The prevalence of type 2 diabetes (T2D) continues to increase, and its association with cardiovascular (CV) disease has led to the inclusion of CV endpoints in clinical trials on the treatment of T2D.
This article explores the various trials already performed and under development in this field, with particular focus on the EMPA-REG OUTCOME trial. In this trial, empagliflozin, a sodium-glucose co-transporter 2 inhibitor, demonstrated a reduction in CV risk in patients with T2D and established CV disease, in addition to CV safety and a decrease in glycated hemoglobin. This represents a paradigm shift that has led to changes in the international guidelines for the treatment of T2D. These results were maintained in subsequent subgroup analysis for heart failure, chronic kidney disease and peripheral arterial disease, although there are many questions concerning the mechanisms involved in these effects, including whether they are hemodynamic, metabolic or due to decreased myocardial cytoplasmic sodium concentrations.
With this reduction in risk for major CV events in patients with T2D, the EMPA-REG OUTCOME trial demonstrated CV protection from a hypoglycemic drug for the first time, and opened a new era in the treatment and management of T2D. This study has led to the development of ongoing trials that will establish which patients will benefit most from this therapy, particularly with regard to comorbidities.
A prevalência da diabetes mellitus tipo 2 (DMT2) continua a aumentar e a sua associação com a doença cardiovascular (CV) tem levado à incorporação e valorização de endpoints CV nos ensaios clínicos sobre o tratamento da DMT2.
Este artigo faz uma revisão dos vários ensaios já realizados e em desenvolvimento, neste âmbito, com especial enfoque no estudo EMPA-REG OUTCOME. Neste estudo, a empagliflozina, um inibidor do cotransportador tipo 2 de sódio-glicose (iSGLT2), demonstrou efeitos benéficos na redução do risco CV em doentes com DMT2 e doença CV estabelecida, para além de segurança CV e diminuição dos valores de HbA1c, representando uma mudança de paradigma com impacto ao nível das recomendações internacionais para o tratamento da DMT2. Estes resultados mantiveram-se em análises de subgrupos posteriores, nomeadamente na insuficiência cardíaca, doença renal crónica e doença arterial periférica, ainda que muitas questões se coloquem sobre os mecanismos envolvidos nestes efeitos - se efeitos hemodinâmicos, efeitos metabólicos ou se a diminuição das concentrações de sódio citoplasmático no miocárdio.
Com a diminuição do risco de eventos CV major em doentes com DMT2, os resultados do estudo EMPA-REG OUTCOME demonstraram, pela primeira vez, proteção CV associada ao efeito de um fármaco anti-hiperglicémico e iniciaram uma nova era no tratamento e gestão da DMT2. Este estudo levou ao desenvolvimento de outros ensaios, ainda a decorrer, que permitirão estabelecer quais os doentes que mais beneficiarão desta terapêutica, nomeadamente na relação à existência de comorbilidades.
Diabetes is among the most important chronic diseases worldwide because of its high prevalence and its associated high direct and indirect economic burden, due in large part to the development of micro- and macrovascular complications. Despite efforts made in terms of health policies and education, the prevalence of both type 1 and type 2 diabetes, especially the latter, continues to rise.1
There is an association between diabetes and cardiovascular (CV) disease, especially myocardial infarction (MI), stroke, peripheral arterial disease (PAD), cardiomyopathy and heart failure (HF).1 CV disease is the leading cause of morbidity and mortality in individuals with diabetes, especially MI and stroke, the prevalences of which are reported to be four times higher in diabetic patients.1–3 In Portugal, according to the National Diabetes Program, around a third of patients admitted for MI or stroke have diabetes, and mortality from MI in these patients is higher than in non-diabetic individuals.4
The prevalence of HF is also higher (2.5 times5) in diabetic patients, one in five of whom have HF.6 Patients with both diabetes and HF have a worse prognosis, with higher CV mortality and more and longer hospitalizations.5,7 However, although HF is known to be among the major CV events related to diabetes, it is frequently overlooked as a therapeutic target in the many trials on these conditions.7
In view of its considerable impact, diabetes is considered a major independent risk factor for CV disease, on a par with smoking, hypertension and dyslipidemia, and treatment of diabetes should include reducing CV risk.2,8 Therapeutic strategies aimed at preventing and reducing CV complications in type 2 diabetes should include glycemic control as well as a multifactorial approach controlling blood pressure, lipid profile and body weight, combating sedentary behavior and promoting smoking cessation.9 Even so, reducing CV events in type 2 diabetes is a complex challenge, since individuals with diabetes usually also have other CV risk factors.1
The standard view is that the genesis of CV risk associated with type 2 diabetes is due to a permanent hyperglycemic state, and that there is an approximately linear relationship between metabolic disturbances and vascular damage.10 However, this view has been contradicted by the fact that drugs such as dipeptidyl peptidase-4 inhibitors, sulfonylureas and insulin, despite optimizing glycemic control, do not in fact provide the hoped-for benefits in reducing CV risk.
