Saphenous vein graft (SVG) anastomotic lesions can have significant fibromuscular hyperplasia and may be resistant to balloon angioplasty alone. Stents have been used successfully to treat these lesions. There are no reports of immediate stent recoil following such treatment in the literature. We describe immediate and persistent stent recoil in an anastomotic SVG lesion even after initial and post-deployment complete balloon dilatation of the stent and its successful treatment by cutting balloon angioplasty.
As lesões de enxerto de veia safena (EVS) anastomótica podem ter hiperplasia fibromuscular significativa e podem ser resistentes à angioplastia de balão. Stents têm sido utilizados com sucesso no tratamento de tais lesões. Apesar de tratar estas lesões com stents, não há relatos na literatura de recoil imediato dos stents. Relata-se um caso de recoil persistente e imediato do stent numa lesão do EVS anastomótica logo após a dilatação inicial do balão e pós-implantação completa do stent e seu tratamento bem-sucedido com angioplastia com cutting balloon.
Anastomotic interventions to saphenous vein grafts (SVG) pose a unique challenge to interventional cardiologists, as the morphologic and pathophysiologic characteristics of these lesions are different from native coronary artery lesions, being more fibrotic from excessive smooth muscle proliferation and less calcific or atherosclerotic, especially when they occur within a year of surgery.1,3
Challenges to interventional cardiologists in treating these lesions include suboptimal response to balloon angioplasty and accelerated lumen loss leading to early restenosis, which can be tackled by stent implantation