In a primary percutaneous coronary intervention (PCI) program, interhospital transfer of patients with ST-elevation myocardial infarction (STEMI) can increase ischemic time, compared to patients who are admitted directly to a catheterization laboratory.
ObjectiveTo assess the impact of interhospital transfer in patients with STEMI undergoing primary PCI, in terms of time to reperfusion and one-year mortality.
MethodsThis was an observational, retrospective, longitudinal study of patients with STEMI admitted to Hospital de Braga between June 2011 and May 2016, who were treated successfully within 12hours of symptom onset. A total of 1222 patients were included and divided into two groups according to admission to Hospital de Braga: direct or interhospital transfer.
ResultsIn this study, 37.0% (n=452) of the population were admitted directly to Hospital de Braga and 63.0% (n=770) were transferred from other hospitals. Although timings (in min) until reperfusion were longer in interhospital transfer patients (symptom onset-first medical contact (median 76.5, IQR 40.3–150 vs. 91.0, IQR 50–180, p=0.002), first medical contact-reperfusion (median 87.5, IQR 69.0–114 vs. 145, IQR 115–199, p<0.001) and symptom onset-reperfusion (median 177, IQR 125–265 vs. 265, IQR 188–400, p<0.001)), one-year mortality did not differ significantly between the groups (53 [11.7 %] vs. 71 [9.2 %], p=0.193). In multivariate analysis, age, symptom onset-reperfusion time and especially Killip class IV at admission (HR 11.2, 95 % CI 6.35–19.8, p<0.001) were the main independent predictors of one-year mortality.
ConclusionInterhospital transfer of patients with STEMI increased the time before PCI. No differences were detected between groups in one-year mortality. This may be related to the fact that the direct admission group had twice as many patients in Killip class IV as the interhospital transfer group.
Num programa de angioplastia primária, a transferência inter-hospitalar dos doentes com enfarte agudo do miocárdio com elevação do segmento ST (EAMcEST) pode aumentar o tempo de isquemia relativamente aos doentes que são admitidos diretamente num laboratório de hemodinâmica.
ObjetivoAvaliar o impacto da transferência inter-hospitalar nos doentes submetidos a intervenção coronária percutânea primária (ICPp), no intervalo de tempo até à reperfusão e na mortalidade a um ano.
MétodosEstudo observacional, retrospetivo e longitudinal. 1222 doentes com EAMcEST admitidos no Hospital de Braga (HB), entre junho/2011 e maio/2016, tratados com sucesso nas primeiras 12 horas de evolução dos sintomas, foram divididos em dois grupos consoante a admissão: direta ou transferência inter-hospitalar.
Resultados37.0 %(n=452) foram admitidos diretamente no HB e 63.0 %(n=770) transferidos de outros hospitais. Apesar do aumento nos intervalos de tempo (em minutos) até à reperfusão nos doentes da transferência inter-hospitalar (início dos sintomas-primeiro contato médico) (Mdn=76,5,AIQ (40,3–150) versus Mdn=91,0,AIQ (50–180), p=0002), primeiro contato médico-reperfusão (Mdn=87,5, AIQ (69,0–114) versus Mdn=145, AIQ (115–199),p<0001) e início dos sintomas-reperfusão (Mdn=177,AIQ(125–265) versus Mdn=265, AIQ (188–400),p=11,2, IC95 % [6,35–19,8], p<0001) revelaram-se os principais preditores independentes de mortalidade a um ano.
ConclusãoA transferência inter-hospitalar dos doentes com EAMcEST aumentou de forma significativa o intervalo de tempo até à realização da ICPp. Relativamente à mortalidade a um ano, não se detetaram diferenças entre grupos. Isto pode estar relacionado com o fato de o grupo da admissão direta ter o dobro de doentes em Killip IV.
Cardiovascular disease (CVD) is the leading cause of death worldwide.1 According to the World Health Organization, 17.7 million people died from CVD in 2015, representing 31 % of overall global mortality, of whom 7.4 million died directly from coronary disease.2 In Europe, CVD is responsible for 3.9 million deaths each year; it is estimated that one in six men, and one in seven women, will die from myocardial infarction (MI).3,4 In Portugal, mortality from CVD has fallen over the years, but it remains the leading cause of death, accounting for 29.7 % of overall mortality in 2015.5
Ischemic heart disease has the highest mortality of all CVD,1 particularly ST-elevation myocardial infarction (STEMI), in both the acute phase and in follow-up. Among the available therapeutic options for this condition, primary percutaneous coronary intervention (PCI) has been shown to be more effective than thrombolysis in reducing mortality and adverse cardiac events, as well as being safer, as it is less likely to be associated with severe bleeding complications.
