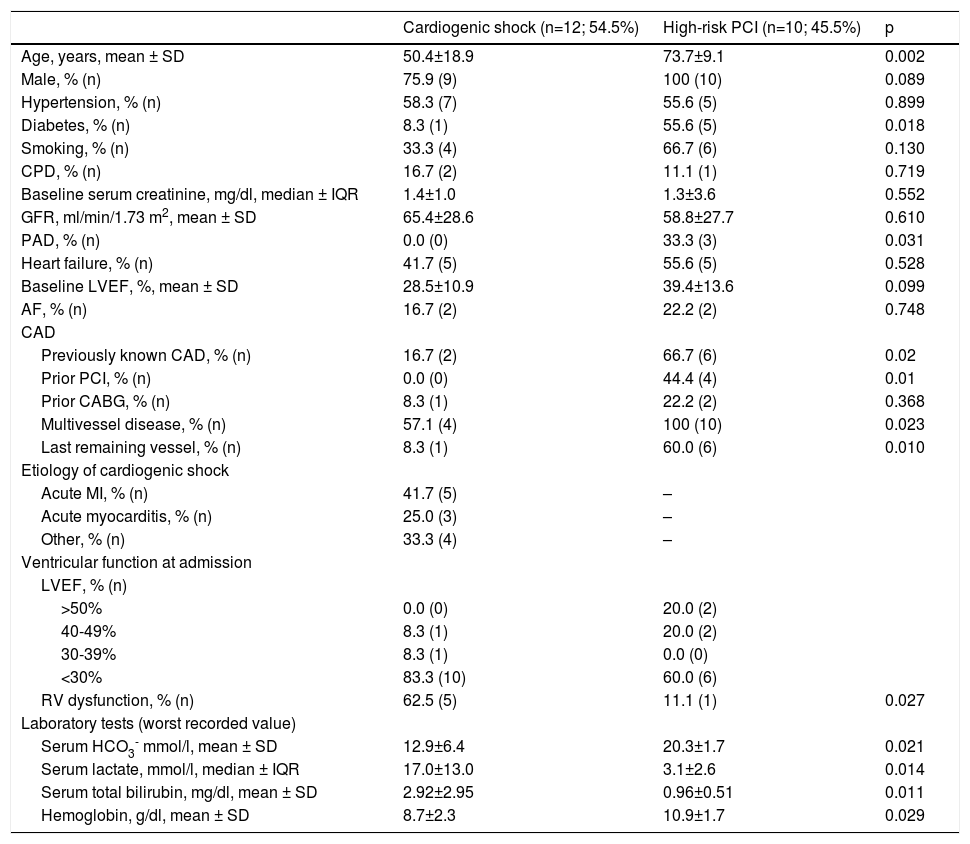
The baseline characteristics of CS patients are presented in Table 2. Twelve patients (mean age 50.4±18.9 years, 75.9% male) with CS were treated with Impella® devices; Impella® CP was used in seven patients. In the CS-treated population, 58.3% had hypertension, 33.3% were smokers and 41.7% had prior heart failure (mean LVEF 28.5±10.9%). Only one patient had diabetes and two patients had previously diagnosed coronary artery disease (CAD).
Baseline characteristics of the study population.
