Response to cardiac resynchronization therapy (CRT) can currently be assessed by clinical or echocardiographic criteria, and there is no strong evidence supporting the use of one rather than the other. Reductions in B-type natriuretic peptide (BNP) and C-reactive protein (CRP) have been shown to be associated with CRT response. This study aims to assess variation in BNP and CRP six months after CRT and to correlate this variation with criteria of functional and echocardiographic response.
MethodsPatients undergoing CRT were prospectively enrolled between 2011 and 2014. CRT response was defined by echocardiography (15% reduction in left ventricular end-systolic volume) and by cardiopulmonary exercise testing (10% increase in peak oxygen consumption) from baseline to six months after device implantation.
ResultsA total of 115 patients were enrolled (68.7% male, mean age 68.6±10.5 years). Echocardiographic response was seen in 51.4% and 59.2% were functional responders. There was no statistical correlation between the two. Functional response was associated with a significantly greater reduction in BNP (-167.6±264.1 vs. -24.9±269.4 pg/ml; p=0.044) and CRP levels (-1.6±4.4 vs. 2.4±9.9 mg/l; p=0.04). Nonetheless, a non-significant reduction in BNP and CRP was observed in echocardiographic responders (BNP -144.7±260.2 vs. -66.1±538.2 pg/ml and CRP -7.1±24.3 vs. 0.8±10.3 mg/l; p>0.05).
ConclusionAn increase in exercise capacity after CRT implantation is associated with improvement in myocardial remodeling and inflammatory biomarkers. This finding highlights the importance of improvement in functional capacity after CRT implantation, not commonly considered a criterion of CRT response.
A avaliação da resposta à terapêutica de ressincronização cardíaca (CRT) assenta em critérios clínicos e ecocardiográficos, sem evidência inequívoca que apoie o uso de uns em relação aos outros. Reduções do péptido natriurético tipo-B (BNP) e da proteína C-reactiva (PCR) associaram-se à resposta à CRT. O objetivo deste estudo é avaliar a variação do BNP e PCR após seis meses de CRT e relacionar essa variação com critérios de resposta funcional e ecocardiográfica.
MétodosDe 2011 a 2014, doentes com indicação para CRT foram incluídos prospetivamente. A resposta à CRT foi definida por ecocardiograma (redução em 15% no volume telessistólico do ventrículo esquerdo) e por prova cardiorrespiratória (aumento de 10% no consumo de oxigénio máximo), aos seis meses.
ResultadosForam incluídos 115 doentes (género masculino: 68,7%, idade média 68,6±10,5 anos); 51,4% apresentaram resposta ecocardiográfica e 59,2% resposta funcional. Não se verificou uma correlação estatisticamente significativa entre esses. Os respondedores funcionais apresentaram reduções estatisticamente significativas de BNP (-167,6±264,1 versus -24,9±269,4; p=0,044) e PCR (-1,6±4,4 versus 2,4±9,9; p=0,04). No grupo de respondedores ecocardiográficos essa redução não atingiu significância estatística [BNP (-144,7±260,2 versus -66,1±538,2) e PCR (-7,1±24,3 versus 0,8±10,3;p>0,05)].
ConclusãoUm aumento da capacidade funcional após implantação de CRT está associado a uma melhoria dos biomarcadores inflamatórios e de remodelagem reversa ventricular. Essa ideia enaltece a importância da melhoria da capacidade funcional após implantação de CRT, pouco considerada como critério de resposta à ressincronização.
B-type natriuretic peptide
chronic heart failure
cardiopulmonary exercise testing
C-reactive protein
cardiac resynchronization therapy
intraclass correlation coefficient
left ventricular end-diastolic volume
left ventricular ejection fraction
left ventricular end-systolic volume
New York Heart Association
peak oxygen uptake
minute ventilation-carbon dioxide production slope
Cardiac resynchronization therapy (CRT) is an established treatment for patients with symptomatic chronic heart failure (CHF) and prolonged QRS despite optimal pharmacological therapy. By restoring the heart's electromechanical synchrony, CRT improves self-reported symptoms and reduces mortality and rehospitalization for heart failure.1–4 Response to CRT is associated with left ventricular reverse remodeling, which is objectively assessed through echocardiographic parameters, particularly improvement in left ventricular ejection fraction (LVEF) and reduction in left ventricular end-systolic volume (LVESV).5 Nonetheless, up to 40% of CRT recipients are considered non-responders.6 Improvement in New York Heart Association (NYHA) functional class and six-minute walk test distance have also been proposed as clinical response criteria in several studies.7,8 Improvement in peak oxygen uptake (VO2 max), a marker of functional status and activity, has been described after CRT device implantation in a small cohort of patients.9 However, there is little agreement between the criteria of response, which suggests that clinical or functional improvement can occur without changes in echocardiographic parameters.10
B-type natriuretic peptide (BNP) is a marker of volume and pressure overload that has been proposed as a diagnostic and prognostic tool in CHF, in which it correlates well with severity. Moreover, pharmacological therapies that improve CHF symptoms and outcomes have been shown to reduce BNP levels.11 Studies have also reported significant reductions in plasma BNP levels after CRT device implantation.12–14 Systemic inflammation is also known to play a role in CHF,15 and increased serum levels of inflammatory markers such as C-reactive protein (CRP) confer a dismal prognosis for CHF patients.16,17
Nonetheless, there are conflicting data concerning reductions in BNP and CRP as markers of neurohormonal and inflammatory status after CRT device implantation.11,12 Moreover, their association with CRT response criteria that assess different pathological pathways of the syndrome is not fully understood.