Whichever method of coronary reperfusion is chosen, it should be performed as rapidly as possible. The patient’s prognosis depends on the extent of infarction, which in turn depends on the time from symptom onset to reperfusion of the myocardial tissue.6 Rapid restoration of coronary flow reduces infarct size and reduces morbidity and mortality.7
Most hospitals in Portugal do not possess a catheterization laboratory, and thus patients with STEMI must be transferred to a referral hospital with this facility. The impact on prognosis in STEMI of delays associated with interhospital transfer has been the subject of considerable research in Europe and efforts have been made to reduce these delays.8,9 Development of clearly defined regional protocols has helped to increase the proportion of patients treated within the specified time windows.10,11 Nevertheless, if a patient goes to a center without a catheterization laboratory and is then transferred to a PCI-capable hospital, reperfusion can be significantly delayed,12,13 which can increase morbidity and mortality. Ideally, all STEMI patients should be taken by the emergency medical services to a hospital capable of performing PCI immediately. However, in practice, many patients travel by their own means to a hospital without a catheterization laboratory, thereby delaying initiation of treatment that is known to reduce mortality.12,13
The aim of this study is to compare treatment delay in patients with STEMI undergoing interhospital transfer and in those admitted directly to Hospital de Braga, and to assess its impact on mortality, in order to obtain data on which to base discussion and development of measures to improve prognosis of STEMI patients in the Minho region of Portugal.
MethodsStudy designThis was an observational, retrospective, longitudinal study with descriptive and analytical components.
Study populationThe initial study population consisted of 1369 patients diagnosed with STEMI and treated successfully by primary PCI in the catheterization laboratory of Hospital de Braga between June 1, 2011 and May 31, 2016. Of these, 115 were found to have late presentation (>12hours after symptom onset) and were excluded from the analysis. To avoid duplication of results, 12 patients with a second STEMI within the selected time frame were also excluded, as were another 20 patients in whom one-year follow-up data were incomplete. The final study population was thus 1222 patients, who were divided into two groups according to mode of admission to our hospital: direct admission or interhospital transfer. The former group (37 %, n=452) consisted of those who went by their own means to the emergency department of Hospital de Braga or were transported to our hospital by the emergency medical service. The interhospital transfer group (63.0 %, n=452) consisted of those who were transferred to Hospital de Braga from regional hospitals without PPCI capability (Figure 1).
Data collectionData were collected for the present study from patients’ medical records using medical information management software (Glintt®, SimmaCardio® and Plataforma de Dados de Saúde®) and entered into a computer database.
Sociodemographic data (age, gender, weight, height, and body mass index) were collected. Clinical data collected included history of diabetes, hypertension, current or previous smoking, chronic kidney disease (CKD), MI, PCI, and coronary artery bypass grafting; type of STEMI (anterior vs. inferior/lateral/posterior); and Killip class at admission and during hospital stay. Angiographic data (presence or absence of multivessel disease) and echocardiographic data (left ventricular ejection fraction based on the last exam before hospital discharge) were also collected. The timings of each patient’s pathway – symptom onset, first medical contact (FMC) and perfusion by PCI – were recorded and the patient’s mode of admission, as defined above, was noted.
In order to analyze patients’ prognosis, data on in-hospital, 30-day, six-month and one-year mortality were collected.
DefinitionsFMC was defined as the patient’s first contact with any health service, including primary health care. For patients attended initially by the national emergency medical service, FMC was defined as the time when a medical emergency response vehicle or advanced life support ambulance arrived at the patient’s location. Time of reperfusion was defined as the time when the angioplasty guidewire crossed the culprit lesion.
Three timings in the patient’s pathway were recorded: symptom onset-FMC, FMC-reperfusion, and symptom onset-reperfusion.
Late-presentation MI was defined as a period of >12hours between symptom onset and reperfusion.