that can increase gain in minimal lumen diameter (MLD) and reduce restenosis rates.4–6 Other complications include perforation leading to hemorrhagic complications or tamponade, distal embolization and no reflow, among others.4,6
We present a unique case of immediate stent recoil after adequate balloon dilation and stent implantation in a distal SVG anastomotic lesion that was successfully treated by cutting balloon angioplasty.
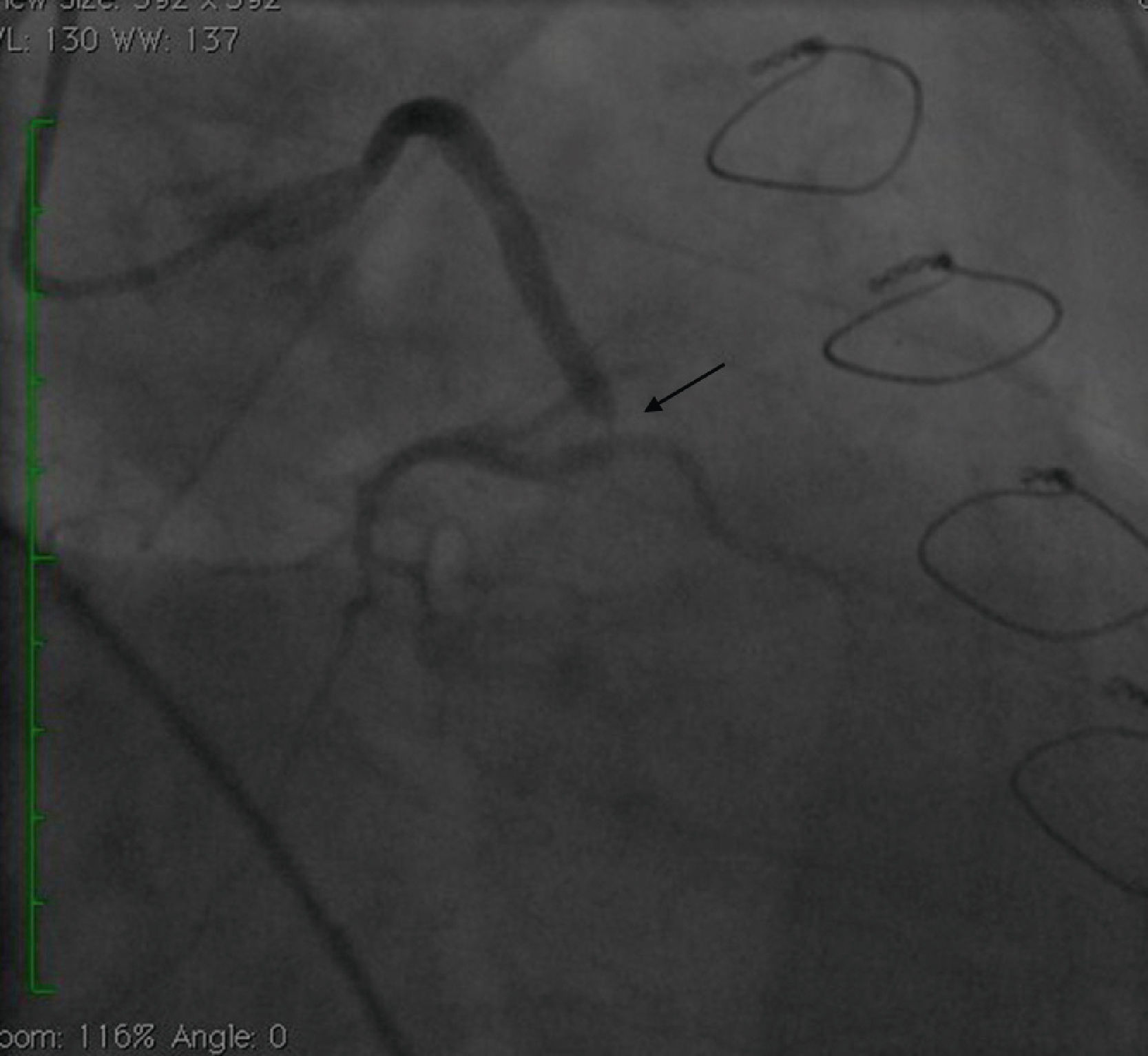
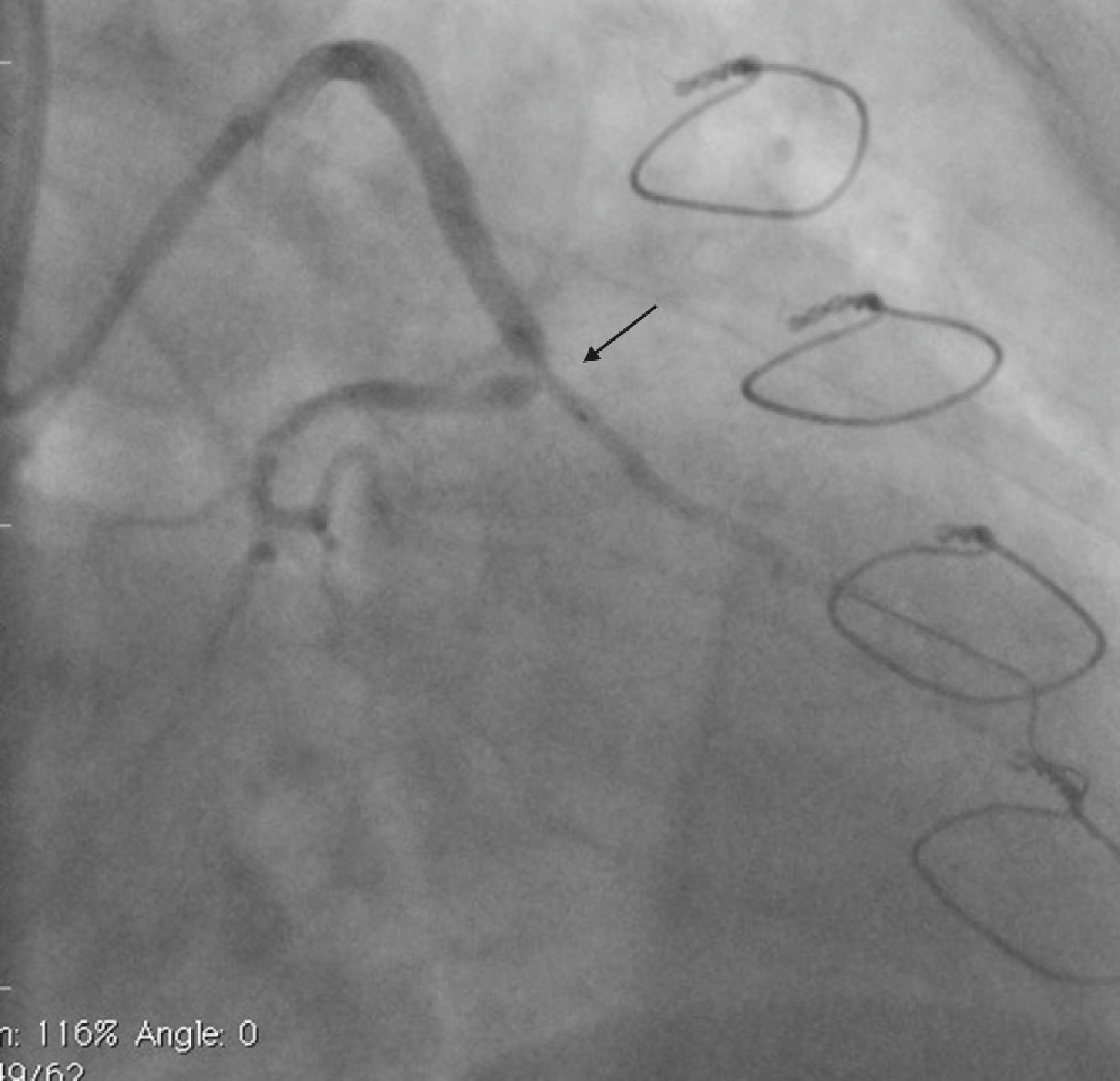
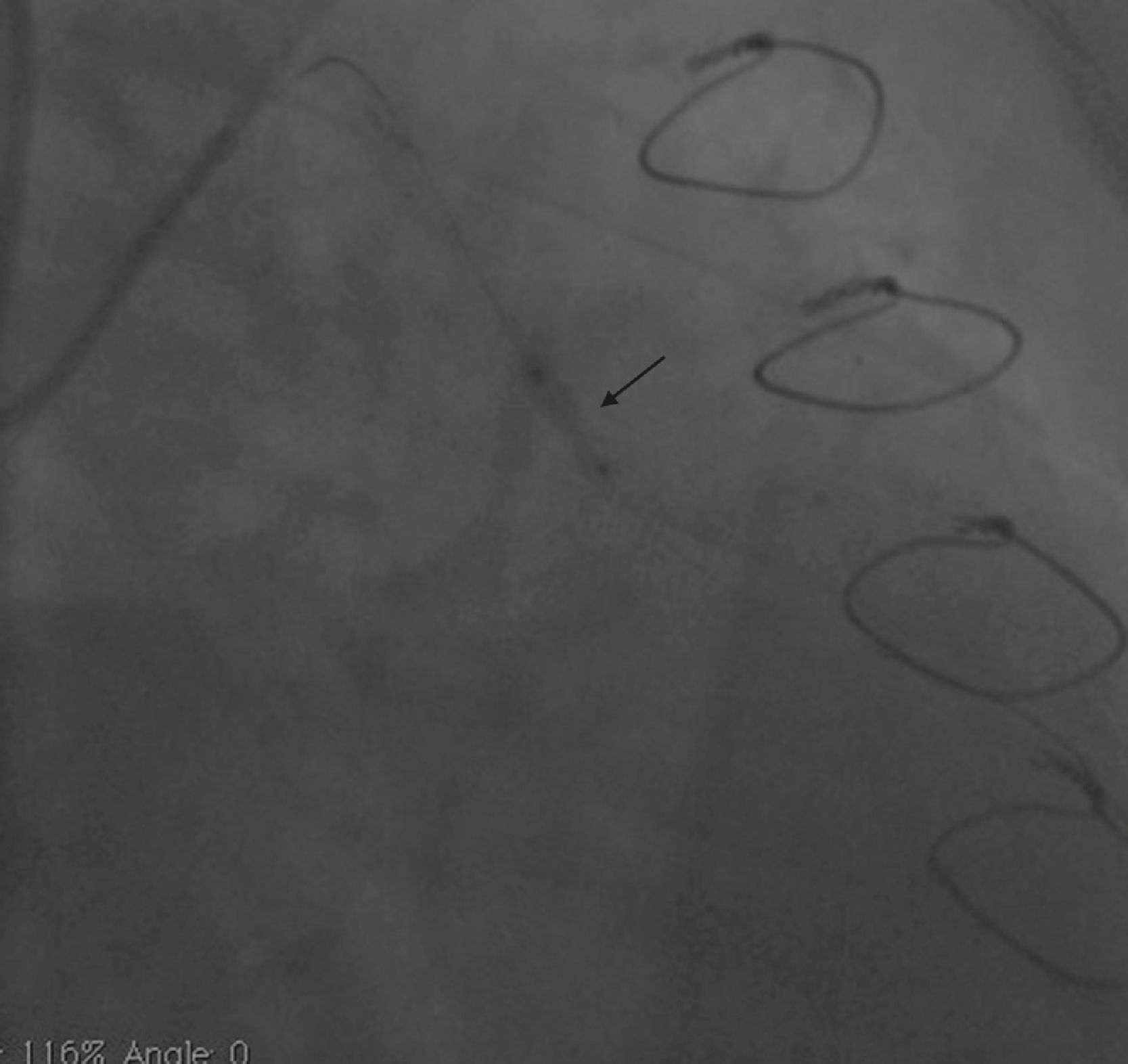
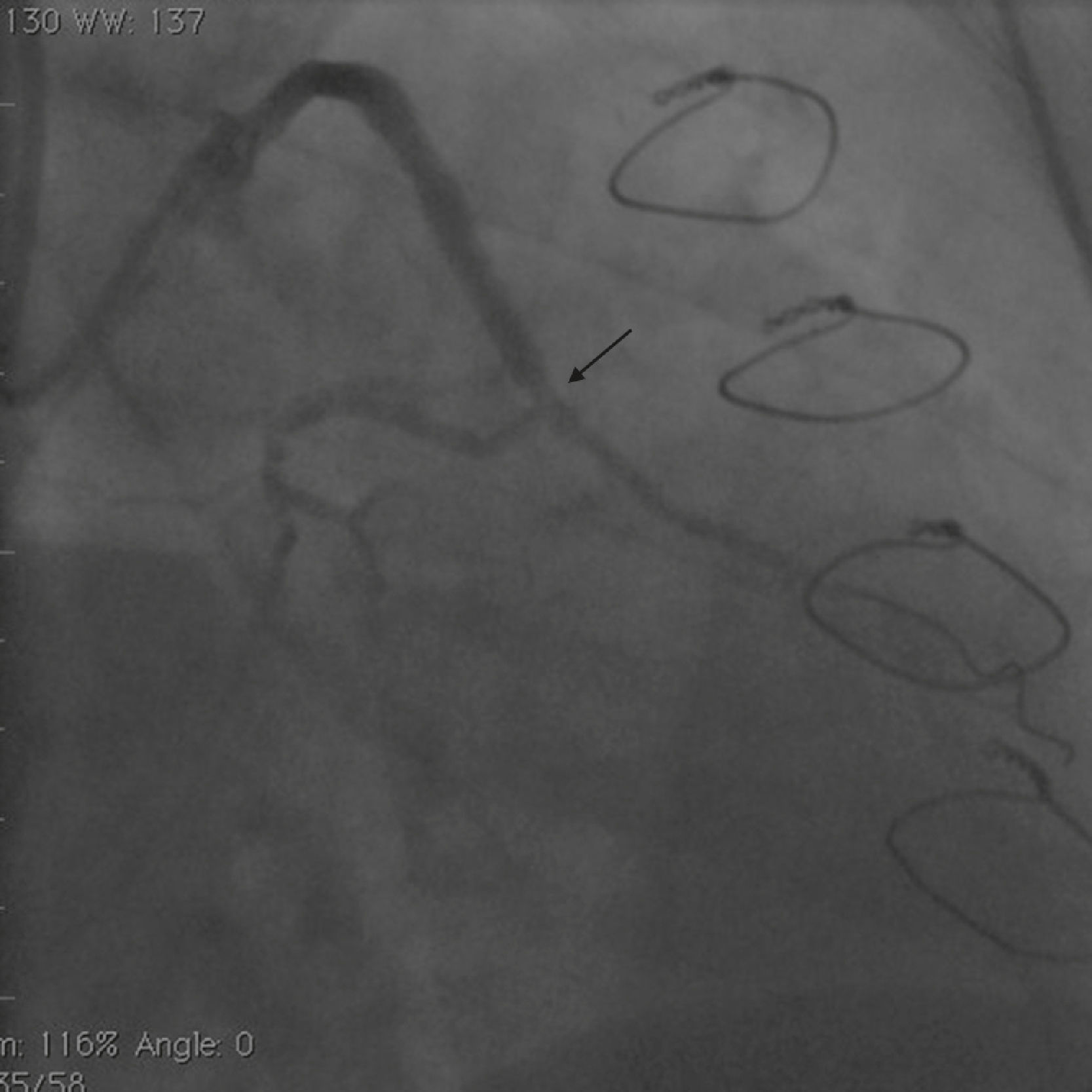
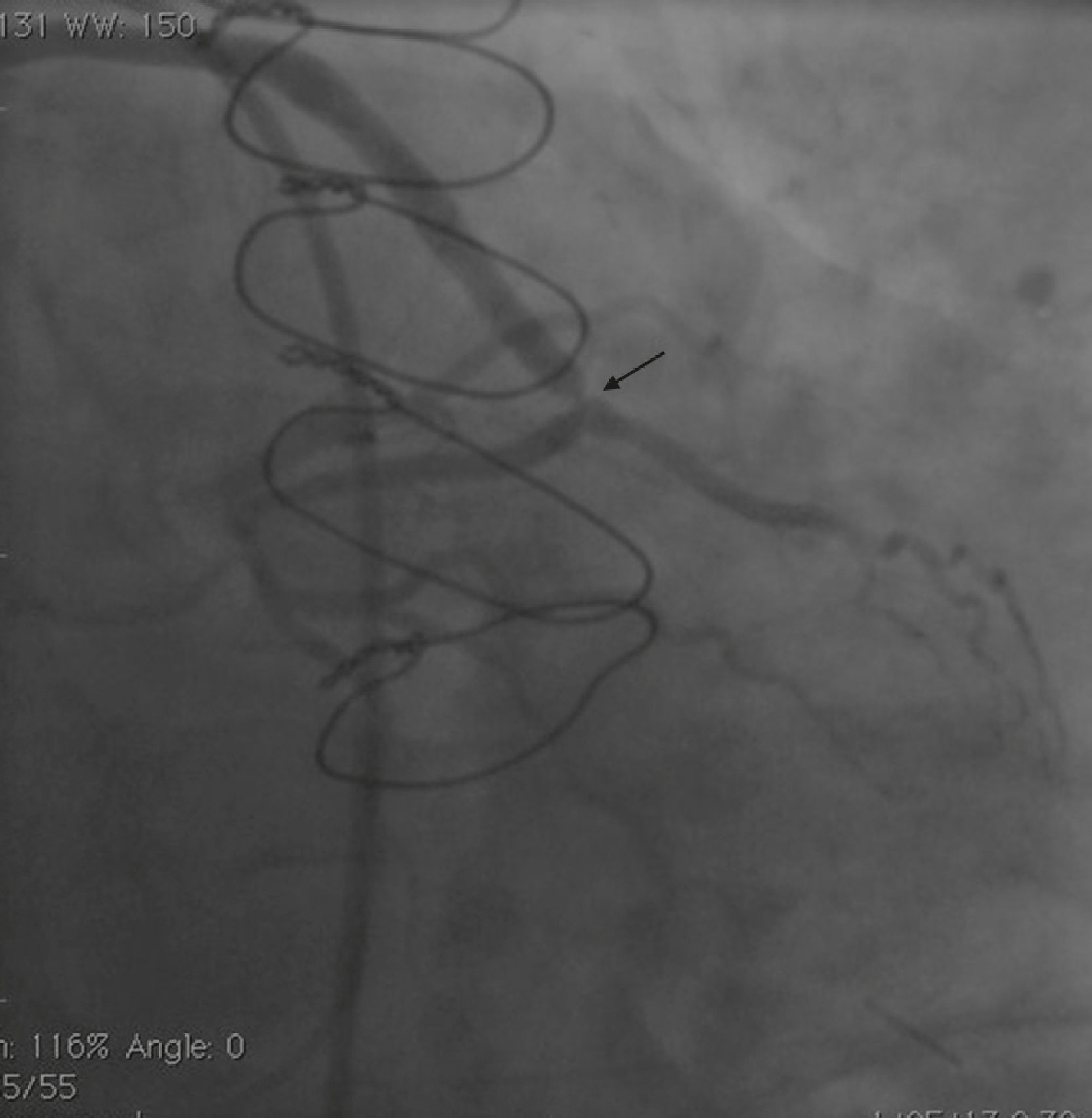
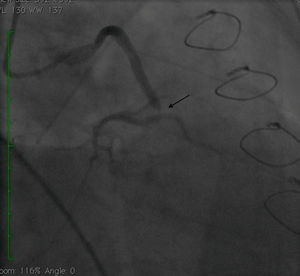
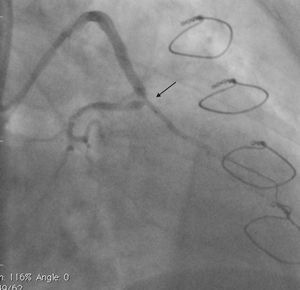
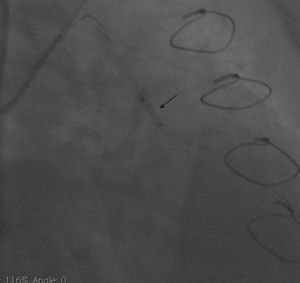
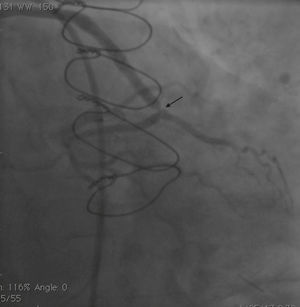