| Cardiogenic shock (n=12; 54.5%) | High-risk PCI (n=10; 45.5%) | p | |
|---|---|---|---|
| Age, years, mean ± SD | 50.4±18.9 | 73.7±9.1 | 0.002 |
| Male, % (n) | 75.9 (9) | 100 (10) | 0.089 |
| Hypertension, % (n) | 58.3 (7) | 55.6 (5) | 0.899 |
| Diabetes, % (n) | 8.3 (1) | 55.6 (5) | 0.018 |
| Smoking, % (n) | 33.3 (4) | 66.7 (6) | 0.130 |
| CPD, % (n) | 16.7 (2) | 11.1 (1) | 0.719 |
| Baseline serum creatinine, mg/dl, median ± IQR | 1.4±1.0 | 1.3±3.6 | 0.552 |
| GFR, ml/min/1.73 m2, mean ± SD | 65.4±28.6 | 58.8±27.7 | 0.610 |
| PAD, % (n) | 0.0 (0) | 33.3 (3) | 0.031 |
| Heart failure, % (n) | 41.7 (5) | 55.6 (5) | 0.528 |
| Baseline LVEF, %, mean ± SD | 28.5±10.9 | 39.4±13.6 | 0.099 |
| AF, % (n) | 16.7 (2) | 22.2 (2) | 0.748 |
| CAD | |||
| Previously known CAD, % (n) | 16.7 (2) | 66.7 (6) | 0.02 |
| Prior PCI, % (n) | 0.0 (0) | 44.4 (4) | 0.01 |
| Prior CABG, % (n) | 8.3 (1) | 22.2 (2) | 0.368 |
| Multivessel disease, % (n) | 57.1 (4) | 100 (10) | 0.023 |
| Last remaining vessel, % (n) | 8.3 (1) | 60.0 (6) | 0.010 |
| Etiology of cardiogenic shock | |||
| Acute MI, % (n) | 41.7 (5) | – | |
| Acute myocarditis, % (n) | 25.0 (3) | – | |
| Other, % (n) | 33.3 (4) | – | |
| Ventricular function at admission | |||
| LVEF, % (n) | |||
| >50% | 0.0 (0) | 20.0 (2) | |
| 40-49% | 8.3 (1) | 20.0 (2) | |
| 30-39% | 8.3 (1) | 0.0 (0) | |
| <30% | 83.3 (10) | 60.0 (6) | |
| RV dysfunction, % (n) | 62.5 (5) | 11.1 (1) | 0.027 |
| Laboratory tests (worst recorded value) | |||
| Serum HCO3- mmol/l, mean ± SD | 12.9±6.4 | 20.3±1.7 | 0.021 |
| Serum lactate, mmol/l, median ± IQR | 17.0±13.0 | 3.1±2.6 | 0.014 |
| Serum total bilirubin, mg/dl, mean ± SD | 2.92±2.95 | 0.96±0.51 | 0.011 |
| Hemoglobin, g/dl, mean ± SD | 8.7±2.3 | 10.9±1.7 | 0.029 |
AF: atrial fibrillation; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CPD: chronic pulmonary disease; GFR: glomerular filtration rate; HCO3-: bicarbonate; IQR: interquartile range; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; RV: right ventricular; SD: standard deviation.
The main cause of CS was MI (41.7%). A quarter of patients presented with acute myocarditis and 33.3% had acute decompensated heart failure. The majority (83.3%) of patients presented with severe LV dysfunction and 62.5% also had impaired right ventricular function. Cardiorespiratory arrest occurred in 83.3% of cases. All patients presented with multiorgan dysfunction prior to device implantation.
All patients received vasopressors or inotropes (median number of drugs 2±1), 91.7% were mechanically ventilated and 33.3% had need for renal replacement therapy. Infection complicated clinical course in six patients.
Concomitant use of ECMO was recorded in five patients. In two cases, the primary indication for Impella® implantation was venting of the LV in patients previously on ECMO support. Three patients who initially received an Impella® needed therapy escalation due to hemodynamic deterioration and were subsequently placed on ECMO support. Five patients were previously under IABP support and were switched to an Impella® device, as they remained in refractory CS. Detailed information on procedural characteristics and supportive therapy is provided in Table 3.
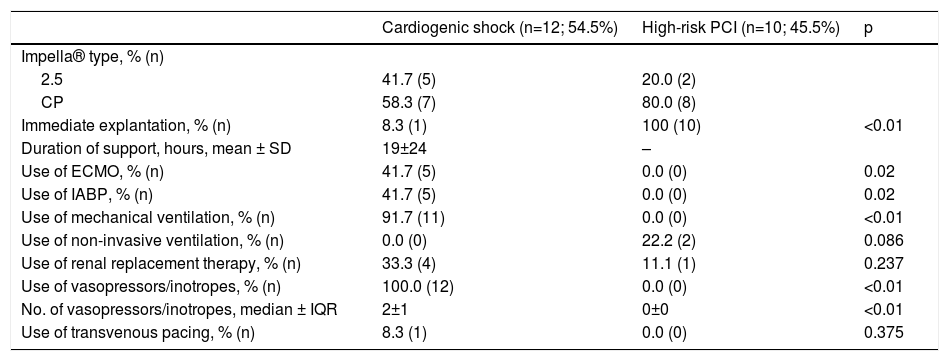
Procedural aspects and supportive care.