Successful PCI was defined as TIMI flow ≥2 and <30 % residual stenosis following the procedure.
Multivessel disease was diagnosed angiographically as the presence of >50 % stenosis in at least one epicardial coronary artery in addition to the culprit artery.
Stratification by Killip class was based on physical examination and the presence of heart failure. Patients in cardiogenic shock was classified as Killip class IV.14,15
Statistical analysisThe statistical analysis was performed using IBM SPSS® version 23.0.
Distribution of continuous variables was assessed for normality using the Kolmogorov-Smirnov test, a normal distribution being defined as p>0.05, complemented with analysis of skewness and kurtosis.16,17 Those with normal distribution (approximately symmetrical) were expressed as mean and standard deviation (SD) and those with non-normal distribution as median and interquartile range (IQR). To compare continuous variables between the two study groups, parametric tests were applied for normally distributed variables and non-parametric tests for non-normally distributed variables. The parametric test used was the t test for independent samples, after assessing equality of variances by Levene’s test.18 Hedges’ g, the most widely used test of effect size in samples of different sizes, was used (Hedges’ g of 0.2,0.5, and 0.8 represent a small, medium, and large effect, respectively). The non-parametric test used was the Mann-Whitney test, expressed as a value of r calculated by the formula r=Z/(√n), with r values of around 0.1, 0.3 and 0.5 representing a small, medium, and large effect, respectively.19
Categorical variables were expressed as absolute (n) and relative (%) frequencies. Proportions in the study groups were compared using the chi-square test, or Fisher’s exact test when the proportion of expected values less than 5 was >20 %. Values of continuity correction were reported in 2×2 contingency tables.18 Effect size for these tests was assessed by the phi coefficient for 2×2 tables, with values of around 0.1, 0.3 and 0.5 representing a small, medium, and large effect, respectively.19
The relationship between one-year cumulative mortality and treatment delay was assessed by means of graphics for the three defined timings (symptom onset-FMC, FMC-reperfusion, and symptom onset-reperfusion).
One-year survival was analyzed by the Kaplan-Meier method and groups were compared using the log-rank test.
Multivariate Cox regression analysis adjusted for confounders that were statistically significant in univariate analysis was used to determine whether mode of admission was an independent predictor of one-year mortality. Hazard ratios (HR) adjusted for confounders and respective 95 % confidence intervals (CI) were calculated.20
The relationship between FMC-reperfusion time and one-year mortality was analyzed as a continuous function by univariate logistic regression, with one-year mortality as the dependent variable and FMC-reperfusion time as the independent variable.
Results with p<0.05 were considered statistically significant in all analyses except for the Kolmogorov-Smirnov and Levene tests, the assumptions of which were considered satisfied for p>0.05.18
Confidentiality and ethical considerationsNo questionnaires were applied in the course of this study. Confidentiality and anonymity of all data collected were ensured by attributing each study participant an identifying code number; the correspondence between the code and the participant was recorded in a document to which only the lead investigator had access.
The study was approved by the ethics committee of Hospital de Braga (CESHB) and the ethics subcommittee for life sciences and health (SECVS) of the Medical School of University of Minho.
Rules of ethical conduct and good practice were followed to ensure compliance with the principles of the Declaration of Helsinki (including the revisions of Tokyo 1975, Venice 1983, Hong Kong 1989, Somerset West 1996, Edinburgh 2000, Washington 2002, Tokyo 2004, Seoul 2008 and Fortaleza 2013), the Convention on Human Rights and Biomedicine, the guidelines of the Council for International Organizations of Medical Sciences, and the Guideline for Good Clinical Practice of the International Conference of Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.20–23
ResultsBaseline characteristics of the study populationTable 1 presents the baseline characteristics of the study population. No statistically significant differences were seen between the two study groups in terms of sociodemographic characteristics. However, although there was no significant difference in overall age between the two groups (mean 62.0 years, SD 14 vs. mean 61.0 years, SD 13.3; t test (n=1220) 1.2, p=0.239, g=0.074), analysis of the subgroup aged ≥75 years showed that direct admission patients were older (mean 82.7 years, SD 5.3 vs. mean 80.8 years, SD 4.6; t (n=183) 2.9, p=0.004, g=0.39). There were no significant differences between groups in other cardiovascular risk factors (obesity, diabetes, hypertension, dyslipidemia, smoking and CKD), history of coronary disease, or type of MI.
