Anemia has been shown to be associated with a worse prognosis, especially higher mortality in various pathological conditions. However, few studies have specifically examined its impact in acute coronary syndrome (ACS) patients. The purpose of our study was to assess the association between different quartiles of hemoglobin on admission and short- and long-term prognosis in patients with ACS.
MethodsWe performed a retrospective analysis of 1303 consecutive ACS patients admitted to a coronary care unit and analyzed the association between baseline hemoglobin and morbidity and mortality, in-hospital and at 12-month follow-up. The population was divided into groups according to quartiles of hemoglobin concentration (Hb): Q1: <10.8g/dl; Q2: 10.8–12.2g/dl; Q3: 12.3–13.2g/dl; Q4: ≥13.3g/dl. Logistic regression analysis was used to identify independent predictors of short- and long-term mortality.
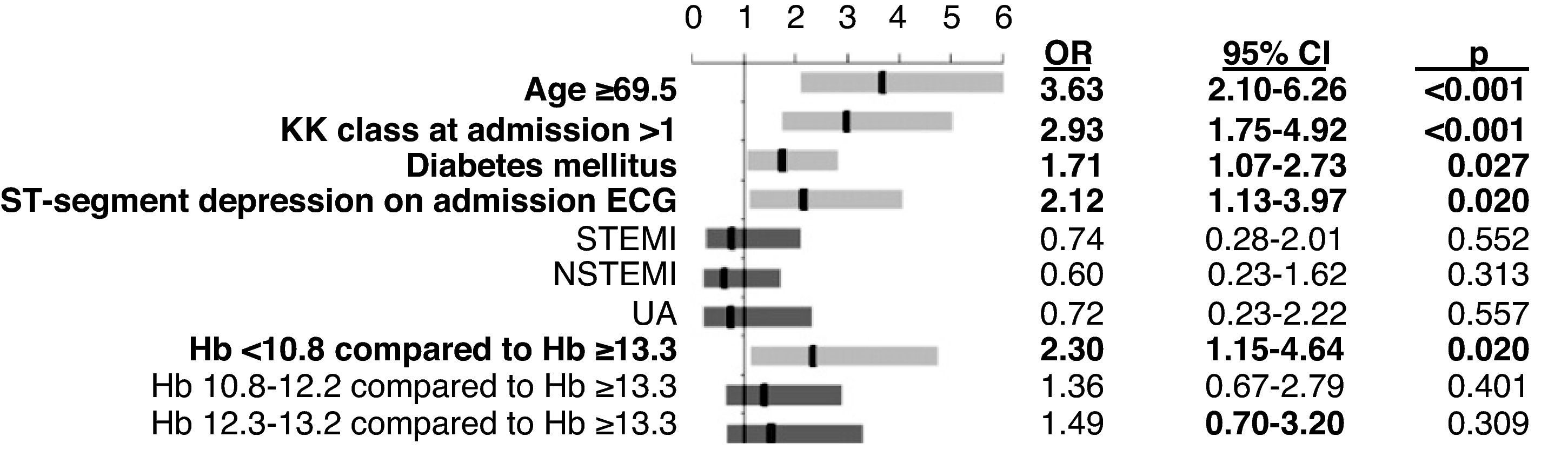
ResultsHypertension and diabetes mellitus were more common in the lower Hb quartiles, while the prevalence of smoking and physical inactivity increased with higher Hb. A higher proportion of patients in the lower quartiles had congestive heart failure, peripheral artery disease and previous stroke or transient ischemic attack. Anemic patients tended to be older, with worse renal function and left ventricular systolic function. Patients in Q1 had significantly higher levels of troponin I and blood glucose on admission. Anemic patients showed significantly higher in-hospital mortality (Q1: 9.8%; Q2: 6.3%; Q3: 4.1%; Q4: 3.6%, p<0.001), longer hospital stay (Q1: 6.1±4.4; Q2: 5.2±3.0; Q3: 4.9±2.7; Q4: 4.3±2.1 days, p<0.001) and higher 1-year mortality (Q1: 23.6%; Q2: 11.6%; Q3: 10.6%; Q4: 5.5%, p<0.001). In multivariate analysis, the only independent predictor of in-hospital mortality was Killip class >1 at admission. The independent predictors of long-term mortality were age ≥69.5 years, Killip class >1 at admission, diabetes mellitus, ST-segment depression on admission ECG and Hb <10.8g/dl.
Discussion and conclusionsLow baseline hemoglobin is associated with more comorbidities and can accurately predict 1-year mortality after an acute coronary syndrome.
A anemia tem sido associada a um pior prognóstico, e particularmente a uma mortalidade mais elevada em diversas condições patológicas. Contudo, poucos estudos analisaram especificamente o seu impacto a curto e a longo prazo, em doentes com Síndrome Coronária Aguda. Este artigo tem como objectivo analisar a associação entre diferentes quartis de hemoglobina na admissão hospitalar e o prognóstico a curto e a longo prazo em doentes com síndrome coronária aguda.
População e MétodosEstudo retrospectivo de 1303 doentes consecutivamente admitidos com síndrome coronária aguda numa unidade de cuidados intensivos coronários, procurando analisar a associação entre os níveis basais de hemoglobina e a morbilidade e mortalidade intra-hospitalar e num seguimento clínico de 12 meses. Esta população de doentes foi dividida em quartis de concentração da hemoglobina ([Hb]): Q1: <10,8g/dL; Q2: 10,8–12,2g/dL; Q3: 12,3–13,2g/dL; Q4: ≥13,3g/dL. Para identificação dos preditores de risco independente de mortalidade a curto e a longo prazo foi usada uma análise de regressão logística.
ResultadosA hipertensão arterial e a diabetes mellitus foram mais comuns nos quartis mais baixos de hemoglobina, enquanto que a proporção de fumadores e de sedentarismo aumentava com o aumento da concentração da hemoglobina. Os doentes com quartis mais baixos de hemoglobina apresentavam também mais frequentemente antecedentes de insuficiência cardíaca congestiva, doença arterial periférica e acidentes vasculares cerebrais/acidentes isquémicos transitórios. Os doentes anémicos tendiam a ser mais idosos, com pior função renal e pior função ventricular esquerda, e apresentavam valores de troponina I e de glicemia na admissão significativamente superiores. Os doentes anémicos mostraram também taxas significativamente superiores de mortalidade intra-hospitalar (Q1: 9,8%; Q2: 6,3%; Q3: 4,1%; Q4: 3,6%, p<0,001), internamentos mais prolongados (Q1: 6,1 ± 4,4; Q2: 5,2 ± 3,0; Q3: 4,9 ± 2,7; Q4: 4,3 ± 2,1, p<0,001) e maior mortalidade aos 12 meses (Q1: 23,6%; Q2: 11,6%; Q3: 10,6%; Q4: 5,5%, p<0,001). Na análise multivariada, o único preditor independente de mortalidade intra-hospitalar foi uma classe Killip na admissão > 1, e os preditores de mortalidade no follow-up foram: idade ≥ 69,5 anos, classe Killip na admissão> 1, diabetes mellitus, existência de depressão do segmento ST no ECG de admissão hospitalar e uma concentração de hemoglobina inferior a 10,8g/dL.