Hence, this study aims primarily to assess variations in BNP and CRP six months after CRT device implantation, and secondarily to assess the association between changes in these laboratory variables and functional and echocardiographic response to CRT.
MethodsPopulation and study designBetween April 2011 and December 2014, consecutive patients referred for CRT device implantation in a tertiary center were systematically included in a prospective cohort study. Indications for CRT followed current international guidelines.18 All patients had been on optimal medical therapy for at least six months and were followed by a specialized heart failure team. A comprehensive assessment including demographic, clinical, laboratory, electrocardiographic, echocardiographic and cardiopulmonary exercise testing (CPET) was performed and data were collected at baseline and six months after device implantation and subsequently analyzed. The ethics committee of our university hospital center approved the study protocol and written consent was obtained for all patients.
Demographic and clinical dataDemographic and clinical data were obtained through medical consultation at baseline and at six-month follow-up. Demographic variables included age and gender. Clinical variables included heart failure etiology, classical cardiovascular risk factors, comorbidities, ongoing medication and NYHA functional class. A 12-lead electrocardiogram was also obtained to determine heart rhythm, QRS width and intraventricular conduction pattern.
Laboratory dataBlood samples were collected through peripheral venous catheterization into blood collection tubes with (serum) or without (plasma) anticoagulant (EDTA). All laboratory tests were performed after at least six hours fasting and at rest in a supine position in the same laboratory using hospital protocol, and assays were determined according to the manufacturer's recommendations. BNP was analyzed from plasma on a Spinchron DLX 800 (Beckman Coulter) centrifuge using a two-step chemiluminescence assay and serum CRP was determined with a nephelometer (Siemens BN ProSpecT).
Transthoracic echocardiographyA complete transthoracic echocardiogram was performed in all patients using a Vivid E9 scanner (GE Healthcare) with a 3 MHz probe, at baseline and at six-month follow-up. M-mode, two-dimensional, color, pulsed, continuous wave and tissue Doppler data were obtained from parasternal and apical views, and the standard echocardiographic parameters were calculated. Acquired cine-loop images with at least three cardiac cycles were analyzed offline (EchoPAC software, GE Healthcare) for additional measures. LVEF, LVESV and left ventricular end-diastolic volume (LVEDV) were determined from apical 4- and 2-chamber views using Simpson's biplane method.19 The mean of three measurements was considered for analysis. Endocardial borders were manually traced and the left ventricular papillary muscles were included in volume acquisition. All measurements were reviewed by the same echocardiographic operator. The intraclass correlation coefficient (ICC) was calculated for LVESV to assess intraobserver variability. Echocardiographic response to CRT was established as a ≥15% reduction in LVESV from baseline to six months after CRT device implantation, as previously published.20
Cardiopulmonary exercise testingCPET with ventilatory expired gas analysis was performed in all patients at baseline and at six-month follow-up using the modified Bruce protocol. Exercise test duration, VO2 max and minute ventilation-carbon dioxide production slope (VE/VCO2) were determined. A significant functional response to CRT was defined as a ≥10% increase in VO2 max from baseline to six-month follow-up.21
Cardiac resynchronization therapy device implantationCRT device implantation was performed through a transvenous approach, using the subclavian and cephalic veins. The right atrial lead was positioned in the right atrial appendage and the right ventricular lead was actively fixed in the right ventricular apex or interventricular septum. The left ventricular lead was introduced using a long guiding sheath in order to cannulate the coronary sinus. An angiogram was performed and then the lead was positioned preferably in a lateral or posterolateral vein.
Statistical analysisBaseline demographic and clinical data were expressed as means and standard deviation for continuous variables and as percentages for categorical variables. Changes in BNP and CRP (Δ BNP and Δ CRP) were established by the arithmetic subtraction of their values at six-month follow-up from baseline. Normality (Gaussian distribution) was tested in all continuous variables using the Shapiro-Wilk test. To assess the association between continuous variables, Pearson's or Spearman's correlation was used for normally or non-normally distributed data, respectively. Δ BNP and Δ CRP were separately compared between echocardiographic and functional responders and non-responders using the Student's t or Mann-Whitney tests for normally or non-normally distributed variables, respectively. The chi-square test was used to compare dichotomous variables. The level of significance considered was α=0.05. Data were analyzed using SPSS for Windows, version 20.0 (IBM SPSS Inc, Chicago, IL).
ResultsBaseline characteristicsA total of 115 patients underwent CRT device implantation for whom complete data were collected at baseline and six-month follow-up. Mean age was 68.6±10.5 years and 68.7% were male. Ischemic heart failure etiology was reported in 29.1%. Mean QRS width at baseline was 172.1±28.8 ms and complete left bundle branch block was present in 56% of patients. Beta-blockers were prescribed in 86.2% of patients and 88.6% were treated with renin-angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers). At baseline, 74.3% patients were in NYHA functional class III or IV. Clinical improvement of at least one NYHA class was observed in 81.3% of patients. CRP and BNP presented a statistically significant reduction from baseline to six-month follow-up (CRP 7.0±15.6 to 6.5±15.3 mg/l, p<0.001, and BNP 533.6±553.6 to 404.9±530.3 pg/ml, p<0.001). Echocardiographic response was observed in 51.4% and functional response in 59.2%, assessed by the above criteria. Response as assessed by echocardiography was not statistically correlated with functional response (r=0.14, p=0.433 and chi-square=0.063, p=0.80).
Table 1 delineates the baseline demographic and clinical characteristics of responders and non-responders assessed by both sets of criteria independently. Table 2 details laboratory, echocardiographic and CPET data at baseline and at six-month follow-up.