Baseline characteristics of the study population.
| DA (n=452) | IT (n=770) | Statistical tests | |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age | 62.0 (14.0) | 61.0 (13.3) | (n=1220) 1.18a, p=0.239, g=0.074 |
| Age ≥75 years | 96 (21.2) | 141 (18.3) | (n=1222) 1.38b, p=0.240, phi=0.036 |
| Male | 365 (80.8) | 588 (76.4) | (n=1222) 2.95b, p=0.086, phi=0.051 |
| Obesity | 81 (18.2) | 129 (16.9) | (n=1208) 0.24b, p=0.621, phi=0.016 |
| Diabetes | 85 (20.4) | 165 (22.2) | (n=1160) 0.38b, p=0.536, phi=0.020 |
| Hypertension | 227 (50.9) | 395 (51.4) | (n=1214) 0.01b, p=0.904, phi=0.005 |
| Dyslipidemia | 210 (47.1) | 343 (44.6) | (n=1215) 0.61b, p=0.437, phi=0.024 |
| Smoking (current or past) | 241 (56.8) | 384 (51.6) | (n=1168) 1.22b, p=0.097, phi=0.050 |
| CKD | 9 (2.0) | 11 (1.4) | (n=1218) 0.29b, p=0.593, phi=0.022 |
| History | |||
| MI | 30 (6.7) | 54 (7.0) | (n=1218) 0.01b, p=0.913, phi=0.006 |
| PCI | 25 (5.6) | 37 (4.8) | (n=1219) 0.20b, p=0.653, phi=0.017 |
| CABG | 4 (0.90) | 7 (0.90) | (n=1219) 0.00b, p>0.999, phi=0.001 |
| Clinical presentation | |||
| Killip class IV at admission | 26 (5.8) | 25 (3.2) | (n=1222) 3.87b, p=0.049, phi=0.079 |
| Killip class IV during hospital stay | 45 (10) | 45 (5.8) | (n=1222) 6.47b, p=0.011, phi=0.076 |
| Type of MI | |||
| Anterior | 209 (46.2) | 371 (48.2) | (n=1222) 0.36b, p=0.550, phi=0.019 |
| Inferior/lateral/posterior | 243 (53.8) | 399 (51.8) | (n=1222) 0.36b, p=0.550, phi=0.019 |
| Angiographic characteristics | |||
| Multivessel disease | 220 (48.7) | 363 (47.1) | (n=1222) 0.21a, p=0.647, phi=0.015b |
| Echocardiographic characteristics | |||
| LVEF ≤40 % during hospital stay | 144 (33.1) | 274 (36.5) | (n=1185) 1.27, p=0.259, phi=0.035b |
| Patients aged ≥75years | (n=96) | (n=141) | |
| 82.7 (5.3) | 80.8 (4.6) | (n=183) 2.93, p=0.004, g=0.39 | |
CABG: coronary artery bypass grafting; CKD: chronic kidney disease; DA: direct admission; IT: interhospital transfer; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention.
The proportion of patients in Killip class IV (cardiogenic shock) at admission was significantly higher in the direct admission group than in those undergoing interhospital transfer (5.8 % vs. 3.2 %, chi-square [n=1222] 5.43, p=0.049, phi=0.079), as was the proportion of patients developing cardiogenic shock during hospital stay (10 % vs. 5.8 %, chi-square [n=1222] 6.47, p=0.011, phi=0.076).
There were no significant differences between the study groups in angiographic or echocardiographic characteristics.
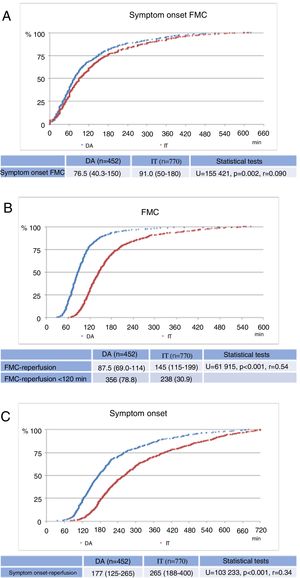
Figure 2 shows the cumulative distribution of patients as a function of the three defined timings, which were shorter in the direct admission group: symptom onset-FMC (median 76.5min, IQR 40.3–150 vs. median 91.0min, IQR 50–180, U=155421, p=0.002, r=0.090); FMC-reperfusion (median 87.5min, IQR 69.0–114 vs. median 145min, IQR 115–199, U=61915, p<0.001, r=0.54); and symptom onset-reperfusion (median 177min, IQR 125–265 vs. median 265min, IQR 188–400, U=103233, p<0.001, r=0.34).