Discussão e conclusõesEm doentes com síndromes coronárias agudas, os valores mais baixos de hemoglobina encontram-se frequentemente associados a outras co-morbilidades, mas por si só, a anemia parece ser um preditor independente de mortalidade um ano após uma síndrome coronária aguda.
There have been important advances in recent years in the management of patients with acute coronary syndrome (ACS), leading to a significant reduction in mortality.1 However, ACS remains one of the main causes of morbidity and mortality in developed countries. Traditionally ACS covers a wide spectrum of clinical entities, which includes unstable angina (UA), non-ST elevation myocardial infarction (NSTEMI), ST-elevation myocardial infarction (STEMI) and sudden cardiac death.2
ACS is frequently complicated by the presence of comorbidities, including anemia, renal failure and diabetes mellitus. Anemia is common in ACS patients at admission, being found in over 15% of patients with myocardial infarction (MI), and in up to 43% of older patients.3
Anemia can exacerbate myocardial ischemia in ACS patients, adversely affecting their prognosis. The harmful effect of anemia is due to various mechanisms: it decreases the blood oxygen saturation required to meet myocardial demand and raises oxygen demand by increasing cardiac ejection volume to maintain an adequate oxygen supply to all systemic tissues. The combination of these two processes may be the pathophysiological basis for the worse outcomes observed in ACS patients with lower baseline hemoglobin (Hb) concentrations.4,5
Although the cardiovascular repercussions of anemia are relatively well known, other questions remain to be clarified, such as the prevalence of anemia in ACS patients at the time of hospital admission, the Hb concentration associated with worse prognosis, and the actual effect of low Hb in the short and long term.5
While the general effect of anemia is decreased myocardial oxygenation, the ideal Hb level has yet to be established. At the other extreme, polycythemia can result in hyperviscosity, which increases coronary vascular resistance and promotes thrombus formation, thus also reducing oxygen supply to ischemic myocardium. Sabatine et al. demonstrated that patients with very high baseline Hb (>16–17g/dl) also presented higher cardiovascular risk.4
The adverse effect of anemia on prognosis in coronary disease has been comprehensively studied. In animal models, higher Hb levels have been shown to prevent myocardial ischemia in the presence of significant coronary disease.4 However, the data available on the real predictive value of anemia in ACS patients are limited and at times inconsistent. Few studies have specifically examined its value as an independent predictor of short- and long-term mortality.
The purpose of this study was to assess the impact of different baseline Hb levels on short- and long-term prognosis in patients with ACS.
MethodsStudy protocol and inclusion criteriaWe performed a retrospective analysis of 1303 consecutive patients admitted to the coronary care unit of the Cardiology Department of Coimbra University Hospitals for ACS between May 2004 and December 2006, with a follow-up of 12 months.
Data analyzed included demographic characteristics, cardiovascular risk factors, history of heart disease, TIMI risk score and Killip class, ECG alterations, markers of myocardial necrosis and other laboratory parameters, treatment, and in-hospital course. Follow-up was by telephone, face-to-face interview or consultation of patients’ medical records, 12 months after discharge.
Admission diagnoses included UA, NSTEMI and STEMI. MI was defined as elevated markers of myocardial necrosis (troponin I ≥0.2μg/l) associated with chest pain, and was classified as STEMI or NSTEMI based on the presence or absence of >1mm ST-segment elevation in contiguous electrocardiographic leads.6 UA was defined as chest pain, without elevated cardiac biomarkers, with at least one of the following characteristics: occurrence at rest (or on minimal exertion) generally lasting more than ten minutes – rest angina; intense pain of recent onset – new-onset angina; or crescendo angina.7
Anemia was defined according to the World Health Organization criteria: Hb <13g/dl in men and <12g/dl in women.
Patients were considered to have a history of hypertension, dyslipidemia or diabetes mellitus if they had a previous diagnosis of the condition. Obesity was defined as a body mass index (BMI) of ≥30kg/m2. Smoking was defined as a history of active smoking, irrespective of daily consumption. Left ventricular function was determined echocardiographically by Simpson's method.
Treatment was in accordance with the current international guidelines for the management of ACS patients.
Statistical analysisThe population was divided into groups according to quartiles of Hb: Q1: <10.8g/dl; Q2: 10.8–12.2g/dl; Q3: 12.3–13.2g/dl; Q4: ≥13.3g/dl. Categorical variables are presented as absolute frequencies and percentages and compared using the chi-square test or Fisher's exact test as appropriate. Continuous variables are presented as means±standard deviation and compared using the ANOVA test. A value of p<0.05 was considered statistically significant.
Multivariate logistic regression analysis was performed to identify independent predictors of in-hospital and one-year mortality, using the Hosmer–Lemeshow test and the c-statistic to calibrate the model and receiver operating characteristic (ROC) curves to determine the best cut-off in order to maximize the sensitivity and specificity of the variables included in the multivariate analysis model.
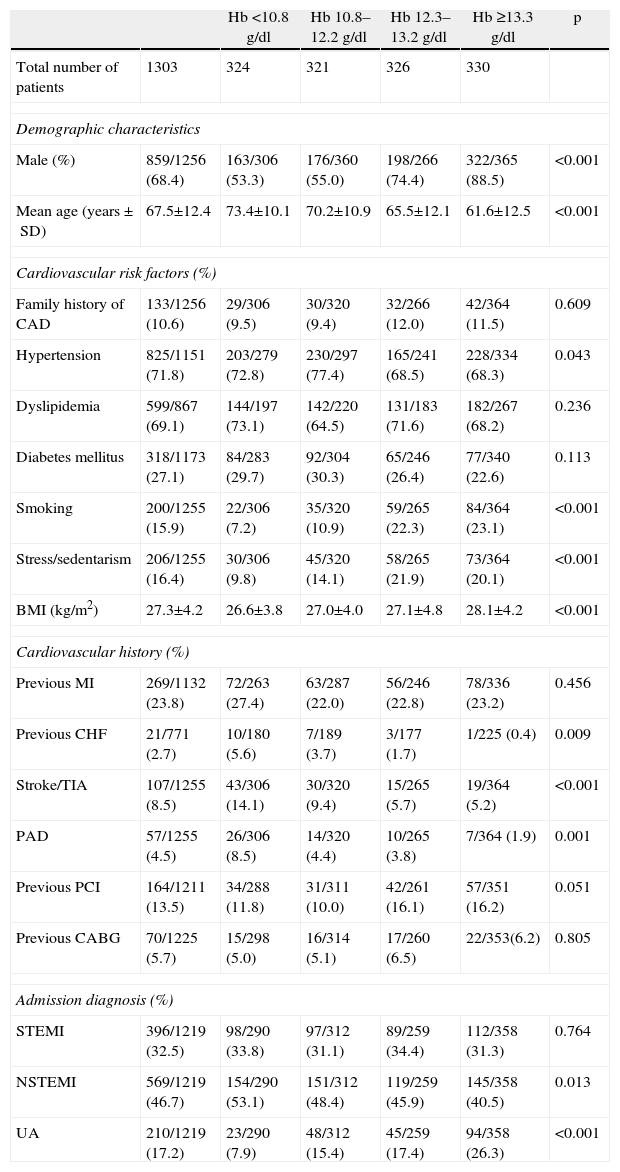
ResultsGeneral characteristics of the study populationMost of the 1303 patients under study were male, and mean age was 67.5±12.4 years (Table 1). The type of ACS was STEMI in 396 patients (32.5%), NSTEMI in 569 (46.7%), and UA in 210 (17.2%).