Case reportA 50-year-old white male with hypertension, hyperlipidemia and coronary artery disease had coronary artery bypass surgery with a left internal mammary artery to left anterior descending artery graft and reverse saphenous graft to the first obtuse marginal (OM1) 13 months previously. He had had a non-ST segment elevation myocardial infarction two months previously with placement of a drug-eluting stent (DES) in the right coronary artery (RCA). He also had 90% focal stenosis at the distal anastomosis of the SVG to the OM1 on coronary angiography (Figure 1). This graft was also filling the left circumflex system retrogradely. The patient continued to have class 2–3 angina since his PCI to the RCA. He had been compliant with his medications: aspirin 81 mg daily, clopidogrel 75 mg daily, carvedilol 12.5 mg twice daily, Lisinopril 5 mg daily, rosuvastatin 40 mg daily, gemfibrozil 600 mg twice daily. With this clinical picture, he was scheduled for percutaneous intervention of the distal anastomotic lesion of the SVG to OM1 graft. The latter was engaged with a 6-F Amplatzer Left 0.75 guide catheter and after therapeutic anticoagulation was achieved with heparin, a Balance Middleweight guidewire (Abbott Vascular) was placed in the distal OM1. The lesion was then dilated with a 2.5 mm × 12 mm Apex balloon catheter (Boston Scientific Corporation) at 12 atm with full balloon inflation. This reduced the lesion from 90% to 40% stenosis. A 2.5 mm × 8 mm Promus stent (Boston Scientific Corporation) was then deployed at 15 atm across the lesion into the native OM1 distally and the stent balloon was reinflated proximally at 18 atm, with full expansion of the stent (Figures 2 and 3). The stent