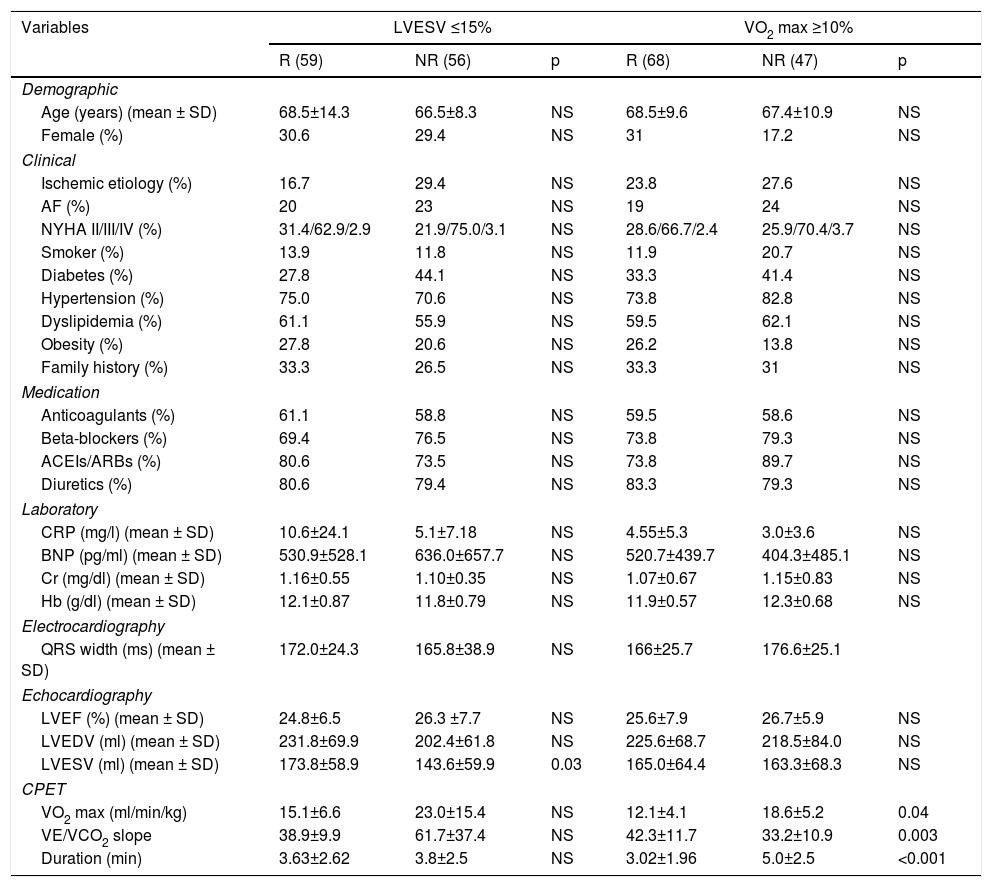
Baseline characteristics of echocardiographic and functional responders and non-responders.
| Variables | LVESV ≤15% | VO2 max ≥10% | ||||
|---|---|---|---|---|---|---|
| R (59) | NR (56) | p | R (68) | NR (47) | p | |
| Demographic | ||||||
| Age (years) (mean ± SD) | 68.5±14.3 | 66.5±8.3 | NS | 68.5±9.6 | 67.4±10.9 | NS |
| Female (%) | 30.6 | 29.4 | NS | 31 | 17.2 | NS |
| Clinical | ||||||
| Ischemic etiology (%) | 16.7 | 29.4 | NS | 23.8 | 27.6 | NS |
| AF (%) | 20 | 23 | NS | 19 | 24 | NS |
| NYHA II/III/IV (%) | 31.4/62.9/2.9 | 21.9/75.0/3.1 | NS | 28.6/66.7/2.4 | 25.9/70.4/3.7 | NS |
| Smoker (%) | 13.9 | 11.8 | NS | 11.9 | 20.7 | NS |
| Diabetes (%) | 27.8 | 44.1 | NS | 33.3 | 41.4 | NS |
| Hypertension (%) | 75.0 | 70.6 | NS | 73.8 | 82.8 | NS |
| Dyslipidemia (%) | 61.1 | 55.9 | NS | 59.5 | 62.1 | NS |
| Obesity (%) | 27.8 | 20.6 | NS | 26.2 | 13.8 | NS |
| Family history (%) | 33.3 | 26.5 | NS | 33.3 | 31 | NS |
| Medication | ||||||
| Anticoagulants (%) | 61.1 | 58.8 | NS | 59.5 | 58.6 | NS |
| Beta-blockers (%) | 69.4 | 76.5 | NS | 73.8 | 79.3 | NS |
| ACEIs/ARBs (%) | 80.6 | 73.5 | NS | 73.8 | 89.7 | NS |
| Diuretics (%) | 80.6 | 79.4 | NS | 83.3 | 79.3 | NS |
| Laboratory | ||||||
| CRP (mg/l) (mean ± SD) | 10.6±24.1 | 5.1±7.18 | NS | 4.55±5.3 | 3.0±3.6 | NS |
| BNP (pg/ml) (mean ± SD) | 530.9±528.1 | 636.0±657.7 | NS | 520.7±439.7 | 404.3±485.1 | NS |
| Cr (mg/dl) (mean ± SD) | 1.16±0.55 | 1.10±0.35 | NS | 1.07±0.67 | 1.15±0.83 | NS |
| Hb (g/dl) (mean ± SD) | 12.1±0.87 | 11.8±0.79 | NS | 11.9±0.57 | 12.3±0.68 | NS |
| Electrocardiography | ||||||
| QRS width (ms) (mean ± SD) | 172.0±24.3 | 165.8±38.9 | NS | 166±25.7 | 176.6±25.1 | |
| Echocardiography | ||||||
| LVEF (%) (mean ± SD) | 24.8±6.5 | 26.3 ±7.7 | NS | 25.6±7.9 | 26.7±5.9 | NS |
| LVEDV (ml) (mean ± SD) | 231.8±69.9 | 202.4±61.8 | NS | 225.6±68.7 | 218.5±84.0 | NS |
| LVESV (ml) (mean ± SD) | 173.8±58.9 | 143.6±59.9 | 0.03 | 165.0±64.4 | 163.3±68.3 | NS |
| CPET | ||||||
| VO2 max (ml/min/kg) | 15.1±6.6 | 23.0±15.4 | NS | 12.1±4.1 | 18.6±5.2 | 0.04 |
| VE/VCO2 slope | 38.9±9.9 | 61.7±37.4 | NS | 42.3±11.7 | 33.2±10.9 | 0.003 |
| Duration (min) | 3.63±2.62 | 3.8±2.5 | NS | 3.02±1.96 | 5.0±2.5 | <0.001 |
ACEIs: angiotensin-converting enzyme inhibitors; AF: atrial fibrillation; ARBs: angiotensin receptor blockers; BNP: B-type natriuretic peptide; CPET: cardiopulmonary exercise test; Cr: creatinine; CRP: C-reactive protein; Hb: hemoglobin; NR: non-responder; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NYHA: New York Heart Association functional class; R: responder; SD: standard deviation; VE/VCO2: minute ventilation-carbon dioxide production slope; VO2 max: peak oxygen consumption.