General characteristics of the study population.
| Hb <10.8g/dl | Hb 10.8–12.2g/dl | Hb 12.3–13.2g/dl | Hb ≥13.3g/dl | p | ||
| Total number of patients | 1303 | 324 | 321 | 326 | 330 | |
| Demographic characteristics | ||||||
| Male (%) | 859/1256 (68.4) | 163/306 (53.3) | 176/360 (55.0) | 198/266 (74.4) | 322/365 (88.5) | <0.001 |
| Mean age (years±SD) | 67.5±12.4 | 73.4±10.1 | 70.2±10.9 | 65.5±12.1 | 61.6±12.5 | <0.001 |
| Cardiovascular risk factors (%) | ||||||
| Family history of CAD | 133/1256 (10.6) | 29/306 (9.5) | 30/320 (9.4) | 32/266 (12.0) | 42/364 (11.5) | 0.609 |
| Hypertension | 825/1151 (71.8) | 203/279 (72.8) | 230/297 (77.4) | 165/241 (68.5) | 228/334 (68.3) | 0.043 |
| Dyslipidemia | 599/867 (69.1) | 144/197 (73.1) | 142/220 (64.5) | 131/183 (71.6) | 182/267 (68.2) | 0.236 |
| Diabetes mellitus | 318/1173 (27.1) | 84/283 (29.7) | 92/304 (30.3) | 65/246 (26.4) | 77/340 (22.6) | 0.113 |
| Smoking | 200/1255 (15.9) | 22/306 (7.2) | 35/320 (10.9) | 59/265 (22.3) | 84/364 (23.1) | <0.001 |
| Stress/sedentarism | 206/1255 (16.4) | 30/306 (9.8) | 45/320 (14.1) | 58/265 (21.9) | 73/364 (20.1) | <0.001 |
| BMI (kg/m2) | 27.3±4.2 | 26.6±3.8 | 27.0±4.0 | 27.1±4.8 | 28.1±4.2 | <0.001 |
| Cardiovascular history (%) | ||||||
| Previous MI | 269/1132 (23.8) | 72/263 (27.4) | 63/287 (22.0) | 56/246 (22.8) | 78/336 (23.2) | 0.456 |
| Previous CHF | 21/771 (2.7) | 10/180 (5.6) | 7/189 (3.7) | 3/177 (1.7) | 1/225 (0.4) | 0.009 |
| Stroke/TIA | 107/1255 (8.5) | 43/306 (14.1) | 30/320 (9.4) | 15/265 (5.7) | 19/364 (5.2) | <0.001 |
| PAD | 57/1255 (4.5) | 26/306 (8.5) | 14/320 (4.4) | 10/265 (3.8) | 7/364 (1.9) | 0.001 |
| Previous PCI | 164/1211 (13.5) | 34/288 (11.8) | 31/311 (10.0) | 42/261 (16.1) | 57/351 (16.2) | 0.051 |
| Previous CABG | 70/1225 (5.7) | 15/298 (5.0) | 16/314 (5.1) | 17/260 (6.5) | 22/353(6.2) | 0.805 |
| Admission diagnosis (%) | ||||||
| STEMI | 396/1219 (32.5) | 98/290 (33.8) | 97/312 (31.1) | 89/259 (34.4) | 112/358 (31.3) | 0.764 |
| NSTEMI | 569/1219 (46.7) | 154/290 (53.1) | 151/312 (48.4) | 119/259 (45.9) | 145/358 (40.5) | 0.013 |
| UA | 210/1219 (17.2) | 23/290 (7.9) | 48/312 (15.4) | 45/259 (17.4) | 94/358 (26.3) | <0.001 |
BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CHF: congestive heart failure; MI: myocardial infarction; NSTEMI: non-ST elevation myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-elevation myocardial infarction; TIA: transient ischemic attack; UA: unstable angina.
With regard to cardiovascular risk factors, most patients had a history of hypertension and dyslipidemia, 27.1% had diabetes mellitus (10% insulin-treated and 15.6% under oral antidiabetics), and 15.9% were smokers. Regarding personal cardiovascular history, 23.8% had had a previous MI, while a history of congestive heart failure (CHF), peripheral arterial disease, and stroke or transient ischemic attack (TIA) was present in 2.7%, 4.5% and 8.5%, respectively (Table 1).
Most patients (44.9%) had single-vessel coronary disease, and 14.0% had no significant coronary lesions.
Most of the study population (84.1%) presented in Killip class 1 at admission, with a TIMI score of 2 (25.9%) or 3 (24.6%).
Comparison of patient baseline characteristics according to quartiles of hemoglobinAnemia is a common comorbidity in ACS patients, and was present in 65.6% of our study population.
Patients in the lower quartiles of Hb tended to be significantly older and more often had hypertension, while the prevalence of smoking and stress/sedentarism increased with higher Hb levels. BMI was lower in patients with lower Hb (Table 1).
The proportion of patients with previous CHF, peripheral arterial disease and stroke or TIA was higher in the lower quartiles of Hb (Table 1).
With regard to risk assessment scores, patients with higher Hb presented lower Killip class at admission (Killip class 1: Q1: 75.4%; Q2: 85.0%; Q3: 85.1%; Q4: 89.9%; p<0.001), while those in the lower Hb quartiles predominated in the higher Killip classes (Killip class 4: Q1: 3.0%; Q2: 2.2%; Q3: 0.8%; Q4: 0.3%; p=0.017). Patients with higher Hb also tended to have lower TIMI risk scores (TIMI 0: Q1: 1.6%; Q2: 2.2%; Q3: 4.5%; Q4: 5.5%; p=0.020; and TIMI 1: Q1: 10.8%; Q2: 13.8%; Q3: 17.7%; Q4: 22.3%; p<0.001). By contrast, anemic patients predominated among those with TIMI 4 (Q1: 20.3%; Q2: 18.4%; Q3: 19.2%; Q4: 12.6%; p=0.040) or TIMI 5 (Q1: 14.1%; Q2: 10.0%; Q3: 7.5%; Q4: 7.1%; p=0.012).
As regards the type of ACS, there was a higher proportion of NSTEMI in the Q1 group and a higher proportion of UA in those with normal Hb (Table 1).