Clinical trials on the treatment of type 2 diabetes now include CV endpoints, particularly the occurrence of MI and stroke and CV mortality, in addition to those related to metabolic control.11
State of the art in cardiovascular outcomes trials in type 2 diabetesConsiderable effort has gone into attempts to find pharmacological agents that indisputably reduce CV outcomes in patients with type 2 diabetes. However, the results of multiple trials on a range of drug classes have generally been inconsistent or insignificant, especially concerning macrovascular complications.12
There have been five landmark trials that compared the effect of intensive glycemic control with standard CV risk control in patients with type 2 diabetes: UKPDS,13 UGDP,14 VADT,15 ADVANCE16 and ACCORD.17 Despite differences between these trials in terms of stage of CV disease and follow-up periods, certain conclusions can be drawn.18
The findings of these trials suggest that intensive glucose reduction has beneficial effects on microvascular risk,13,15,16 but that there is disagreement concerning macrovascular events and mortality.14–17 All-cause mortality, mortality associated with diabetes and occurrence of MI were all decreased in overweight individuals,13 while a long-term retrospective analysis of patients in the UKPDS19 and VADT15 trials showed reductions in CV events with intensive compared to standard therapy.
The publication of a meta-analysis associating rosiglitazone, a thiazolidinedione, with a significant increase in CV events and mortality compared to other oral antidiabetic drugs, prompted debate concerning the need for a more detailed assessment of the CV effects of antidiabetic therapies.20
In view of uncertainties regarding these effects, in 2008 the US Food and Drug Administration (FDA),21 followed by the European Medicines Agency in 2012,22 issued guidance for the pharmaceutical industry on conducting clinical trials on new drugs for treating type 2 diabetes, focusing on CV outcomes. Since then, various cardiovascular outcome trials have assessed the risks and benefits of new antidiabetic therapies. Designed to assess CV safety and efficacy, mainly in type 2 diabetes, these trials include among their endpoints CV mortality, MI, stroke, hospitalization for acute coronary syndrome and urgent revascularization procedures in populations at high CV risk, including elderly patients and those with renal failure.23,24Table 1 summarizes CVOTs on antidiabetic drugs carried out since the publication of the FDA’s guidance in 2008.