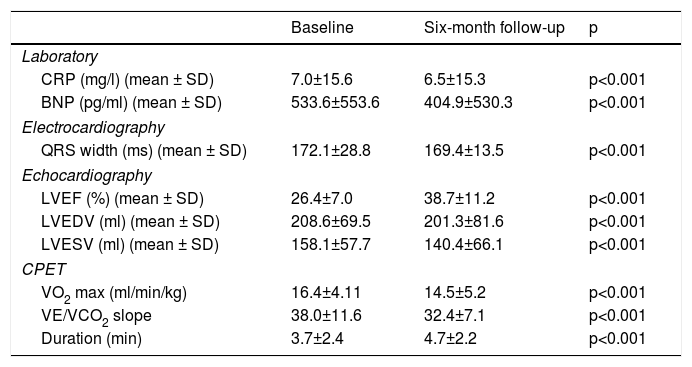
Laboratory, electrocardiographic, echocardiographic and cardiopulmonary exercise test data at baseline and six-month follow-up.
| Baseline | Six-month follow-up | p | |
|---|---|---|---|
| Laboratory | |||
| CRP (mg/l) (mean ± SD) | 7.0±15.6 | 6.5±15.3 | p<0.001 |
| BNP (pg/ml) (mean ± SD) | 533.6±553.6 | 404.9±530.3 | p<0.001 |
| Electrocardiography | |||
| QRS width (ms) (mean ± SD) | 172.1±28.8 | 169.4±13.5 | p<0.001 |
| Echocardiography | |||
| LVEF (%) (mean ± SD) | 26.4±7.0 | 38.7±11.2 | p<0.001 |
| LVEDV (ml) (mean ± SD) | 208.6±69.5 | 201.3±81.6 | p<0.001 |
| LVESV (ml) (mean ± SD) | 158.1±57.7 | 140.4±66.1 | p<0.001 |
| CPET | |||
| VO2 max (ml/min/kg) | 16.4±4.11 | 14.5±5.2 | p<0.001 |
| VE/VCO2 slope | 38.0±11.6 | 32.4±7.1 | p<0.001 |
| Duration (min) | 3.7±2.4 | 4.7±2.2 | p<0.001 |
BNP: B-type natriuretic peptide; CPET: cardiopulmonary exercise testing; CRP: C-reactive protein; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; VE/VCO2: minute ventilation-carbon dioxide production slope; VO2 max: peak oxygen consumption.