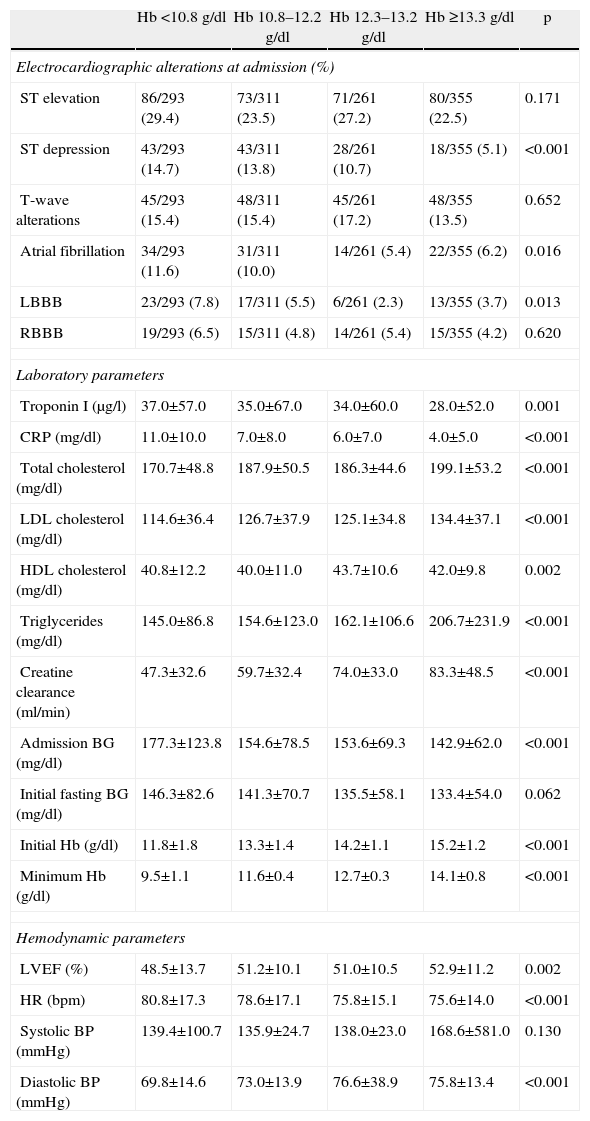
In terms of differences in baseline ECG, atrial fibrillation and complete left bundle branch block were more common in individuals with low Hb levels; also such individuals had a higher incidence of ST-segment depression (Table 2).
Electrocardiographic, laboratory and hemodynamic parameters.
| Hb <10.8g/dl | Hb 10.8–12.2g/dl | Hb 12.3–13.2g/dl | Hb ≥13.3g/dl | p | |
| Electrocardiographic alterations at admission (%) | |||||
| ST elevation | 86/293 (29.4) | 73/311 (23.5) | 71/261 (27.2) | 80/355 (22.5) | 0.171 |
| ST depression | 43/293 (14.7) | 43/311 (13.8) | 28/261 (10.7) | 18/355 (5.1) | <0.001 |
| T-wave alterations | 45/293 (15.4) | 48/311 (15.4) | 45/261 (17.2) | 48/355 (13.5) | 0.652 |
| Atrial fibrillation | 34/293 (11.6) | 31/311 (10.0) | 14/261 (5.4) | 22/355 (6.2) | 0.016 |
| LBBB | 23/293 (7.8) | 17/311 (5.5) | 6/261 (2.3) | 13/355 (3.7) | 0.013 |
| RBBB | 19/293 (6.5) | 15/311 (4.8) | 14/261 (5.4) | 15/355 (4.2) | 0.620 |
| Laboratory parameters | |||||
| Troponin I (μg/l) | 37.0±57.0 | 35.0±67.0 | 34.0±60.0 | 28.0±52.0 | 0.001 |
| CRP (mg/dl) | 11.0±10.0 | 7.0±8.0 | 6.0±7.0 | 4.0±5.0 | <0.001 |
| Total cholesterol (mg/dl) | 170.7±48.8 | 187.9±50.5 | 186.3±44.6 | 199.1±53.2 | <0.001 |
| LDL cholesterol (mg/dl) | 114.6±36.4 | 126.7±37.9 | 125.1±34.8 | 134.4±37.1 | <0.001 |
| HDL cholesterol (mg/dl) | 40.8±12.2 | 40.0±11.0 | 43.7±10.6 | 42.0±9.8 | 0.002 |
| Triglycerides (mg/dl) | 145.0±86.8 | 154.6±123.0 | 162.1±106.6 | 206.7±231.9 | <0.001 |
| Creatine clearance (ml/min) | 47.3±32.6 | 59.7±32.4 | 74.0±33.0 | 83.3±48.5 | <0.001 |
| Admission BG (mg/dl) | 177.3±123.8 | 154.6±78.5 | 153.6±69.3 | 142.9±62.0 | <0.001 |
| Initial fasting BG (mg/dl) | 146.3±82.6 | 141.3±70.7 | 135.5±58.1 | 133.4±54.0 | 0.062 |
| Initial Hb (g/dl) | 11.8±1.8 | 13.3±1.4 | 14.2±1.1 | 15.2±1.2 | <0.001 |
| Minimum Hb (g/dl) | 9.5±1.1 | 11.6±0.4 | 12.7±0.3 | 14.1±0.8 | <0.001 |
| Hemodynamic parameters | |||||
| LVEF (%) | 48.5±13.7 | 51.2±10.1 | 51.0±10.5 | 52.9±11.2 | 0.002 |
| HR (bpm) | 80.8±17.3 | 78.6±17.1 | 75.8±15.1 | 75.6±14.0 | <0.001 |
| Systolic BP (mmHg) | 139.4±100.7 | 135.9±24.7 | 138.0±23.0 | 168.6±581.0 | 0.130 |
| Diastolic BP (mmHg) | 69.8±14.6 | 73.0±13.9 | 76.6±38.9 | 75.8±13.4 | <0.001 |
BG: blood glucose; BP: blood pressure; CRP: C-reactive protein; Hb: hemoglobin; HR: heart rate; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; RBBB: right bundle branch block.
Patients in the lowest quartile of Hb had significantly higher troponin I and C-reactive protein and worse renal function. They also presented significantly higher blood glucose at admission and on initial fasting glucose measurement, although they did not have more previous diabetes mellitus (Table 2).
Left ventricular function was worse among those in the lower quartiles, while heart rate was higher, and diastolic blood pressure tended to be lower with lower Hb (Table 2).
There was also a tendency for a higher percentage of patients without significant coronary lesions in the higher quartiles, while anemic individuals tended to have more left main disease, although the differences did not reach statistical significance (p>0.05).