Mortality during follow-upNo significant differences between the two groups were observed in 30-day, six-month or one-year mortality (11.7 % vs. 9.2 %; chi-square [n=1222] 1.70, p=0.193, phi=0.040), or in in-hospital and post-discharge mortality (Table 2).
Mortality during follow-up.
| DA (n=452) | IT (n=770) | Statistical tests | |
|---|---|---|---|
| In-hospital mortality | 27 (6) | 33 (4.3) | (n=1222) 1.40a, p=0.238, phi=0.038 |
| 30 days | 33 (7.3) | 44 (5.7) | (n=1222) 0.96a, p=0.327, phi=0.032 |
| 6 months | 42 (9.3) | 61 (7.9) | (n=1222) 0.53a, p=0.468, phi=0.024 |
| 1 year | 53 (11.7) | 71 (9.2) | (n=1222) 1.70a, p=0.193, phi=0.040 |
| Post-discharge mortality | |||
| 30 days | 6 (1.4) | 11 (1.5) | (n=1162) 0.00a, p>0.999, phi=0.003 |
| 6 months | 15 (3.5) | 28 (3.8) | (n=1162) 0.01a, p=0.942, phi=0.007 |
| 1 year | 26 (6.1) | 38 (5.2) | (n=1162) 0.31a, p=0.576, phi=0.020 |
DA: direct admission; IT: interhospital transfer.
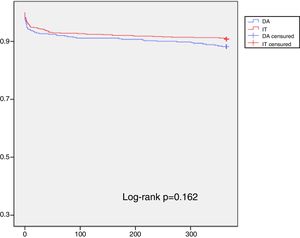
Kaplan-Meier curves for one-year survival also showed no significant differences between the two study groups (log-rank p=0.162) (Figure 3).
The mode of admission was not an independent predictor of one-year mortality on multivariate analysis (HR=0.99, 95 % CI 0.63–1.55, p=0.946). However, age (HR=1.09, 95 % CI 1.07–1.11, p<0.001), CKD (HR=3.05, 95 % CI 1.38–6.78 p=0.006), Killip class IV at admission (HR=11.2, 95 % CI 6.35–19.8, p<0.001), and symptom onset-reperfusion time (HR=1.001, 95 % CI 1.001–1.003, p=0.034), were independent predictors of one-year mortality (Table 3).
Multivariate analysis of predictors of one-year mortality.
| Variable | HR | 95 % CI | p |
|---|---|---|---|
| Interhospital transfer | 0.99 | 0.63-1.55 | 0.946 |
| Age | 1.09 | 1.07-1.11 | <0.001 |
| Male gender | 1.09 | 0.66-1.79 | 0.737 |
| CKD | 3.05 | 1.38–6.78 | 0.006 |
| Diabetes | 1.25 | 0.77-2.03 | 0.377 |
| Smoking | 1.16 | 0.68-2.20 | 0.586 |
| Hypertension | 0.88 | 0.55-1.41 | 0.592 |
| Multivessel disease | 1.08 | 0.70-1.65 | 0.735 |
| Killip class IV at admission | 11.2 | 6.35-19.8 | <0.001 |
| LVEF ≤40 % during hospital stay | 3.02 | 1.91-4.76 | <0.001 |
| Symptom onset-reperfusion time | 1.001 | 1.001-1.003 | 0.034 |
CI: confidence interval; CKD: chronic kidney disease; HR: hazard ratio; LVEF: left ventricular ejection fraction.
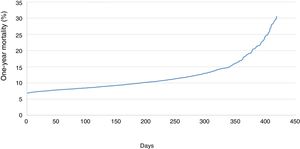
When analyzed as a continuous function by univariate logistic regression, FMC-reperfusion time was inversely correlated with one-year mortality (odds ratio [OR] 1.003, 95 % CI 1.002–1.005, p<0.001) (Figure 4). Analysis of the curve shows that the effect on mortality increased only slightly during the first 210min, but more sharply thereafter.