| Cardiogenic shock (n=12; 54.5%) | High-risk PCI (n=10; 45.5%) | p | |
|---|---|---|---|
| Impella® type, % (n) | |||
| 2.5 | 41.7 (5) | 20.0 (2) | |
| CP | 58.3 (7) | 80.0 (8) | |
| Immediate explantation, % (n) | 8.3 (1) | 100 (10) | <0.01 |
| Duration of support, hours, mean ± SD | 19±24 | – | |
| Use of ECMO, % (n) | 41.7 (5) | 0.0 (0) | 0.02 |
| Use of IABP, % (n) | 41.7 (5) | 0.0 (0) | 0.02 |
| Use of mechanical ventilation, % (n) | 91.7 (11) | 0.0 (0) | <0.01 |
| Use of non-invasive ventilation, % (n) | 0.0 (0) | 22.2 (2) | 0.086 |
| Use of renal replacement therapy, % (n) | 33.3 (4) | 11.1 (1) | 0.237 |
| Use of vasopressors/inotropes, % (n) | 100.0 (12) | 0.0 (0) | <0.01 |
| No. of vasopressors/inotropes, median ± IQR | 2±1 | 0±0 | <0.01 |
| Use of transvenous pacing, % (n) | 8.3 (1) | 0.0 (0) | 0.375 |
ECMO: extracorporeal membrane oxygenation. IABP: intra-aortic balloon pump; IQR: interquartile range; SD: standard deviation.
One patient was referred for surgical implantation of a ventricular assist device (INCOR®, Berlin Heart) and three patients were transferred to a transplantation center. Mean duration of Impella® support was 19±24 hours.
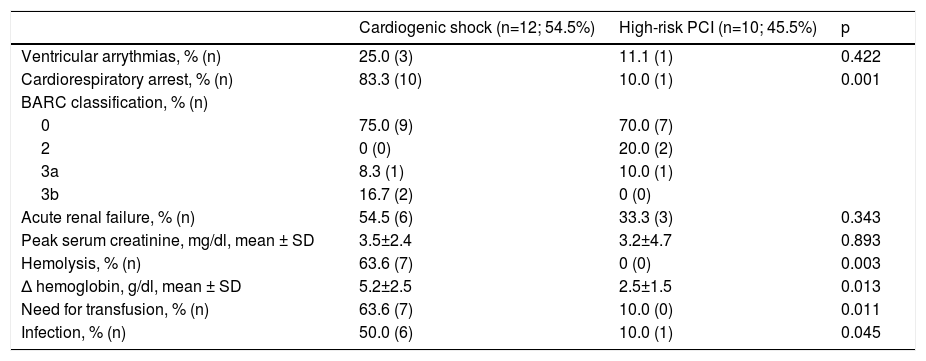
Safety endpoints and adverse events are shown in Table 4. Hemolysis was the most frequent device-related complication (63.7%) and was an indication for transfusion in four patients. Six of the patients presented with acute renal failure. One patient presented with a BARC type 3a vascular complication and two patients exhibited BARC type 3b complications.
Safety endpoints and adverse events.