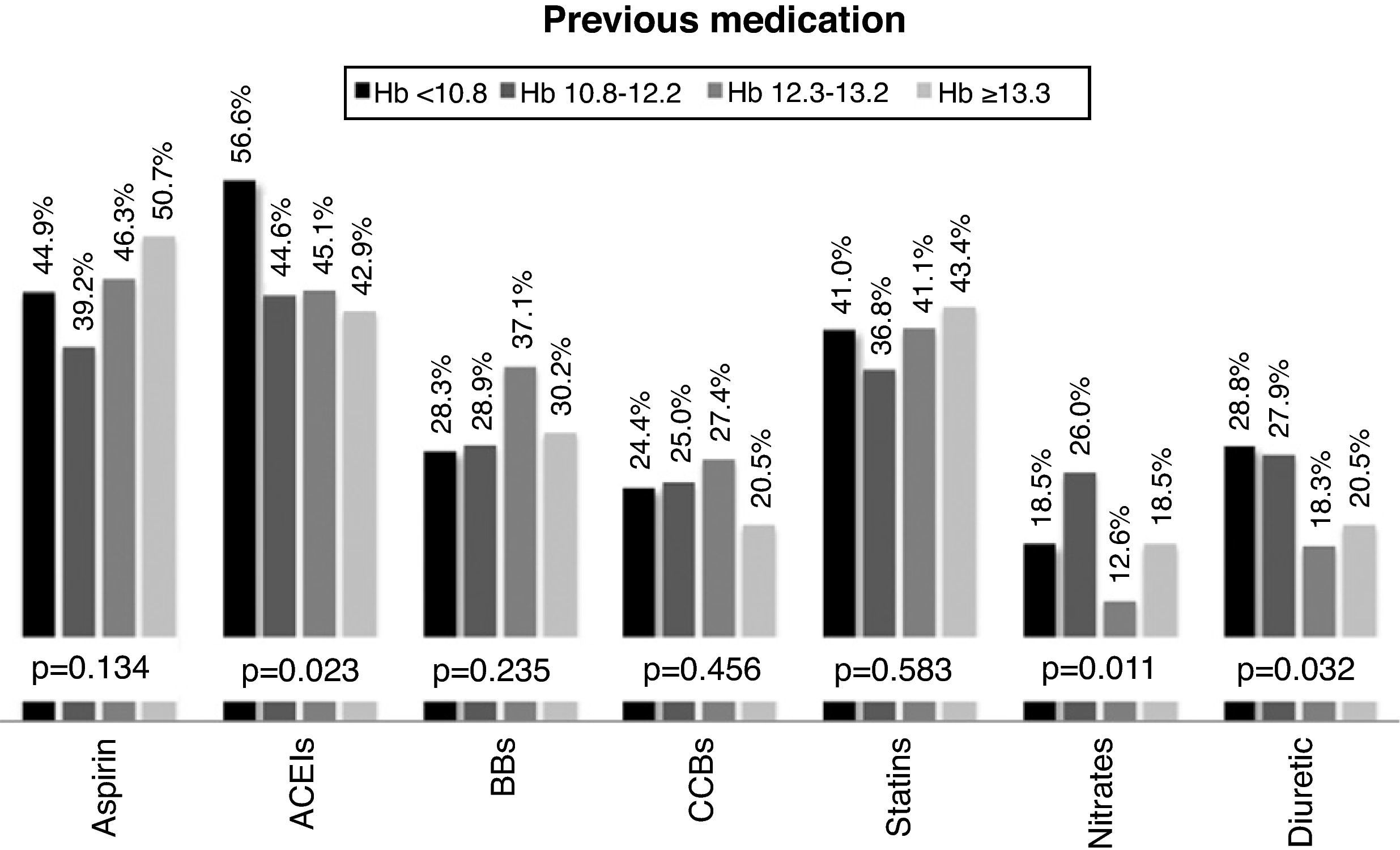
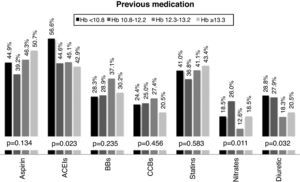
Therapeutic managementPrevious medication was highly variable in the overall population: aspirin in 45.2%, angiotensin-converting enzyme (ACE) inhibitors in 47.5%, beta-blockers in 30.9%, calcium channel blockers in 24.2%, statins in 40.6%, nitrates in 19.1%, and diuretics in 24.1%. However, patients in the lower quartiles of Hb were more frequently medicated with ACE inhibitors and diuretics at the time of admission (Figure 1).
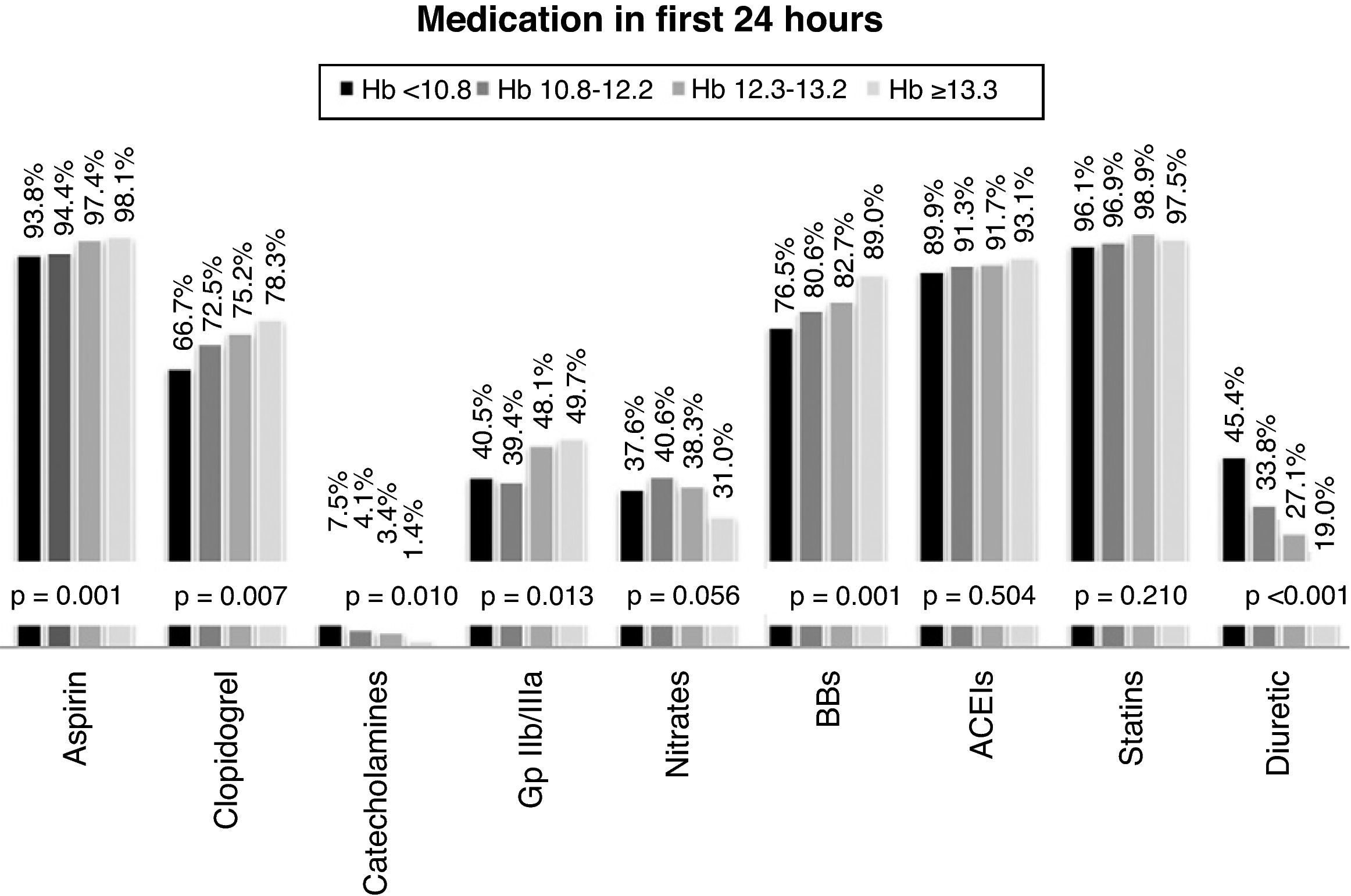
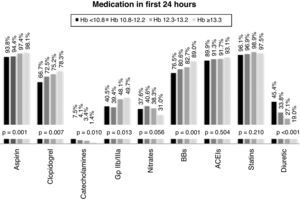
With regard to medical therapy instituted in the first 24hours after admission, most of the overall population were medicated with aspirin (95%), beta-blockers (92.5%), ACE inhibitors (91.6%), statins (97.3%) and low molecular weight heparin (96.7%). Only 44.5% were treated with glycoprotein IIb/IIIa inhibitors, and prescription of nitrates (36.6%), diuretics (30.9%) and catecholamines (4.0%) was also low. Prescription of antiplatelets (aspirin and clopidogrel) and glycoprotein IIb/IIIa inhibitors was significantly more common in individuals with higher Hb (≥13.3g/dl), as was the use of beta-blockers. By contrast, diuretics and catecholamines were prescribed significantly more often in those with the lowest Hb level (Figure 2).