Cardiovascular outcome trials carried out since the publication of Food and Drug Administration guidance in 2008.21
| Trial | Status | Drug | Therapeutic class | Intervention | Primary endpoint | No. of patients | Clinical Trials.gov identifier |
|---|---|---|---|---|---|---|---|
| EXAMINE | Completed | Alogliptin | DPP-4i | Alogliptin vs. placebo | CV death, MI, or stroke | 5380 | NCT00968708 |
| SAVOR-TIMI53 | Completed | Saxagliptin | DPP-4i | Saxagliptin vs. placebo | CV death, MI, or stroke | 18206 | NCT01107886 |
| EMPA-REG OUTCOME | Completed | Empagliflozin | SGLT2i | Empagliflozin 10 mg vs. empagliflozin 25 mg vs. placebo | CV death, MI, or stroke | 7064 | NCT01131676 |
| ELIXA | Completed | Lixisenatide | GLP-1 RA | Lixisenatide vs. placebo | CV death, MI, UA, or stroke | 6068 | NCT01147250 |
| TECOS | Completed | Sitagliptin | DPP-4i | Sitagliptin vs. placebo | CV death, MI, UA, or stroke | 14671 | NCT00790205 |
| LEADER | Completed | Liraglutide | GLP-1 RA | Liraglutide vs. placebo | CV death, MI, or stroke | 9340 | NCT01179048 |
| SUSTAIN-6 | Completed | Semaglutide | GLP-1 RA | Semaglutide 0.5 mg vs. semaglutide 1.0 mg vs. placebo | CV death, MI, or stroke | 3297 | NCT01720446 |
| FREEDOM-CVO | Completed | Exenatide via DUROS® | GLP-1 RA | ITCA 650 (exenatide via DUROS®) vs. placebo | CV death, MI, stroke or hospitalization for UA | 4156 | NCT01455896 |
| CANVAS | Completed | Canagliflozin | SGLT2i | Canagliflozin 100 mg vs. canagliflozin 300 mg vs. placebo | CV death, MI, UA, or stroke | 4418 | NCT01032629 |
| EXSCEL | Completed | Exenatide | GLP-1 RA | Exenatide once weekly vs. placebo | CV death, MI, or stroke | 14752 | NCT01144338 |
| CARMELINA | Ongoing (2018) | Linagliptin | DPP-4i | Linagliptin vs.placebo | CV death, MI, UA, or stroke | 8000 | NCT01897532 |
| PIONEER | Ongoing (2018) | Semaglutide | GLP-1 RA | Semaglutide vs. placebo | CV death, MI, or stroke | 3176 | NCT02692716 |
| TOSCA-IT | Ongoing (2018) | Pioglitazone | PPAR-γ agonist | Pioglitazone vs. sulfonylurea | CV death, MI, stroke, or coronary revascularization | 3371 | NCT00700856 |
| HARMONY Outcomes | Ongoing (2019) | Albiglutide | GLP-1 RA | Albiglutide vs. placebo | CV death, MI, or stroke | 9400 | NCT02465515 |
| REWIND | Ongoing (estimated 2019) | Dulaglutide | GLP-1 RA | Dulaglutide vs. placebo | CV death, MI, or stroke | 9622 | NCT01394952 |
| VERTIS CV | Ongoing (2019) | Ertugliflozin | SGLT2i | Ertugliflozin vs. placebo | CV death, MI, or stroke | 8000 | NCT01986881 |
| DAPA-HF | Ongoing (2019) | Dapagliflozin | SGLT2i | Dapagliflozin vs. placebo | CV death, hospitalization for HF, or an urgent HF visit | 4500 | NCT03036124 |
| CAROLINA | Ongoing (2019) | Linagliptin | DPP-4i | Linagliptin vs. glimepiride vs. placebo | CV death, MI, UA, or stroke | 6072 | NCT01243424 |
| DECLARE-TIMI58 | Ongoing (2019) | Dapagliflozin | SGLT2i | Dapagliflozin 10 mg vs. placebo | CV death, MI, or stroke | 17276 | NCT01730534 |
| CREDENCE | Ongoing (2019) | Canagliflozin | SGLT2i | Canagliflozin 300 mg vs. placebo | CKD + CV death and hospitalization for HF | 4331 | NCT02065791 |
| EMPEROR-Preserved | Ongoing (2020) | Empagliflozin | SGLT2i | Empagliflozin vs. placebo | CV death or hospitalization for HF | 4126 | NCT03057951 |
| EMPEROR-Reduced | Ongoing (2020) | Empagliflozin | SGLT2i | Empagliflozin vs. placebo | CV death or hospitalization for HF | 2850 | NCT03057977 |
| DAPA-CKD | Ongoing (2020) | Dapagliflozin | SGLT2i | Dapagliflozin vs. placebo | ≥50% sustained decline in eGFR or reaching ESRD or CV death or renal death | 4000 | NCT03036150 |
CKD: chronic kidney disease; CV: cardiovascular; DPP-4i: dipeptidyl peptidase 4 inhibitor; eGFR: estimated glomerular filtration rate; ESRD: end-stage renal disease; GLP-1 RA: glucagon-like peptide-1 receptor agonist; PPAR-γ: peroxisome proliferator-activated receptor gamma; SGLT2i: sodium-glucose transport protein 2 inhibitor; UA: unstable angina.