| Cardiogenic shock (n=12; 54.5%) | High-risk PCI (n=10; 45.5%) | p | |
|---|---|---|---|
| Ventricular arrythmias, % (n) | 25.0 (3) | 11.1 (1) | 0.422 |
| Cardiorespiratory arrest, % (n) | 83.3 (10) | 10.0 (1) | 0.001 |
| BARC classification, % (n) | |||
| 0 | 75.0 (9) | 70.0 (7) | |
| 2 | 0 (0) | 20.0 (2) | |
| 3a | 8.3 (1) | 10.0 (1) | |
| 3b | 16.7 (2) | 0 (0) | |
| Acute renal failure, % (n) | 54.5 (6) | 33.3 (3) | 0.343 |
| Peak serum creatinine, mg/dl, mean ± SD | 3.5±2.4 | 3.2±4.7 | 0.893 |
| Hemolysis, % (n) | 63.6 (7) | 0 (0) | 0.003 |
| Δ hemoglobin, g/dl, mean ± SD | 5.2±2.5 | 2.5±1.5 | 0.013 |
| Need for transfusion, % (n) | 63.6 (7) | 10.0 (0) | 0.011 |
| Infection, % (n) | 50.0 (6) | 10.0 (1) | 0.045 |
Δ: difference; BARC: Bleeding Academic Research Consortium vascular complications; IQR: interquartile range; PCI: percutaneous coronary intervention; SD: standard deviation.
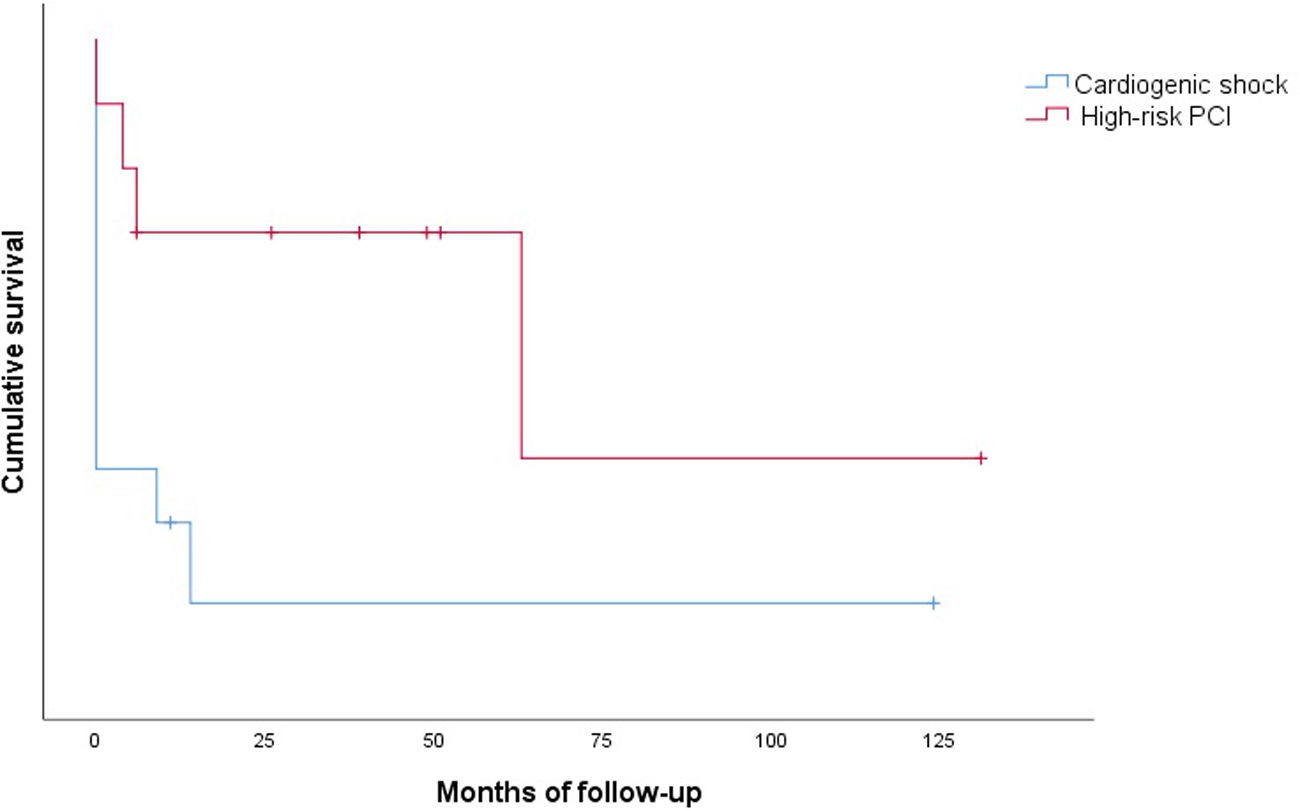
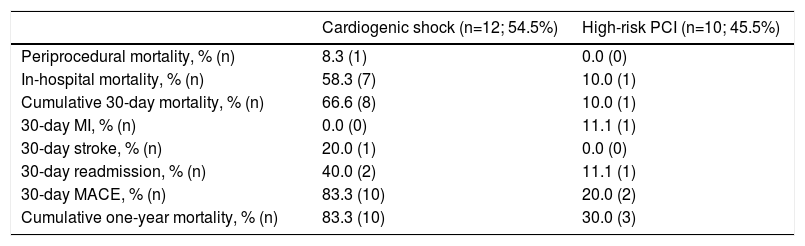
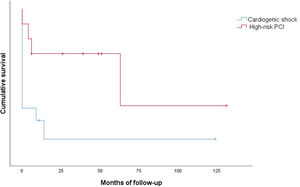
Clinical outcomes are reported in Table 5. Only one intraprocedural death was recorded, which was not device- or technique-related. In-hospital mortality was 58.3% and cumulative 30-day mortality was 66.6%. The 30-day MACE rate was 83.3%, with two patients readmitted during the first month. Only one patient presented with stroke. Cumulative one-year mortality was 83.3%. The mean follow-up in the CS group was 13.2±35.3 months (Figure 2).
Clinical outcomes.