was then post-dilated with a 3.5 mm × 6 mm Sprinter Legend noncompliant balloon (Medtronic) inflated at 12 atm in the anastomosis and at 18 atm in the vein graft. Initially, the stent appeared to have expanded well (Figure 4), but, while more angiographic images were being taken, the stent recoiled in the vein graft at the distal anastomosis with reappearance of 80–90% focal stenosis inside the stent (Figure 5).
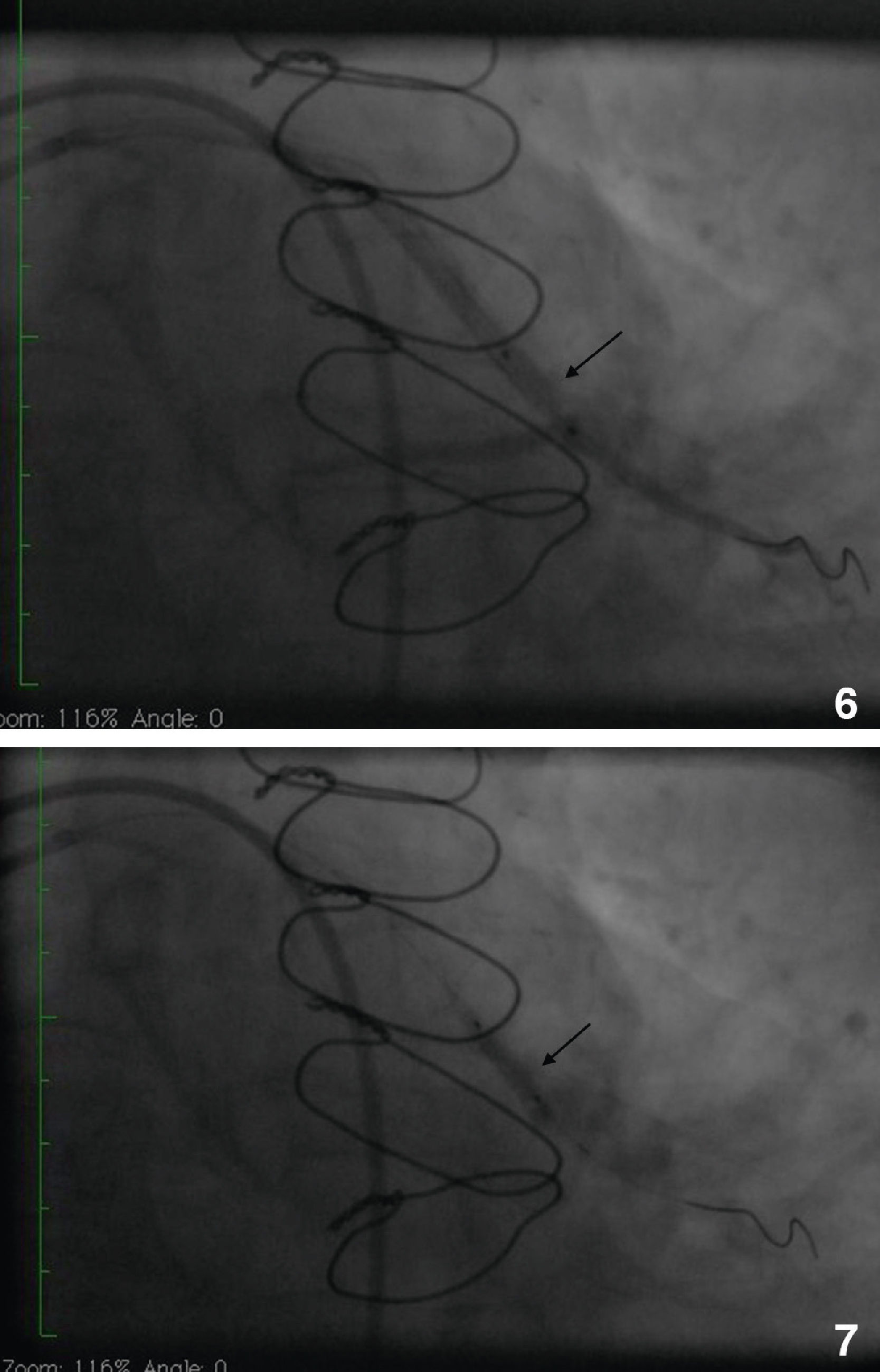
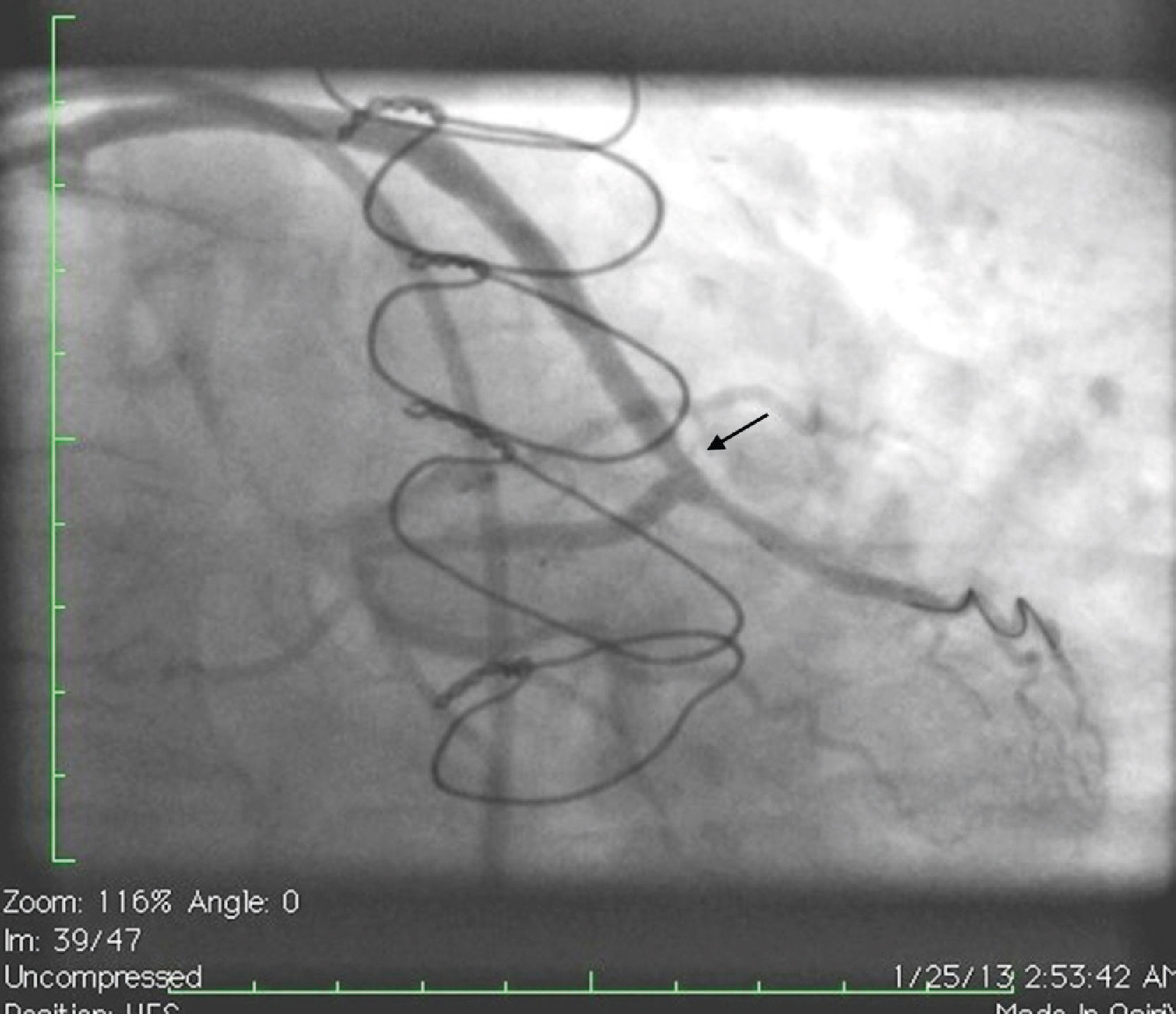
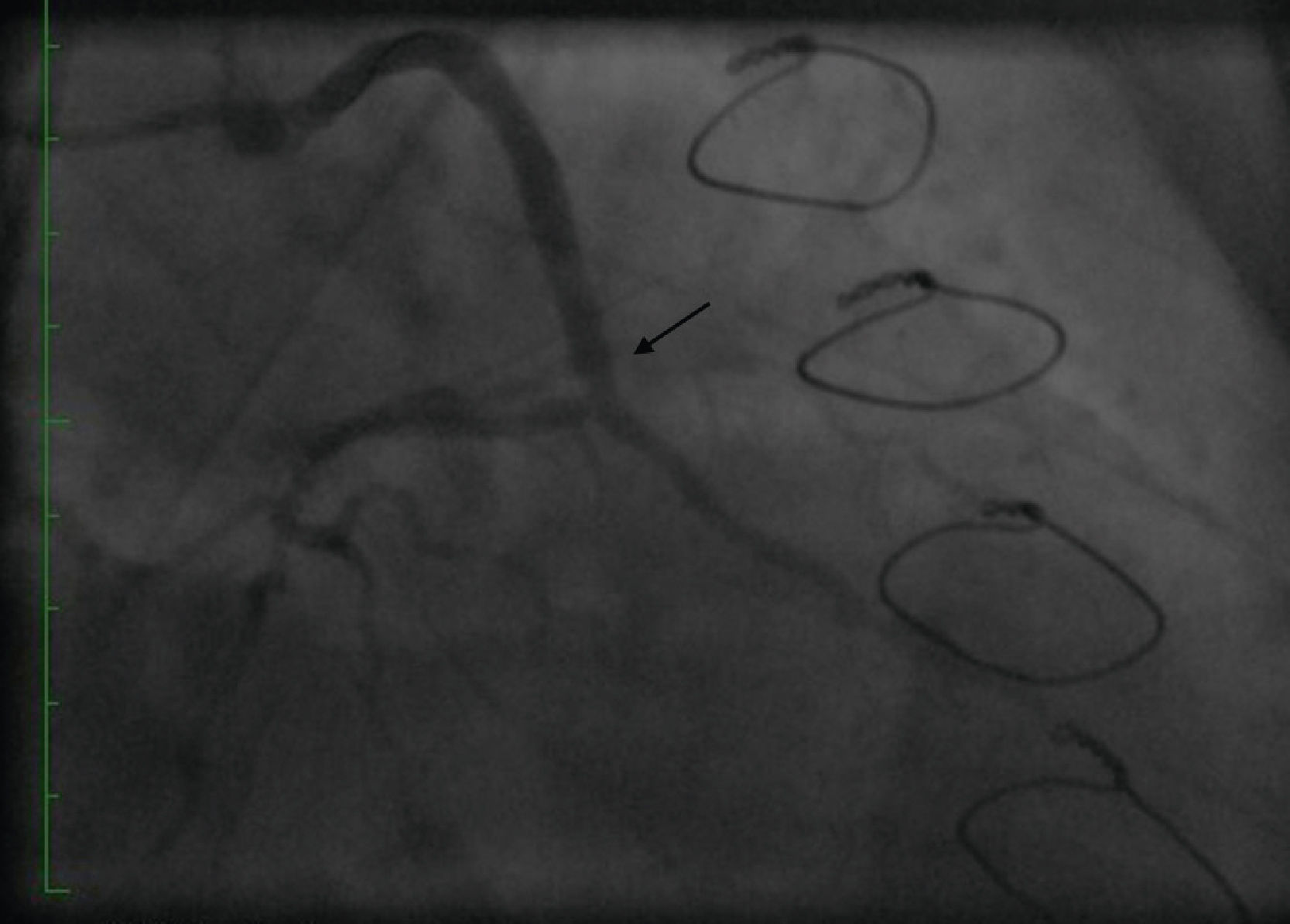
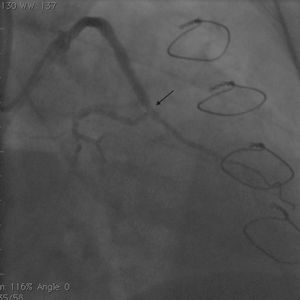
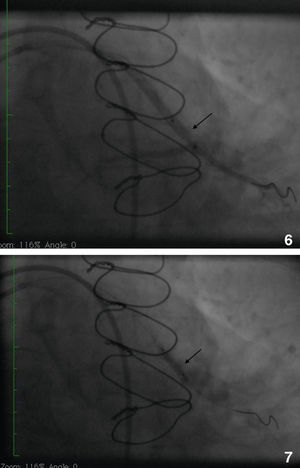
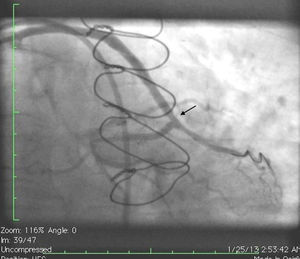
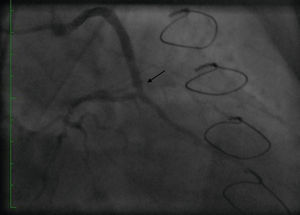
Initially we reused the 2.5 mm × 12 mm Apex balloon catheter (Boston Scientific Corporation) inflated at 18 atm with full expansion of the stent, but once the balloon deflated there was still stent recoil. Because of concerns that plaque protrusion or rigid fibrotic tissue surrounding the stent were causing the recoil, we decided to use a cutting balloon for plaque modification. A slightly longer (10 mm) cutting balloon was chosen for additional penetration of the tissue outside the stent. A 2.75 mm × 10 mm Flextome Cutting Balloon (Boston Scientific Corporation) was slowly inflated in the lesion at 12 atm with good re-expansion of the stent (Figures 6–8). Finally, the stent was again post-dilated with a 3.25 mm × 8 mm noncompliant Apex balloon (Boston Scientific Corporation) at 18 atm and the lesion was reduced to 0% (Figure 9). There was no further recoil of the stent. The patient had an uneventful recovery and was discharged home on dual antiplatelet therapy.