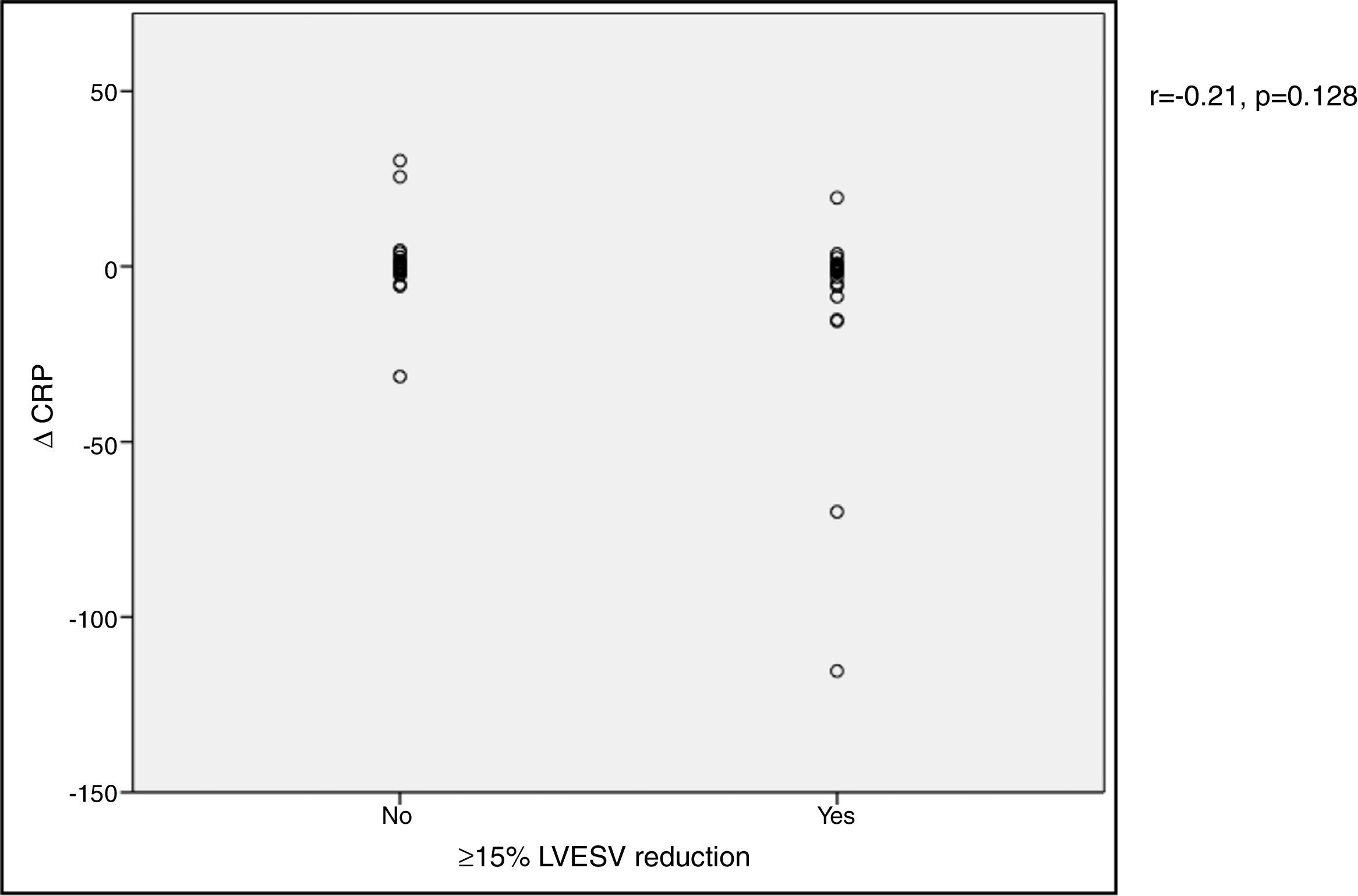
In our cohort, severely reduced mean LVEF at baseline was observed (26.4±7.0%). A significant improvement in mean LVEF and reduction in mean LVEDV and LVESV were noted after CRT device implantation. Additionally, 63.4% of patients showed more than 10% improvement in LVEF and in 51.4% of patients LVESV decreased by more than 15% compared to baseline (echocardiographic responders). An ICC of 0.89 at baseline and 0.87 at six-month follow-up was calculated for intraobserver variability of LVESV. BNP reduction was more pronounced in responders than in non-responders as assessed by echocardiography, although this did not achieve statistical significance (-144.7±260.2 vs. -66.1±538.2 pg/ml, p>0.05) (Figure 1). Echocardiographic responders also presented a non-significant reduction in serum CRP levels after CRT (-7.1±24.3 vs. 0.7±10.3 mg/l, p>0.05) (Figure 2).
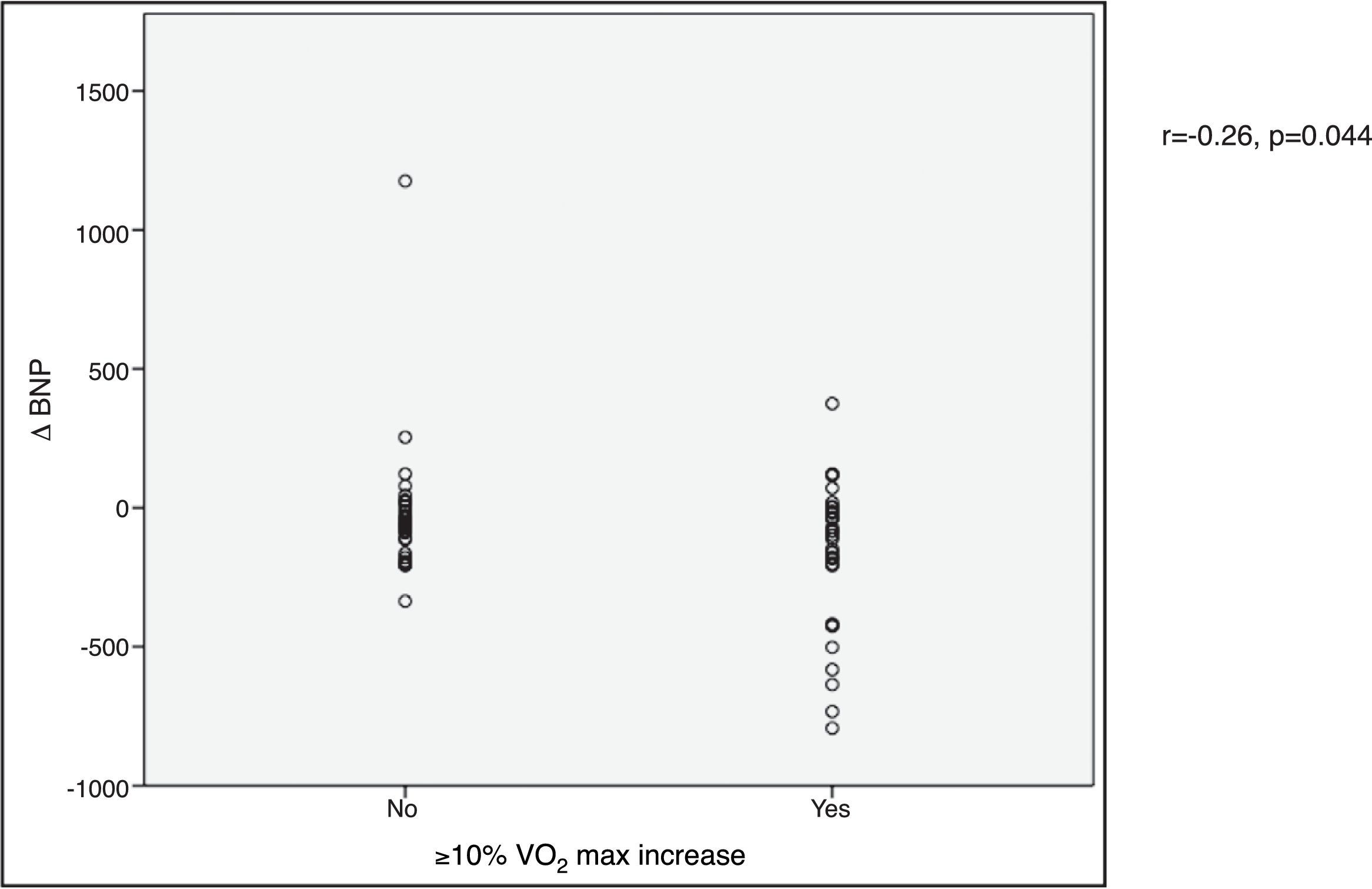