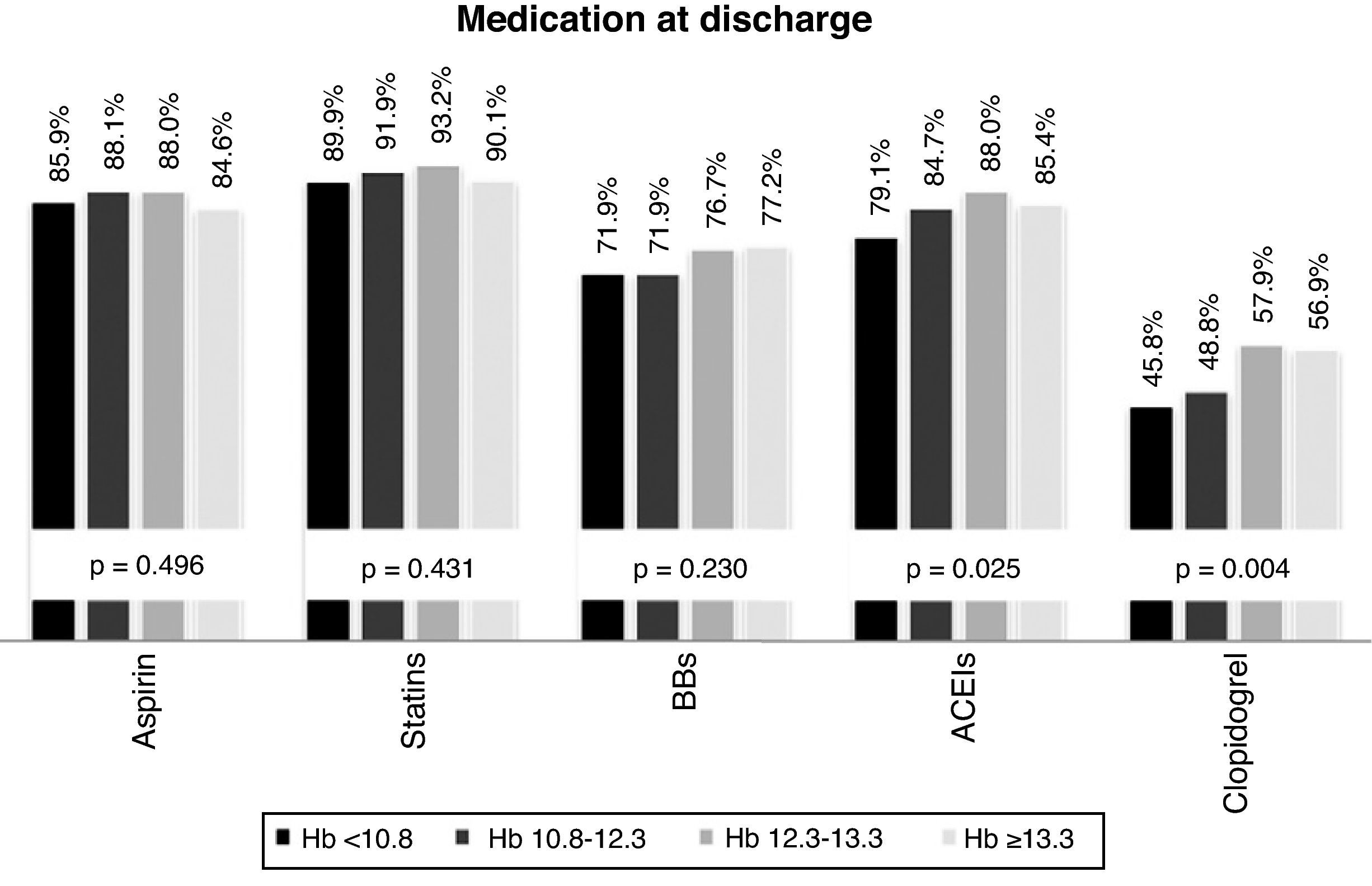
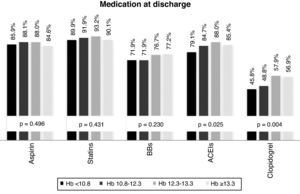
The drugs most frequently prescribed at discharge were, in descending order: statins (94.2%), aspirin (86.5%), ACE inhibitors (84.2%), beta-blockers (74.4%) and clopidogrel (52.3%). Prescription rates of ACE inhibitors and clopidogrel were lower in individuals with lower Hb levels (Figure 3).
In-hospital prognosisMean hospital stay for the total population was 5.1±3.2 days, with an overall rate of complications of 5.1% and mortality of 5.9% (9.3% in STEMI, 5.1% in NSTEMI and 1.9% in UA).
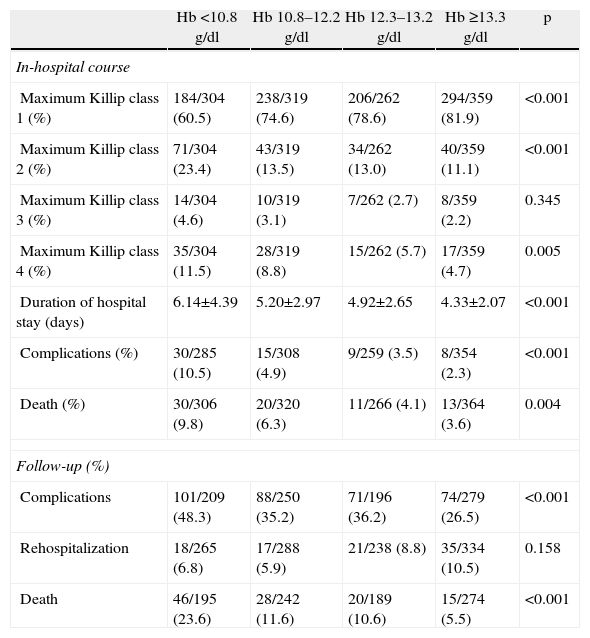
Anemic patients had significantly higher in-hospital mortality (irrespective of ACS type), longer hospital stay and more in-hospital complications. Patients in the higher quartiles of Hb more often remained in Killip class 1 during hospital stay, while those with lower Hb had higher maximum Killip class (Table 3).