| Cardiogenic shock (n=12; 54.5%) | High-risk PCI (n=10; 45.5%) | |
|---|---|---|
| Periprocedural mortality, % (n) | 8.3 (1) | 0.0 (0) |
| In-hospital mortality, % (n) | 58.3 (7) | 10.0 (1) |
| Cumulative 30-day mortality, % (n) | 66.6 (8) | 10.0 (1) |
| 30-day MI, % (n) | 0.0 (0) | 11.1 (1) |
| 30-day stroke, % (n) | 20.0 (1) | 0.0 (0) |
| 30-day readmission, % (n) | 40.0 (2) | 11.1 (1) |
| 30-day MACE, % (n) | 83.3 (10) | 20.0 (2) |
| Cumulative one-year mortality, % (n) | 83.3 (10) | 30.0 (3) |
IQR: interquartile range; MACE: major adverse cardiovascular events; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation.
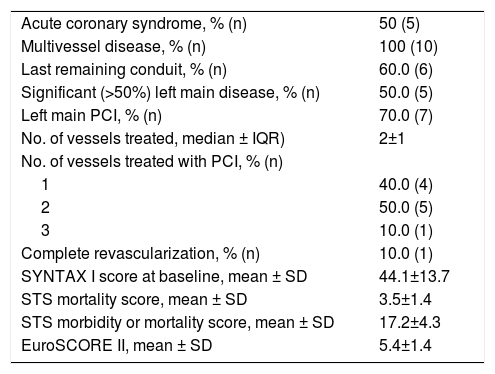
Baseline characteristics of the HR-PCI group are shown in Table 1. Ten patients were referred for HR-PCI after the heart team considered them ineligible for surgery due to prohibitively high risk. All but two patients were treated with an Impella® CP device. HR-PCI patients were significantly older than CS patients (mean age 73.7±9.1 years, p=0.002). All subjects were male, with a prevalence of hypertension and diabetes of 55.6%. Most patients (66.7%) were smokers and one patient had chronic pulmonary disease. Most individuals (55.6%) had previous heart failure (half with LVEF≤35%) and CAD (66.7%). Three patients (33.3%) had peripheral arterial disease. Two patients had previous coronary artery bypass graft (CABG) surgery and four had a prior PCI procedure. Details of the group's coronary anatomy and PCI procedural aspects are reported in Table 6.