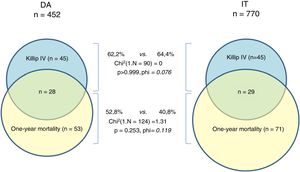
Mortality in patients who developed cardiogenic shock during hospital stay was over 60 % in both groups (62.2 % vs. 64.4 %, chi-square [n=90] 0, p>0.999, phi=0.076). However, one-year mortality in those who developed cardiogenic shock during hospital stay was 52.8 % in the direct admission group, as opposed to 40.8 % in the interhospital transfer group (chi-square [n=124] 1.31, p=0.253, phi=0.119) (Figure 5).
DiscussionThe results obtained in the present study for the two components of total ischemic time (symptom onset-FMC and FMC-reperfusion) can be considered to be generally acceptable. Although significant differences do exist, patients in both groups were prompt in seeking assistance from the health system following symptom onset, with a median delay of 76.5min (40.3–150) in the direct admission group and 91.0min (50–180) in the interhospital transfer group (Figure 2A). However, these medians cannot conceal the fact that a significant proportion of patients are unacceptably late in contacting the health services, with times in the last quartile of 150 and 180min, respectively, in the direct admission and interhospital transfer groups. There is an urgent need to improve this situation, potentially through large-scale ongoing public information campaigns aiming to alert the general population to the symptoms of MI and to the actions that should be taken when it occurs.8,24
Regarding FMC-reperfusion time, the direct admission group presented good results, with a median of 87.5min (69.0–114), and 78.8 % of patients being treated within the maximum time recommended by the guidelines (120min). By contrast, in the interhospital transfer group, the median FMC-reperfusion time was 145min (115–199), and only 30.9 % were treated within the recommended 120min (Figure 2B), a modest result compared to the direct admission group. However, considering all the logistical difficulties involved in transferring a patient with STEMI, an additional 56.5min can be taken to be acceptable, especially when compared to results from other series.7,25,26–28 There are, nevertheless, several aspects that could be improved. Efforts to reduce system delays for STEMI patients should focus on two points. Firstly, FMC-reperfusion time could be reducing through faster diagnosis of STEMI, with an electrocardiogram performed by a cardiopulmonary technician associated with assessment by the Manchester triage system in all patients with chest or abdominal pain.29 Secondly, patient transfer protocols should be established in centers without PCI facilities that include immediate availability of ambulances (either local or belonging to the national emergency medical services), staffed by a physician and a nurse, that can safely and rapidly transport the patient directly to the catheterization laboratory, avoiding the delays that currently occur due to the need to wait for an ambulance, lack of availability of emergency medical staff, and other factors.10 In addition, the above-mentioned public campaigns should educate the population that when the signs and symptoms of MI are detected, they should call the national emergency number immediately, thereby bypassing centers without PCI capability. This would lead to considerable gains in terms of reducing ischemic time.8,24
Adding the time taken to transport patients between hospitals to total ischemic time would be expected to result in increased mortality in these patients. However, one-year mortality was no higher in the interhospital transfer group in our population, with no statistically significant difference between the groups (11.7 % vs. 9.2 %, p=0.193). In our opinion, the main reason for this is the marked differences between the groups in terms of Killip class IV at admission, which was almost twice as frequent in the direct admission group as in the interhospital transfer group (5.8 % vs. 3.2 %, p=0.049) (Table 1). This finding is unexpected, given that the baseline characteristics of the two groups did not differ significantly. It is possible, although we have no specific evidence to support the idea, that the difference in Killip class may have been due to a subgroup of patients with severe STEMI who were first attended in centers without PCI capability, developed cardiogenic shock and died before or during transfer to the catheterization laboratory. Since these patients would not have been included in our analysis, they may have constituted a source of positive selection bias in the interhospital transfer group, leading to underestimation of the prevalence of Killip class IV and mortality in this group.