In-hospital course and at one-year follow-up.
| Hb <10.8g/dl | Hb 10.8–12.2g/dl | Hb 12.3–13.2g/dl | Hb ≥13.3g/dl | p | |
| In-hospital course | |||||
| Maximum Killip class 1 (%) | 184/304 (60.5) | 238/319 (74.6) | 206/262 (78.6) | 294/359 (81.9) | <0.001 |
| Maximum Killip class 2 (%) | 71/304 (23.4) | 43/319 (13.5) | 34/262 (13.0) | 40/359 (11.1) | <0.001 |
| Maximum Killip class 3 (%) | 14/304 (4.6) | 10/319 (3.1) | 7/262 (2.7) | 8/359 (2.2) | 0.345 |
| Maximum Killip class 4 (%) | 35/304 (11.5) | 28/319 (8.8) | 15/262 (5.7) | 17/359 (4.7) | 0.005 |
| Duration of hospital stay (days) | 6.14±4.39 | 5.20±2.97 | 4.92±2.65 | 4.33±2.07 | <0.001 |
| Complications (%) | 30/285 (10.5) | 15/308 (4.9) | 9/259 (3.5) | 8/354 (2.3) | <0.001 |
| Death (%) | 30/306 (9.8) | 20/320 (6.3) | 11/266 (4.1) | 13/364 (3.6) | 0.004 |
| Follow-up (%) | |||||
| Complications | 101/209 (48.3) | 88/250 (35.2) | 71/196 (36.2) | 74/279 (26.5) | <0.001 |
| Rehospitalization | 18/265 (6.8) | 17/288 (5.9) | 21/238 (8.8) | 35/334 (10.5) | 0.158 |
| Death | 46/195 (23.6) | 28/242 (11.6) | 20/189 (10.6) | 15/274 (5.5) | <0.001 |
Complications occurred in 35.8% of the study population during follow-up, and 8.1% required rehospitalization. Overall mortality at one-year follow-up was 12.1%.
Comparing different quartiles of Hb, anemic patients tended to have more complications during follow-up and higher one-year mortality (Table 3).
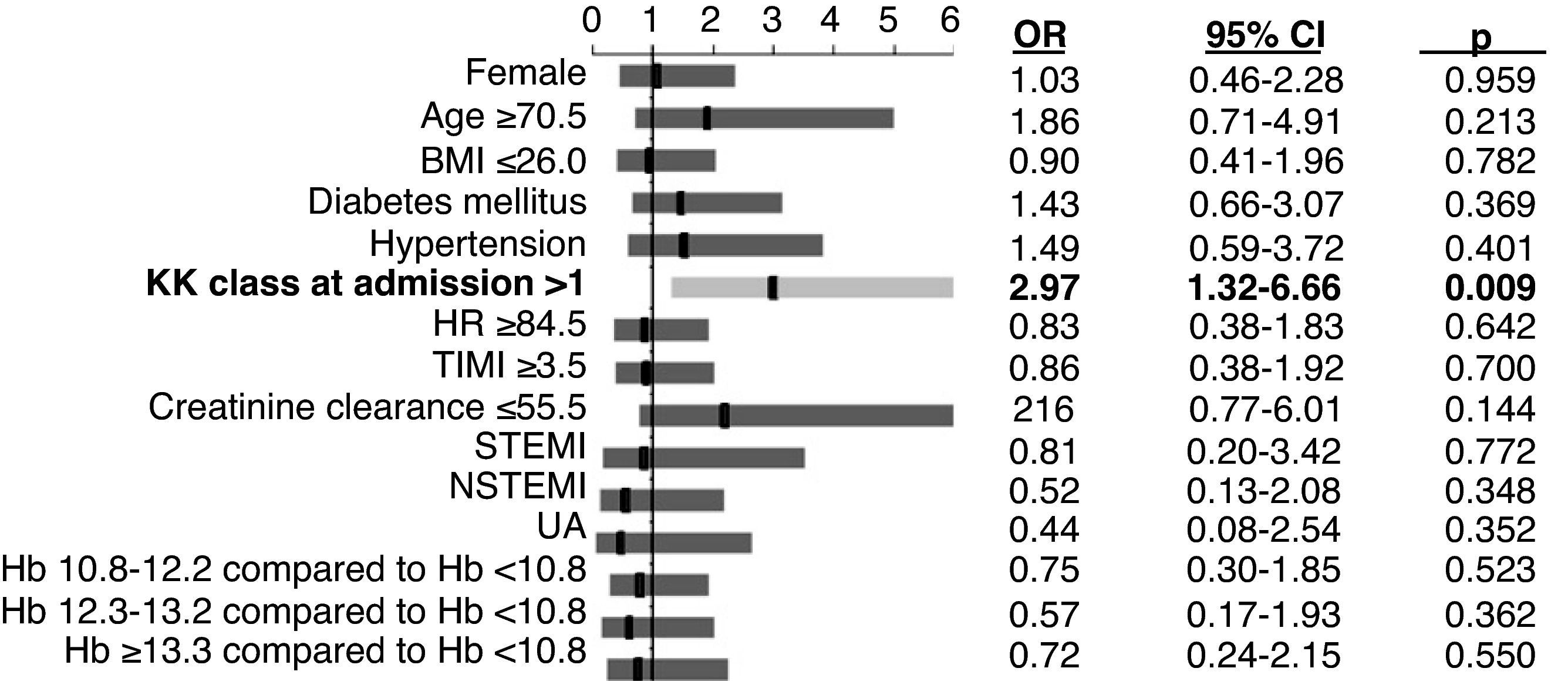
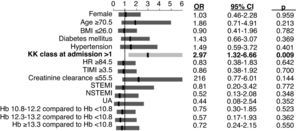
Independent predictors of mortalityIn multivariate logistic regression analysis, the only independent predictor of in-hospital mortality was Killip class >1 at admission (Figure 4).
Hb <10.8g/dl was one of the strongest independent predictors of long-term mortality, the others being age ≥69.5 years, Killip class >1 at admission, diabetes mellitus and ST-segment depression on admission ECG (Figure 5).
DiscussionOur population was similar in terms of age and gender distribution, prevalence of diabetes mellitus and cardiovascular history to those in international multicenter studies on ACS.1,8
The present study confirmed that anemia is a common comorbidity in ACS patients, and is associated with greater morbidity and cardiovascular mortality. In this unselected real-world population, the prevalence of anemia at admission for ACS (65.6%) was above the highest values in published series. In most studies in this area the incidence of anemia ranges between 23% and 42%, lower rates being reported in clinical trials with selective patient recruitment.3,9–12 One possible explanation for the higher incidence in our population may be the fact that it was a single-center study in a tertiary hospital, which thus frequently receives more severe cases referred from other hospitals.