The first drug class on which results were reported under the new guidance was the dipeptidyl peptidase 4 inhibitors. Trials on saxagliptin,25 alogliptin26 and sitagliptin27 demonstrated CV safety, although with neutral effects on the primary CV endpoint and, in the case of saxagliptin, an increased risk of hospitalization for HF.25 Forthcoming results from the CAROLINA (linagliptin vs. glimepiride)28 and CARMELINA (linagliptin vs. placebo)29 trials may provide further information on the CV risk associated with this therapeutic class.
Publication of these trials led to a certain degree of disappointment in the medical community, since none of these antidiabetic therapies appeared to improve CV prognosis. However, the results of the EMPA-REG OUTCOME trial30 represented a paradigm shift. In this trial, empagliflozin, a sodium-glucose transport protein 2 (SLG2) inhibitor, demonstrated a reduction in CV risk in patients with type 2 diabetes and established CV disease, in addition to CV safety. This benefit was seen only three months into the study period and increased during follow-up, with a significant reduction of 38% in relative risk (RR) for CV death.30
This double-blind randomized clinical trial set out to test the noninferiority, and the possibility of superiority, of empagliflozin (10 mg or 25 mg once daily) compared to placebo, both dosages in addition to standard care (antidiabetic, antihypertensive and lipid-lowering drugs), regarding the primary outcome of CV death, nonfatal MI, or nonfatal stroke.
The results demonstrated a significant reduction in the empagliflozin group at both dosages compared to placebo in the primary outcome (10.5% vs. 12.1%; p = 0.04), mainly due to a reduction in CV death (3.7% vs. 5.9%; p < 0.001). There were also significant reductions of 32% in RR of death from any cause and of 35% in RR of hospitalization for HF in the empagliflozin group (5.7% vs. 8.3%; p < 0.001 and 2.7% vs. 4.1%; p = 0.002, respectively).30
Subsequently, the CANVAS Program31 demonstrated that another SGLT2 inhibitor, canagliflozin, reduces CV events and HF hospitalizations, evidence of the consistent benefits of this drug class. The two trials in this program enrolled patients with type 2 diabetes and high CV risk (established CV disease or multiple risk factors) who received either canagliflozin or placebo. The primary outcome was a composite of CV death, nonfatal MI or nonfatal stroke, while the secondary outcomes were death from any cause, CV death, progression of albuminuria, and a composite of CV death and hospitalization for HF. A statistically significant reduction was seen in the primary outcome (26.9 vs. 31.5 participants per 1000 patient-years; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.75-0.97; p < 0.001 for noninferiority; p = 0.02 for superiority). However, while there were reductions in the other CV outcomes (CV death, MI and fatal and nonfatal stroke), these were not statistically superior to placebo except for a lower rate of HF hospitalization in the treatment group. There was a higher risk of amputation of toes, feet, or legs and of fracture with canagliflozin than with placebo. This, and the nonsignificant reduction in CV death, were the main differences between CANVAS and EMPA-REG OUTCOME.30,32
Results are awaited from trials of other SGLT2 inhibitors (dapagliflozin and ertugliflozin) to assess possible class effects.33,34
Among the glucagon-like peptide-1 receptor agonist (GLP-1 RA) drug class, trials of liraglutide35 and semaglutide36 also confirm the beneficial effects of new drugs for the treatment of type 2 diabetes by showing reductions in risk for CV complications, although this is based on 12-month results only. Both trials showed benefits in the primary composite outcome of CV death, nonfatal MI and nonfatal stroke, although there were differences in some of these endpoints when analyzed separately, especially nonfatal MI.35,36
However, there were no significant differences from placebo in the CV endpoints assessed in the EXSCEL trial on exenatide37 or in the ELIXA trial,38 in which lixisenatide failed to show superiority to placebo in patients recently diagnosed with acute coronary syndrome. These results demonstrate that the members of the GLP-1 RA drug class have different effects and that it is therefore difficult to speak of a class effect. Results on CV safety are awaited from trials on dulaglutide39 and albiglutide,40 which may shed more light on the effects of this drug class.