High-risk percutaneous coronary intervention group (n=10): coronary anatomy and procedural aspects.
| Acute coronary syndrome, % (n) | 50 (5) |
| Multivessel disease, % (n) | 100 (10) |
| Last remaining conduit, % (n) | 60.0 (6) |
| Significant (>50%) left main disease, % (n) | 50.0 (5) |
| Left main PCI, % (n) | 70.0 (7) |
| No. of vessels treated, median ± IQR) | 2±1 |
| No. of vessels treated with PCI, % (n) | |
| 1 | 40.0 (4) |
| 2 | 50.0 (5) |
| 3 | 10.0 (1) |
| Complete revascularization, % (n) | 10.0 (1) |
| SYNTAX I score at baseline, mean ± SD | 44.1±13.7 |
| STS mortality score, mean ± SD | 3.5±1.4 |
| STS morbidity or mortality score, mean ± SD | 17.2±4.3 |
| EuroSCORE II, mean ± SD | 5.4±1.4 |
IQR: interquartile range; PCI: percutaneous coronary intervention; STS: Society of Thoracic Surgeons; SD: standard deviation.
The mean baseline SYNTAX I score was 44.1±13.7. The SYNTAX score was not calculated for patients with previous CABG. The mean STS mortality and morbidity or mortality scores were 3.5±1.4 and 17.2±4.3, respectively. The mean EUROSCORE II was estimated at 5.4±1.4. The mean British Columbia PCI risk score was 13.6±22.4.
All patients presented with multivessel disease and six had a last patent conduit at the time of the procedure. The median number of vessels treated was 2±1. Seven patients underwent PCI of a left main lesion and, since none of these patients had previous bypass grafts, they were all cases of unprotected left main angioplasty. The Impella® device was explanted immediately after the procedure in all patients.
There were no intraprocedural or device-related deaths. Two patients experienced a BARC type 2 vascular complication (groin hematoma) and one patient presented with a type 3a complication. Three patients presented with acute renal failure. One patient died during hospitalization. Only one patient died during the first 30 days of follow-up. The 30-day MACE rate was 20.0%, with one patient readmitted for MI in this period. Cumulative mortality at one year was 30.0% (all deaths were of cardiovascular cause). The mean follow-up in the HR-PCI group was 37.5±39.9 months (Figure 2).
Predictors of mortalityPredictors of mortality identified by univariate analysis included a lower baseline GFR (hazard ratio [HR] 0.974, 95% confidence interval [CI] 0.851-0.998, p=0.031), higher lactate level (HR 1.131, 95% CI 1.008-1.269, p=0.036) and lower bicarbonate level (HR 0.848, 95% CI 0.736-0.977, p=0.022). Cardiorespiratory arrest during the clinical course (HR 5.540, 95% CI 1.677-18.295, p=0.005) and the concomitant use of ECMO (HR 8.288, 95% CI 1.948-35.259, p=0.004) and mechanical ventilation (HR 5.407, 95% CI 1.467-19.934, p=0.011) also predicted mortality in our cohort. There were no independent predictors of mortality on multivariate analysis.
DiscussionThis is a report and analysis of the largest Portuguese series of patients treated with the Impella® device. In our registry, the main indication for Impella® use was CS (54.5%), mostly in the setting of an acute coronary event. The other 45.5% of patients underwent implantation of the device for HR-PCI. All of these patients presented with multivessel disease and complex coronary anatomies, as expressed by their extremely high SYNTAX scores.
In our center, in contrast with other reports, the largest experience is with the Impella® CP, the most commonly used device for both indications. Of note, no device-related deaths were recorded in the 12 years of experience with the Impella®. Major vascular complications were only reported in two patients, a lower rate than described in larger series,6,15 very likely related to the preferential use of the 14-F device in our center.
Cardiogenic shockDespite early revascularization and advances in treatment, CS remains a leading cause of death in acute MI.2 Since the publication of the SHOCK trial, which showed improved outcomes with early reperfusion,21 all other tested therapies have failed to extend survival.
Despite the lack of evidence from RCTs, the use of MCS for CS is increasing.6 The IABP is the most used device in this setting. It decreases afterload by unloading the left ventricle and modestly increases cardiac output.1 However, the IABP failed to reduce 30-day mortality in patients with CS complicating acute MI and was not superior to best medical treatment in the IABP-SHOCK II trial.4 This led to a downgrade in the ESC guidelines, which no longer recommend its routine use.5
The Impella® provides greater hemodynamic support and, unlike the IABP, does not rely on a stable rhythm or trigger,1 making it an attractive alternative for patients with profound CS, who often present with transient arrhythmias.