In our study, anemia was associated with more elevated troponin I, higher Killip class at admission and a greater tendency for ST-segment depression on ECG, which when taken together suggest that patients with ACS and anemia may suffer more severe ischemia, and hence have worse short- and long-term prognosis.
Low Hb was frequently associated with other comorbidities in our analysis. The greater incidence of anemia in diabetic patients may be explained by the high prevalence of renal dysfunction in diabetes, associated with reduced erythropoietin production and an impaired hemopoietic response to this hormone. The prevalence of diabetes increases with advancing age, which may also help explain the association between anemia and diabetes, both of which are more common in the elderly,10 as are hemopoietic disorders and other conditions such as cancer and bleeding diathesis.
We also found that systemic inflammation, as reflected in C-reactive protein levels, was more marked among anemic patients. Elevated inflammatory parameters may have some pathophysiological significance in this instance, since pro-inflammatory cytokines inhibit erythropoietin production, leading to reduced red blood cell production. At the same time, inflammation by itself carries a higher risk of atherothrombosis and thus worse long-term cardiovascular prognosis.5
Chronic use of certain drugs may also have contributed to low Hb concentrations in our population. Use of ACE inhibitors was significantly more common in patients with low Hb, and the link between these agents and anemia is well established, which is probably related to increased metabolism of erythropoietin mediated by ACE inhibitors.13 Prior to admission 45% of patients were also taking aspirin, an important factor in these patients’ higher risk of bleeding complications. The literature also describes the possible suppression of bone marrow activity and decreased red blood cell production as a rare secondary effect of certain antiplatelet agents such as clopidogrel.5,14
In recent years, several studies have investigated the possible association between low Hb levels and worse short- and long-term cardiovascular prognosis. As found in our study, various analyses have reported higher rates of ischemic events with lower Hb levels.5,14 Our results regarding long-term mortality are generally in agreement with most studies to date.
In contrast to our analysis, Nikolsky et al. and Meneveau et al. concluded that anemia was an independent predictor of in-hospital mortality, with odds ratios (OR) of 1.43, 3.26 and 2.27, respectively.12,15 Archbold et al. demonstrated that anemia was an independent predictor of left ventricular dysfunction and thus of worse prognosis, but as in our study, no independent association was found between low Hb levels and in-hospital mortality.9
Few studies have specifically analyzed the link between anemia and long-term mortality. In agreement with our results, Nikolsky et al. demonstrated that the presence of anemia in ACS patients undergoing percutaneous coronary intervention was an independent predictor of one-year mortality, with an OR of 2.38.15
Our study found that anemia was associated with increased risk of in-hospital and long-term mortalities, longer hospital stays and a higher rate of rehospitalizations. It should be noted that although anemia was associated with classic factors of poor prognosis such as diabetes mellitus, advanced age and renal dysfunction, multivariate analysis showed Hb <10.8g/dl to be one of the strongest independent predictors of mortality throughout 12-month follow-up (OR=2.3), but it was not a predictor of in-hospital mortality.
The physiological effect on the heart of anemia is due to the cardiovascular system's hemodynamic response to oxygen deprivation, by reducing afterload, increasing preload and activating the sympathetic nervous system, which in turn increases heart rate. These changes are intended to increase cardiac ejection volume in order to meet the oxygen demands of all systemic tissues, but in chronic situations they can lead to left ventricular dilatation and eccentric hypertrophy. Continuous increases in blood flow can also predispose to adaptive arterial remodeling and hypertrophy, resulting in arterial wall thickening and systolic hypertension. All these changes may be directly linked to increased risk for chronic arterial disease and thus worsen prognosis in patients with ACS and anemia.10,16
Diabetes mellitus was also identified as a predictor of one-year mortality, which highlights the importance of changes in glucose metabolism and their control for prognosis in these patients. Other independent predictors of one-year mortality were ST-segment depression, probably due to more severe ischemia, age ≥69.5 years (after which the presence of several comorbidities is common), and Killip class >1 at admission. The latter was also the only predictor of in-hospital mortality in our study.
Despite advances in management of ACS patients in recent years, most studies report mortality of between 2.0% and 13.6%.4,8–11
In-hospital mortality in our population was 5.9%, while at 12-month follow-up it was 12.1%. The higher in-hospital mortality in our study compared to the 4.4% reported in the last Euro Heart Survey on ACS (ACS-II, 2004) may be explained by the high incidence of anemia in our population, the fact that all forms of ACS presentation (STEMI, NSTEMI and UA) were included, and that it was performed in a tertiary hospital that would tend to receive more severe cases.18
There was greater use of most drugs recommended for ACS in our population than in international multicenter studies.4,8 However, as in other reference studies, antiplatelets (aspirin and clopidogrel) and beta-blockers were prescribed less in patients with low Hb levels, prescription rates of antiplatelets still being lower in these patients at discharge. Antiplatelet therapy has been recommended for some years in the guidelines, but although it reduces ischemic events, it is also associated with a greater risk of bleeding complications, which may mean that patients with anemia receive suboptimal treatment.12,17
Our study confirmed that anemia is common in ACS patients and identified it as a potentially modifiable risk factor. However, the question remains as to whether correcting anemia during the acute phase of ACS improves cardiovascular outcomes. Blood transfusions can in fact have an immunomodulatory effect that results in increased risk of infection, hemolytic reactions, volume overload, and infusion of procoagulant and proinflammatory cell products, promoting arterial thrombosis.14,16
While uncertainties remain as to the ideal Hb level in patients with ACS, anemia does appear to be associated with adverse short- and long-term cardiovascular outcomes, and so its inclusion in risk scores could be advantageous.
We believe that assessing Hb concentration, an accessible laboratory parameter that is usually measured in all patients at admission, could enable therapeutic strategies to be tailored to the individual, and improve the poor prognosis of ACS patients with anemia.
Study limitationsThis was a single-center study based on data collected retrospectively of patients admitted to a teaching hospital, who probably represented more severe and complex cases of ischemic heart disease, and so may not accurately reflect the majority of ACS patients.
Furthermore, the etiology of anemia in our study population was not specified, and it was therefore not possible to establish a causal relationship between different underlying pathophysiological entities and different types of anemia (microcytic, normochromic and macrocytic), and worse prognosis.
Notwithstanding these limitations, we consider that the study makes an important contribution, since similar studies are few and their results are inconsistent.
ConclusionDespite advances in the management of ACS patients, anemia is common and associated morbidity and mortality remain high. The present study identified anemia as an independent predictor of long-term mortality, highlighting the importance of identifying individuals with low Hb levels during initial assessment of patients with ACS.
Conflicts of interestThe authors have no conflicts of interest to declare.
The statistical analysis was performed by the National Cardiology Data Collection Center (CNCDC) of the Portuguese Society of Cardiology.
Please cite this article as: Ferreira M. Hemoglobina: um mero valor analítico ou um poderoso preditor de risco em doentes com síndromes coronárias agudas? Rev Port Cardiol; 2012. doi:10.1016/j.repc.2011.12.013.