Support for this idea comes from the multivariate analysis (Table 3), which shows that the strongest predictor of one-year mortality was in fact Killip class IV at admission (HR 11.2, 95 % CI 6.35–19.8, p<0.001). Another predictor was age, and although there was no significant difference in overall age between the two groups, in the subgroup aged ≥75 years there were significantly more older patients in the direct admission group (Table 1). This could also help explain the absence of difference in one-year mortality between the groups.
Treatment delays in STEMI patients and their effect on mortality have been the subject of much analysis by a range of research groups and PCI programs, with conflicting results.10,11,25 Although the concept that ‘time is muscle’ is important, and current guidelines strongly recommend that FMC-reperfusion time should be kept below 120min, primary PCI after this time has undoubted benefits.29–32 Furthermore, data from high-volume primary angioplasty centers with well-organized patient transfer systems tend to show that the additional delay caused by interhospital transfer has a limited effect on patient prognosis.33 Since the referring hospitals in the Minho region are all close to Hospital de Braga and its catheterization laboratory, and are all linked by fast road networks, delays due to interhospital transfer in this case are likely to be less than in other regions, with correspondingly less impact on one-year mortality. Analysis of the graphic showing the relationship between FMC-reperfusion time and one-year mortality (Figure 4) supports this idea; mortality increased only slightly for the first 210min, and then more sharply thereafter. It can thus be assumed that the additional time required for interhospital transfer has little impact on patients who do in fact arrive at the catheterization laboratory and undergo primary PCI (<0.5 % difference in one-year mortality).
Analysis of the relationship between one-year mortality and Killip class IV during hospital stay (Figure 5) shows that over half of deaths in the direct admission group occurred in patients in cardiogenic shock during hospital stay, while in the interhospital transfer group this figure was lower, although the difference did not reach statistical significance (52.8 vs. 40.8 %, p=0.253). Another finding from this analysis was the high mortality of patients in cardiogenic shock, which exceeded 60 % in both groups (62.2 % vs. 64.4 %, p>0.999) (Figure 5). Although these figures are in line with those in the literature,34,35 they are still a cause for concern, as they demonstrate that in such cases it is not sufficient to perform successful PCI: these are highly unstable patients who often deteriorate immediately after PCI and need close monitoring. Their very high risk, frequently accompanied by multiple organ failure, necessitates a range of treatments (including circulatory support, mechanical ventilation, renal replacement therapy and others) and a multidisciplinary therapeutic approach. If these are applied promptly, they can help to reduce the high mortality seen in these patients.36,12
Although this study is the largest ever carried out in STEMI patients in Portugal in terms of both number of patients and time period covered, it has certain limitations that should be borne in mind. Firstly, it was a retrospective observational study in a single catheterization laboratory and is thus subject to the limitations and biases inherent to retrospective single-center studies. Secondly, it was not possible to analyze cardiovascular mortality separately from overall mortality, and thus the possibility cannot be excluded that some patients died from causes unrelated to STEMI. Thirdly, as stated above, the lack of information on patients who died before reaching the catheterization laboratory – in the referring hospitals, during transfer, or in our hospital – hampers interpretation of the true relationship between Killip class and mortality. Finally, our results pertain to the Minho and, given the particular geographic and sociodemographic characteristics of the region, as well as its well-developed road network, they may not be applicable to the rest of the country.
ConclusionIn conclusion, it is fair to say that results obtained by the primary PCI program of the Minho region during the five-year study period were acceptable in terms of the time taken to treat STEMI patients.
Most patients undergoing primary PCI in the catheterization laboratory of Hospital de Braga come from other hospitals in the region. Although the time taken to transfer them significantly delayed reperfusion therapy, this delay did not increase one-year mortality in these patients. However, the significant difference in the proportion of patients admitted in Killip class IV in two groups with similar baseline characteristics leads us to believe that some patients transferred from referring hospitals may have died before arrival at the catheterization laboratory, thus affecting mortality in the interhospital transfer group.
We have also identified weak points in the patient pathway from symptom onset to reperfusion, and have discussed measures that could help to resolve them.
Please cite this article as: Ferreira AS, Costa J, Braga CG, Marques J. Impacto na Mortalidade da Admissão Direta versus Transferência Inter-hospitalar nos Doentes com Enfarte Agudo do Miocárdio com Elevação do Segmento ST Submetidos a Intervenção Coronária Percutânea Primária. Rev Port Cardiol. 2019;38:621–631.