Previous studies compared the Impella® with the IABP.6,7,22 In the IMPRESS in Severe Shock trial, which enrolled 48 patients with CS complicating ST-elevation MI, 30-day mortality was similar in patients treated with Impella® and with IABP (46% vs. 50%, p=0.92).22 Schrage et al., in a recent matched-pair analysis of the IABP-SHOCK II trial, showed an absence of survival benefit with the Impella® device.6 Use of the Impella® did not reduce 30-day all-cause mortality compared with matched patients from the IABP-SHOCK II trial treated with IABP or medical therapy.6 The pVAD was, however, associated with significantly higher rates of severe or life-threatening bleeding, peripheral vascular complications and sepsis.6 By contrast, a sub-analysis of the USpella registry suggested that early initiation of hemodynamic support with Impella® prior to PCI is associated with more complete revascularization and improved survival in the setting of refractory CS complicating acute MI.9
ECMO was introduced in our center in 2011. Previously, the IABP and Impella® were the only devices available for patients requiring MCS. This contributed to the heterogeneity of patients and CS etiologies in this cohort. Since ECMO became available at our institution, it has been the device of choice for most patients with CS and in cases of cardiac arrest. Only patients with CS complicating MI and ECMO-supported patients with need for LV venting have since been preferentially implanted with the Impella®.
In line with the reviewed literature, in-hospital and 30-day mortality were high in our registry. These disappointing results express the severity and heterogeneity of the patients treated. Nevertheless, selection bias may have influenced the results, and it is impossible to predict the outcome if the patients had not received the pVAD. Moreover, in two of the patients, the primary indication for Impella® placement was venting of the left ventricle following ECMO implantation; for both patients, the clinical presentation was severe shock with multiorgan failure. This represents a distinct subset of patients and a different indication for the device. However, we decided to include them in the analysis of CS patients since the clinical scenario and support therapy were similar. Also contributing to the poor prognosis, most patients developed cardiorespiratory arrest during the clinical course.
A significant percentage of patients in our cohort presented with hemolysis. This rate was higher than in previous reports, which may be explained by our shorter experience with the device and the influence of a learning curve. Also, the criteria for hemolysis were not uniform and in some cases were based solely on medical notes, since serial haptoglobin and lactate dehydrogenase levels were not available in all patients. However, the large IMP-IT registry also reported a 20.5% rate of hemolysis in patients treated with the Impella® for CS.15 No cases of aortic valve injury or device-related death occurred during the period analyzed.
Three of our patients survived until transfer to a transplantation center. Unfortunately, none of these patients were actually transplanted. While support with the Impella® contributed to immediate stabilization and survival to admission to an institution with the aim of transplantation, it failed to impact on their long-term prognosis. Transplantation is, in our experience, regrettably, an almost inaccessible treatment nowadays. This prompts discussion regarding a possibly unmet need for medium- and long-term left ventricular assist device therapy in Portugal.
High-risk percutaneous coronary interventionPatients with multivessel disease or high-risk coronary lesions represent a complex subset. Although CABG is the standard option for severe left main or multivessel disease, particularly in patients with left ventricular dysfunction,23 surgery is sometimes not feasible due to frailty and high risk of periprocedural morbidity and mortality. While the European guidelines give a class III recommendation for PCI in cases of multivessel disease with a SYNTAX score >22 (or >32 for left main disease),23 PCI may be the only available option for these patients when they are refused for surgery for anatomic or clinical reasons.
Although there is no universal definition of HR-PCI, certain anatomic features – multivessel disease, left main lesions, last remaining conduits, severely calcified or chronically occluded arteries – represent an obvious therapeutic challenge, especially if combined with LV dysfunction or severe comorbidities. With the increasing complexity of coronary lesions (and patients) presenting to the cardiac catheterization lab, the prophylactic use of MCS to minimize periprocedural hemodynamic instability during percutaneous revascularization is rising.