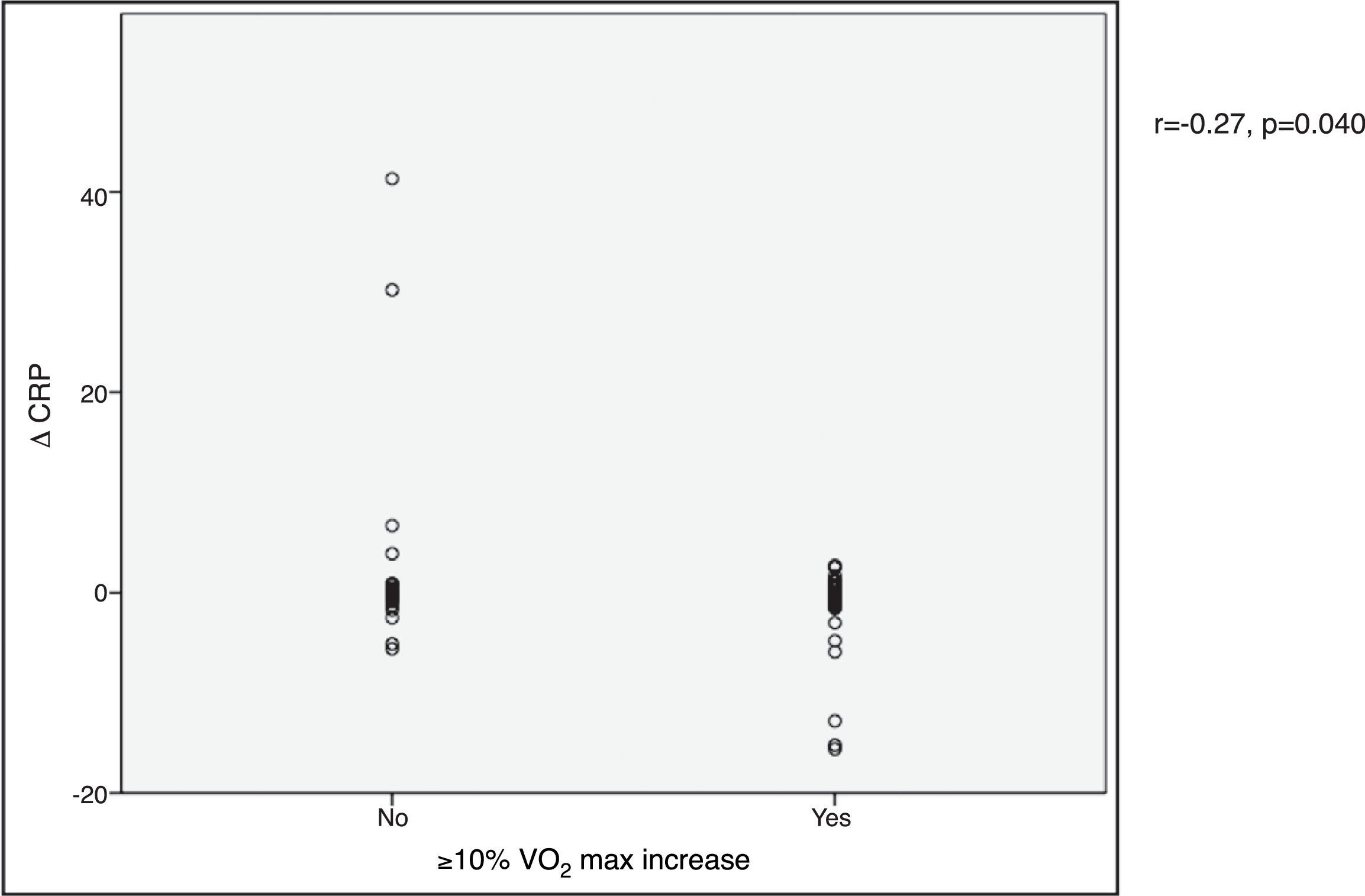
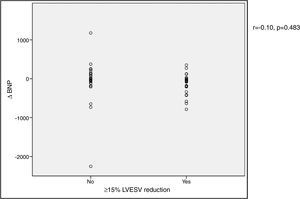
VO2 max during CPET was severely depressed at baseline (mean 14.5±5.2 ml/min/kg). However, a statistically significant improvement (14.5±5.2 to 16.4±4.1 ml/min/kg, p<0.001) was observed after CRT device implantation. Improvement in exercise capacity was also shown by a significant reduction in VE/VCO2 slope and longer CPET duration (38.0±11.6 vs. 32.4±7.1 and 3.7±2.4 vs. 4.7±2.2 ms, respectively, all p<0.001) at six-month follow-up. BNP reduction was significantly more pronounced in those classified as functional responders (-167.6±264.1 pg/ml vs. -24.9±269.4 pg/ml, p=0.044) (Figure 3). CRP reduction was seen in functional responders, while non-responders presented elevation in mean CRP levels six months after CRT device implantation (-1.6±4.4 vs. 2.4±9.9 mg/l, p=0.040) (Figure 4). A significant reduction in VE/VCO2 slope was also noted in functional responders (-9.8±10.5 vs. 1.9±7.2, p=0.001).