Cardiovascular benefits of empagliflozin in patients with type 2 diabetes and established cardiovascular diseaseCardiovascular outcomes in the EMPA-REG OUTCOME trialThe EMPA-REG OUTCOME clinical trial30 included 7020 individuals with type 2 diabetes (glycated hemoglobin between 7.0% and 9.0% for those not treated with glucose-lowering agents and between 7.0% and 10.0% for those receiving glucose-lowering therapy before randomization) and established CV disease (coronary artery disease, PAD or history of MI or stroke), estimated glomerular filtration rate (eGFR) at least 30 ml/min/1.73 m2 and body mass index ≤45 kg/m2.
The primary composite outcome was 3-point major adverse cardiovascular events (MACE) (defined as CV death, nonfatal MI and nonfatal stroke), and the key secondary outcome was the primary outcome plus hospitalization for unstable angina. CV death, nonfatal MI, nonfatal stroke, hospitalization for HF and death from any cause were also analyzed separately.
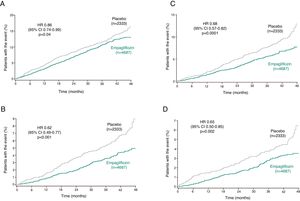
The results (Fig. 1) show a significant reduction in risk for the primary outcome in the empagliflozin group compared to placebo (HR 0.86; 95% CI 0.74-0.99; p < 0.001 for noninferiority and p = 0.04 for superiority). Compared with placebo, empagliflozin also resulted in a significantly lower risk of CV death (HR 0.62; 95% CI 0.49-0.77; p < 0.001), death from any cause (HR 0.68; 95% CI 0.57-0.82; p < 0.001) and hospitalization for HF (HR 0.65; 95% CI 0.50-0.85; p = 0.002).30
Primary and secondary outcomes of the EMPA-REG OUTCOME trial (adapted from Zinman et al.30). CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio; MI: myocardial infarction.
Empagliflozin, as compared with placebo, was also associated with reduced glycated hemoglobin as well as small reductions in weight, waist circumference, uric acid level, and systolic and diastolic blood pressure.30
Empagliflozin showed good safety and tolerability, with a similar incidence of adverse events to the placebo group, except for a higher incidence of genital infections; there were, however, no differences in overall rates of urinary infection.30
The statistically significant reductions seen in the EMPA-REG OUTCOME trial of 38% in RR for CV death, 35% for HF hospitalization and 32% for death from any cause were surprising, in terms of both their magnitude and the speed with which the curves of the two study arms diverged (within six to twelve weeks of the beginning of the trial).30
In view of the high prevalence of HF associated with diabetes and the lack of antidiabetic drugs that have been shown to reduce HF-related outcomes (occurrence, hospitalization and death),41 a post-trial analysis set out to assess HF outcomes in EMPA-REG OUTCOME in greater detail, dividing patients into subgroups of those with and without baseline HF.42
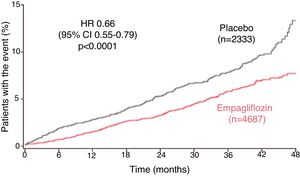
Around 10% (n = 706) of the EMPA-REG OUTCOME study population had HF at baseline. The subanalysis showed that a composite outcome of HF hospitalization or CV death occurred in a lower percentage of patients treated with empagliflozin (5.7% vs. 8.5% in the placebo group; HR 0.66; 95% CI 0.55–0.79; p < 0.001; Fig. 2Figure 2).42 This reduction was maintained after adjustment for gender, age, race, eGFR, previous HF, classes of CV medications, blood pressure and previous antidiabetic medication, demonstrating that empagliflozin’s effect is consistent among subgroups (Table 2).42 With an overall reduction in HF hospitalization and CV death of 34% (28% in those with HF at baseline and 37% in those without), according to this analysis 35 patients would have to be treated for three years to prevent one of these events.42
Time to first heart failure hospitalization or cardiovascular death in the EMPA-REG OUTCOME trial (adapted from Fitchett et al.42). CI: confidence interval; HR: hazard ratio.