In our registry, protected PCI was the indication for Impella® implantation in 45.5% of patients. This accounts for only 0.09% of the total number of angioplasties performed at our institution in the period analyzed, which reinforces the need for careful selection of patients receiving prophylactic support for HR-PCI. The complexity of CAD was the main criterion for pVAD implantation. All patients presented with multivessel disease, and six had a last patent conduit at the time of intervention. The mean SYNTAX score was 44.1±13.7, the highest among the reviewed literature, including the large German Impella®14 and USpella24 registries (SYNTAX scores of 33.0 and 31.4, respectively). Furthermore, the figure in our study probably underestimates the complexity of the population's coronary anatomy, since the SYNTAX score was not calculated in the two patients who presented perhaps the most challenging disease, due to the presence of bypass grafts. In both cases, the bypass graft treated by PCI was the last patent conduit. By contrast, the mean LVEF of our HR-PCI cohort was higher (39.4±13.6%) than in the above registries.
The IABP is still the most widely used support device in HR-PCI. The PROTECT I trial confirmed the safety and feasibility of Impella® support during complex PCI procedures.8 Subsequently, PROTECT II, the largest trial of HR-PCI using MCS, compared the use of the IABP to the Impella® in this setting.10 At 90 days, in the per-protocol population, a trend toward decreased major adverse events was observed in Impella®-supported patients, compared to those implanted with an IABP.10
A joint expert consensus from the American College of Cardiology and the Society for Cardiovascular Angiography and Interventions recommends the use of the Impella® to support PCI in patients with left main disease, last remaining conduit or severe multivessel CAD, in the presence of severe LV dysfunction (LVEF<35%) or decompensated heart failure.1 By contrast, to date, ESC guidelines have not included recommendations regarding the use of pVAD for HR-PCI.
Throughout our center's experience with the Impella® for HR-PCI, overall outcomes were favorable in this subset of patients and in line with the results from larger series.9,14,24 Only one patient died during the first month of follow-up and 70% survived the first year post-PCI. In fact, 30-day mortality was lower than predicted by the British Columbia PCI risk score (10.0% vs. 13.6%). Complication rates were lower in this group, probably related to the shorter duration of use. Our promising results confirm the safety of the Impella® for this specific indication, even in a center with limited experience.
Tools to assess the need for MCS during HR-PCI are currently not available and would be useful to appropriately select patients who would likely benefit from intraprocedural hemodynamic support. RCTs in this field are needed to further assist clinicians on the optimal approach to CS.
LimitationsThere are several limitations to our study. Given its retrospective nature, during data collection, complete retrieval of information regarding patients transferred from other institutions was challenging, especially in the deceased.
The analyzed timeframe included the learning curve in use of the Impella®, and two different versions of the device were used (2.5 and CP).
Moreover, the decision to implant the Impella® was not based on a structured protocol. Instead, it resulted from discussions between the referring physician and the interventional cardiologist and/or the CICU attending cardiologist. Therefore, our cohort does not exhibit a homogeneous clinical presentation or indication for pVAD. The development of a formal shock team is imperative as experience with the device and the number of potential candidates grow.
Although this is a small cohort with heterogeneous patients and treatment indications, it represents, to our knowledge, the largest Portuguese series of patients treated with the Impella®. We feel it is important to report our results and hope this will lead to a wider discussion among the national cardiology community and, eventually, result in the adoption of a multicenter collaborative protocol for a registry concerning these severely ill patients.
ConclusionIn our experience, the use of the Impella® to provide hemodynamic support during HR-PCI, in a selected group of extremely complex coronary disease patients, was feasible and safe. Long-term results are not satisfactory but are comparable to the reported literature and, in our opinion, attributable to the severity of the underlying disease.
In the CS group, in-hospital and 30-day outcomes were poor, with high mortality and non-negligible complication rates, illustrating the severity, complexity and non-uniformity of this particular clinical scenario. With the expanding use of the device, the experience and skill of acute cardiac care teams in the management and troubleshooting of patients under Impella® support will likely improve. This will, hopefully, positively impact the prognosis of this subset of patients. Larger studies are needed to better identify the most suitable candidates for this device and the optimal timing for implantation.
Conflicts of interestThe authors have no conflicts of interest to declare.