In our study population a significant reduction of CRP and BNP was observed six months after CRT (CRP 7.0±15.6 to 6.5±15.3 mg/l, p<0.001 and BNP 533.6±553.6 to 404.9±530.3 pg/ml, p<0.001). The CRP and BNP reductions were significantly higher in the functional responder group, whereas they did not achieve statistical significance in the echocardiographic responder group.
Heart failure is a systemic condition with increased levels of natriuretic peptides and inflammatory markers.12,13 Therapies targeting these pathways have shown positive prognostic impact in this syndrome.11,17
As described, our cohort represents a severe heart failure population characterized by poor self-reported functional status, reduced exercise capacity and low mean LVEF. Moreover, compared to other studies, our population presented a higher proportion of nonischemic heart failure etiology, such as idiopathic, valvular and alcoholic, only 29.1% of patients having ischemic heart failure.18 The heart failure etiology did not significantly differ between functional or echocardiographic responders and non-responders. Patients in our population presented advanced age, which excludes them from a heart transplantation program, and they were already on optimal medical therapy. Additionally, the high mean BNP and CRP levels presented at baseline support the concept of an advanced heart failure population, and significant reductions in BNP and CRP levels were observed at six-month follow-up after CRT device implantation, which is in line with previous studies.22–24,12
Response to CRT is the subject of considerable debate, since there is no established definition for therapeutic response.20 Improvement in LVEF and reductions in LVESV and LVEDV are the result of left ventricular reverse remodeling. Pharmacological therapies targeting this effect have been shown to be associated with better long-term outcomes in heart failure patients.25–28 The cut-off of a ≥15% reduction in LVESV has been described as a more specific surrogate of left ventricular reverse remodeling than LVEF, and has been used to define those responding to CRT therapy by echocardiography.10,11,14,21 In our study, only 51.4% of patients achieved this demanding criterion at six months. These echocardiographic responders had a more pronounced reduction in BNP at six-month follow-up than non-responders, but this did not achieve statistical significance. Despite targeting the same mechanism, the demanding cut-off for LVESV reduction, the short follow-up time for structural changes and variability in BNP could explain this phenomenon. Regarding the changes in CRP, a widely used marker of systemic inflammation, there was no significant association with reduced LVESV, perhaps because they reflect different pathways of the pathological disease process. The association reported in the literature between reduced LVESV and changes in BNP or CRP after CRT has in fact been variable.14,28,29
Exercise capacity, measured through VO2 max during CPET, is known to be a major prognostic indicator in heart failure patients.30,31 It has been assessed in conjunction with pharmacological therapies for heart failure patients.32 However, its use for determining functional response after CRT is not routinely considered and lacks clinical evidence. We used a cut-off of ≥10% improvement in VO2 max to define functional responders, and our study revealed significantly greater reductions in BNP and CRP among CRT patients in whom VO2 max improved substantially at six-month follow-up. An association between reductions in neurohormonal and inflammatory levels and a ≥10% improvement in VO2 max has not previously been reported in this population. Volume overload in heart failure is responsible for raised left ventricular and atrial pressure and consequently elevation of pulmonary capillary wedge pressure, which is associated with functional exercise impairment.13 Moreover, systemic inflammatory markers increase oxygen demand, depress myocardial function and disturb homeostasis in the pulmonary vasculature, influencing the interaction between heart and lungs.33 This finding suggests that electromechanical resynchronization may have a role in reversing these pathological processes, which highlights the importance of considering improvement in VO2 max after CRT as a response criterion.
In our study, the association between reduced LVESV and improved VO2 max was non-significant, suggesting that those who improved by one criterion may not have improved by the other. Similarly, Fornwalt et al. showed that agreement between criteria of clinical, echocardiographic and functional exercise response after CRT device implantation is poor,11 indicating that CRT may lead to improvements in different parameters in different patients. Functional response supported by reduction in BNP and CRP could be related to CRT response independently of significant left ventricular reverse remodeling at six-month follow-up.
Study limitationsFollow-up time in this study was set at six months after CRT implantation, which may be insufficient to assess the long-term structural changes of left ventricular reverse remodeling. Moreover, inferences cannot be made about the lasting clinical, laboratory or echocardiographic alterations in this population after this time. Additionally, CRP levels are susceptible to variability, and ongoing inflammatory processes that influenced CRP levels cannot be excluded.
ConclusionCRT was associated with important reductions in BNP and CRP levels at six-month follow-up. Patients presenting significantly improved functional capacity, considered functional responders, showed significant reductions in serum BNP and CRP levels at six-month follow-up, reflecting its benefit on ventricular remodeling and inflammation. In echocardiographic responders this effect was smaller and non-significant, which calls attention to the importance of assessing functional response in CRT patients.
Conflicts of interestThe authors have no conflicts of interest to declare.