Cardiovascular outcomes in patients with and without heart failure at baseline (adapted from Fitchett et al.42).
| Placebo, n (%) | Empagliflozin, n (%) | HR (95% CI) | |
|---|---|---|---|
| HF hospitalization or CV death | |||
| All patients | 198 (8.5) | 265 (5.7) | 0.66 (0.55-0.79) |
| HF at baseline | 149 (7.1) | 190 (4.5) | 0.63 (0.51-0.78) |
| No HF at baseline | 49 (20.1) | 75 (16.2 | 0.72 (0.50-1.04) |
| HF hospitalization | |||
| All patients | 95 (4.1) | 126 (2.7) | 0.65 (0.50-0.85) |
| HF at baseline | 65 (3.1) | 78 (1.8) | 0.59 (0.43-0.82) |
| No HF at baseline | 30 (12.3) | 48 (10.4) | 0.75 (0.48-1.19) |
| CV death | |||
| All patients | 137 (5.9) | 172 (3.7 | 0.62 (0.49-0.77) |
| HF at baseline | 110 (5.3) | 134 (3.2) | 0.60 (0.47-0.77) |
| No HF at baseline | 27 (11.1) | 38 (8.2) | 0.71 (0.43-1.16) |
| Death from any cause | |||
| All patients | 194 (8.3) | 269 (5.7) | 0.68 (0.57-0.82) |
| HF at baseline | 159 (7.6) | 213 (5.0) | 0.66 (0.51-0.81) |
| No HF at baseline | 35 (14.3) | 56 (12.1) | 0.79 (0.52-1.20) |
CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio.
p < 0.05 for interaction between subgroups in the different outcomes.
Another subanalysis of EMPA-REG OUTCOME assessed whether the benefit of empagliflozin was maintained, irrespective of estimated risk of HF, in patients without HF at baseline, using the Health ABC HF risk score.43 In patients taking empagliflozin without previous HF there was a reduction in HF hospitalizations and CV deaths independently of their risk category, unlike in the placebo group, in whom the number of HF hospitalizations and CV deaths increased in line with higher HF risk43 (Figure 3). The favorable effects of empagliflozin were maintained with regard to the endpoint of CV death, with a reduction in absolute risk for CV death of 4.9% in patients with baseline or incident HF and of 1.5% in those without HF burden (Table 3). A higher proportion of adverse events was seen in patients with baseline HF than in those without HF in both treatment and placebo arms.43
Five-year risk for incident HF using the Health ABC HF score at baseline assessment (adapted from Fitchett et al.43). CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio; p < 0.05 for the interaction between subgroups in the different outcomes.
Cardiovascular deaths within various subgroups of patients with baseline and/or incident heart failure in EMPA-REG OUTCOME (adapted from Fitchett et al.43).
| HF subgroup | Placebo | Empagliflozin | HR (95% CI) | Percentage of all CV deaths |
|---|---|---|---|---|
| HF at baseline (n = 706) | 11.1% | 8.2% | 0.71 (0.43–1.16) | 21.0% |
| HF hospitalization (n = 221) | 24.2% | 14.3% | 0.65 (0.35–1.22) | 13.3% |
| HF reported as AE by the investigator (n = 347) | 26.6% | 17.6% | 0.73 (0.46–1.16) | 23.9% |
| Patients with HF burden (n = 958) | 15.3% | 10.4% | 0.67 (0.47–0.97) | 37.9% |
| Patients without HF burden (n = 6062) | 4.2 | 2.7% | 0.63 (0.48–0.84) | 62.1% |
AE: adverse event; CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio.