SVG lesions occur through three distinct mechanisms: within the first month postoperatively, thrombosis predominates; fibrointimal hyperplasia is the most common finding from one month to one year; and atherosclerosis is the major cause of lesions after the first year.1–3
Fibrotic lesions from neointimal and smooth muscle proliferation at the anastomotic site are promoted by alterations in hemodynamics and subsequent endothelial damage to the saphenous vein, which is exposed to arterial blood flow. This can be partly due to poor surgical technique, such as a tight suture, and technical factors relating to vein graft harvesting, preparation and grafting, which can aggravate turbulent flow by contributing to endothelial damage and subsequent smooth muscle proliferation.2,3
There are also specific features of the walls of these SVGs, which are more muscular and elastin-rich compared to a typical vein wall. Untouched native saphenous veins may show mild intimal and/or medial fibrosis even before grafting. Studies on unused harvested grafts revealed that approximately 1% of saphenous veins have >50% stenosis before grafting.2,3
Surgical preparation techniques will affect the phenotype of smooth muscle cells (SMC), causing SMC migration and intimal thickening. Matrix metalloproteinase activity is also enhanced after preparation. Surgical manipulation before anastomosis leads to loss of the endothelium's integrity and antithrombogenic properties, leading to subsequent occlusive intimal hyperplasia and/or thrombus formation.
Moreover, the vasa vasorum of the veins are disrupted during harvesting, making the SVG dependent solely on diffusion for the first week after implantation. It may take up to six months for adequate circulation to the grafted vessel to be re-established. Ischemic insult and diminished nitric oxide and adenosine production may also aggravate SMC proliferation.2,3
There have been no reports of immediate stent recoil in treatment of these lesions after adequate balloon dilation and stent implantation. As stated above, these lesions may be resistant to balloon angioplasty alone, and there are some reports of cutting balloon and directional atherectomy being used to treat them.4–6 Stenting has been shown to increase gain in MLD and reduce restenosis rates.5,6
The cutting balloon consists of a balloon catheter with three (<3.5 mm) or four (3.5–4.0 mm) blades. These blades initially create small regular longitudinal incisions in the atherosclerotic lesion, then the lumen is enlarged during balloon inflation with widening of the cuts. Cutting the calcified, fibrotic plaque reduces the rigidity and continuity of the plaque and hence the circumferential (hoop) stress of the vessel. Then with balloon inflation and resulting pressure these cracks propagate, resulting in lumen enlargement.7 The incisions created by the blades also prevent late elastic recoil. Cutting balloons have been shown to be effective for resistant dense fibrotic ostial lesions.8 For treatment of in-stent restenosis, the cutting balloon also has advantages over plain balloon angioplasty by reducing the recoil of neointimal tissue into the lumen, as well as reducing hoop stress in the neointimal tissue.9
However, there is no literature on the use of the cutting balloon for immediate stent recoil as seen in our patient. The recoil at the anastomotic lesion suggests significant hoop stress at the lesion, which could be secondary to significant surrounding fibrointimal tissue, tight sutures, or other factors. While the initial plain high-pressure angioplasty (with a balloon shorter or longer than the stent) inside the stent was not successful, cutting balloon angioplasty successfully expanded the recoiled stent, with immediate relief of the tension. Use of a cutting balloon longer than the stent might also have helped to achieve effective tissue penetration. Unfortunately we did not perform intravascular ultrasound (IVUS) to further evaluate the mechanism of the stent recoil; it would be also reasonable to perform IVUS or optical coherence tomography imaging to further assess stent recoil in similar anastomotic lesions.
In conclusion, our case illustrates an uncommon complication of immediate stent recoil in a distal anastomotic vein graft intervention and its successful treatment by cutting balloon angioplasty.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.