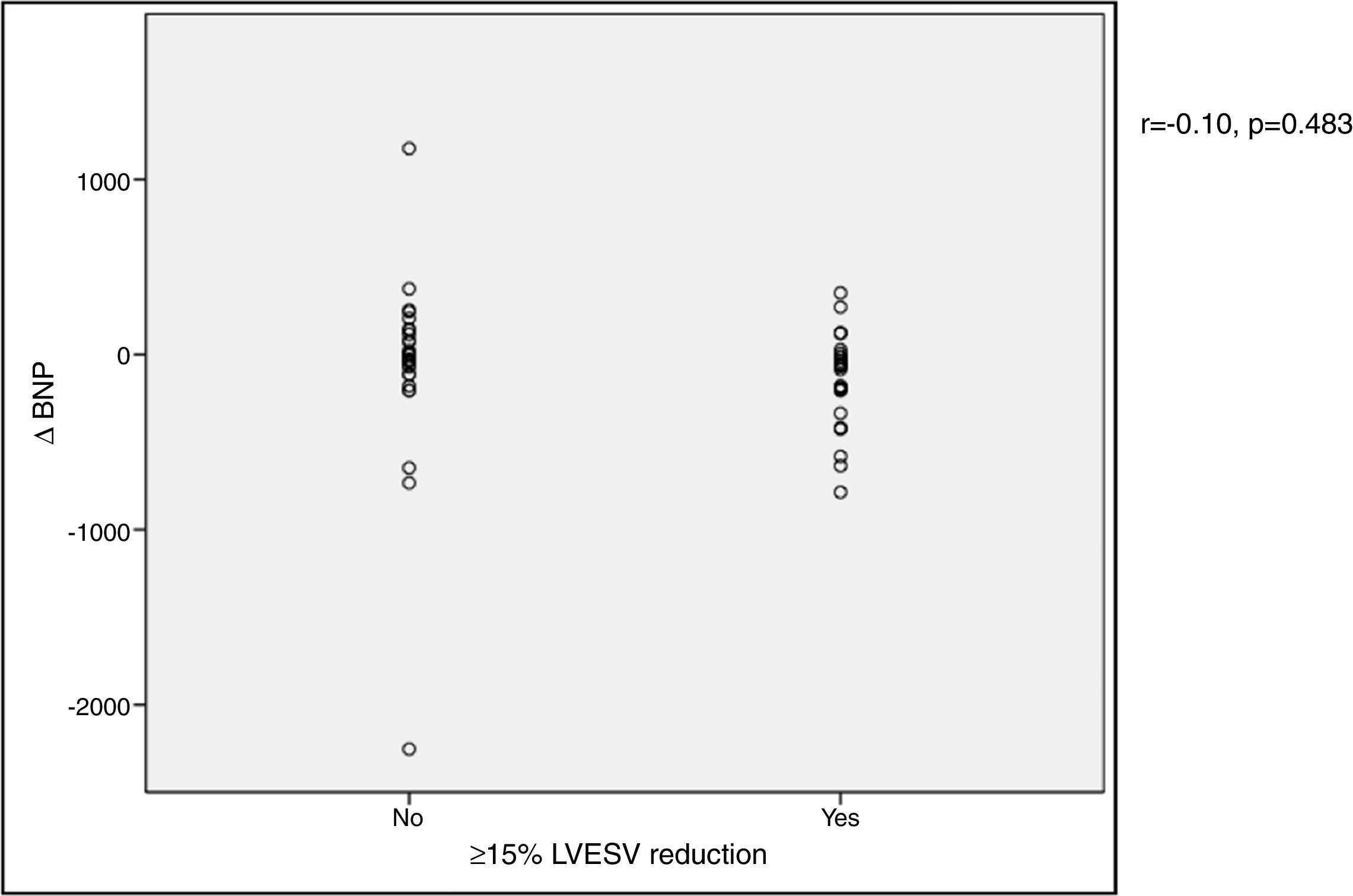
 BNP) levels and echocardiographic response to cardiac resynchronization therapy. Response was defined as a ≥15% reduction in left ventricular end-systolic volume (
BNP) levels and echocardiographic response to cardiac resynchronization therapy. Response was defined as a ≥15% reduction in left ventricular end-systolic volume (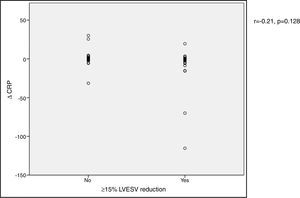 CRP) levels and echocardiographic response to cardiac resynchronization therapy. Response was defined as a ≥15% decrease in left ventricular end-systolic volume (
CRP) levels and echocardiographic response to cardiac resynchronization therapy. Response was defined as a ≥15% decrease in left ventricular end-systolic volume (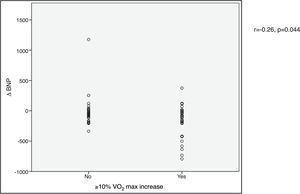 BNP) levels and functional response to cardiac resynchronization therapy. Response was defined as a ≥10% increase in peak oxygen consumption (
BNP) levels and functional response to cardiac resynchronization therapy. Response was defined as a ≥10% increase in peak oxygen consumption (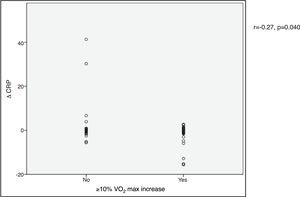 CRP) levels and functional response to cardiac resynchronization therapy. Response was defined as a ≥10% increase in peak oxygen consumption (
CRP) levels and functional response to cardiac resynchronization therapy. Response was defined as a ≥10% increase in peak oxygen consumption (