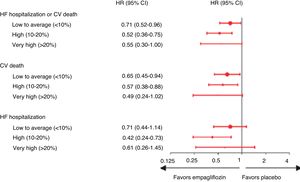
Another subanalysis of EMPA-REG OUTCOME focused on the effect of empagliflozin on the 2250 participants with chronic kidney disease (CKD) at screening.44 In these patients, empagliflozin reduced the risk of CV death by 29% compared with placebo (HR 0.71; 95% CI 0.52–0.98), the risk of all-cause mortality by 24% (HR 0.76; 95% CI 0.59–0.99), the risk of hospitalization for HF by 39% (HR 0.61; 95% CI 0.42–0.87), and the risk of all-cause hospitalization by 19% (HR 0.81; 95% CI 0.72–0.92) (Fig. 4). The occurrence of adverse events was similar in subgroups with different levels of renal function at enrollment, and urinary tract infection, acute renal failure, hyperkalemia, fractures, lower limb amputations, and hypoglycemia were not increased with empagliflozin compared with placebo.44
Cardiovascular death, hospitalization for heart failure, all-cause mortality and all-cause hospitalization in patients with and without chronic kidney disease (estimated glomerular filtration rate <60 ml/min/1.73 m2 and/or macroalbuminuria [urine albumin-to-creatinine ratio >300 mg/g]) at baseline in the EMPA-REG OUTCOME trial (adapted from Wanner et al.44). CI: confidence interval; CKD: chronic kidney disease; CV: cardiovascular; HF: heart failure; HR: hazard ratio; p < 0.05 for the interaction between subgroups in the different outcomes.
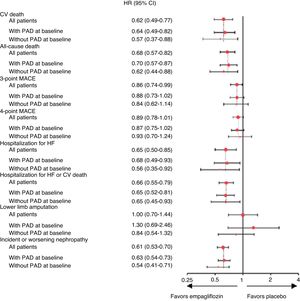
In subgroup analysis of EMPA-REG OUTCOME participants with PAD (n = 1461),45 empagliflozin also reduced CV outcomes, both 3-point MACE (defined as CV death, nonfatal MI and nonfatal stroke) and 4-point MACE (3-point MACE plus hospitalization for unstable angina) (16%, HR 0.84; 95% CI 0.62–1.14 and 7%, HR 0.93; 95% CI 0.70–1.24, respectively), as well as individual endpoints, such as reductions of 43% in CV death (HR 0.57; 95% 0.37-0.88), 38% in all-cause mortality (HR 0.62; 95% CI 0.44–0.88), 44% in HF hospitalization (HR 0.56; 95% CI 0.35–0.92) and 46% in incident or worsening nephropathy (HR 0.54; 95% CI 0.41–0.71). These results were consistent with those in the group without PAD (Fig. 5). The reduction in CV death with empagliflozin in patients with type 2 diabetes and PAD translates to a number needed to treat of 29 patients over 3.1 years to prevent one event.45 In terms of safety, empagliflozin was similar to placebo in patients both with and without baseline PAD, with no increase observed in risk for amputation.45
Cardiovascular outcomes, mortality, lower limb amputations and incident or worsening nephropathy in patients with and without baseline peripheral arterial disease in the EMPA-REG OUTCOME trial (adapted from Verma et al.45). 3-point MACE: major adverse cardiovascular effects (cardiovascular death, nonfatal myocardial infarction and nonfatal stroke); 4-point MACE: 3-point MACE plus hospitalization for unstable angina; CI: confidence interval; CV: cardiovascular; HF: heart failure; PAD: peripheral arterial disease; p < 0.05 for the interaction between subgroups in the different outcomes.
All these analyses appear to demonstrate that the beneficial results of empagliflozin in CV outcomes are also seen in more vulnerable subgroups at higher risk of CV morbidity and mortality. However, many questions remain concerning the mechanisms involved in these effects.
Possible mechanisms underlying the cardiovascular benefits of empagliflozinThe results of the EMPA-REG OUTCOME trial immediately raised questions concerning the mechanisms underlying the CV benefits of empagliflozin. Although definite proof is still lacking, proposed explanations include hemodynamic and metabolic effects and decreased myocardial cytoplasmic sodium concentrations.46,47
The immediate hemodynamic effects of volume depletion and increased hematocrit are plausible explanations for the rapidity with which the benefits of empagliflozin are observed. There appears to be agreement that hemodynamic effects are mainly responsible for the early CV benefits of empagliflozin, as reflected by reductions in blood pressure and intravascular volume and induction of osmotic diuresis.48–50 Increased diuresis may reduce circulating volume and hence cardiac pre- and afterload, increasing hemoconcentration and release of oxygen in the tissues, resulting in lower risk for HF and CV death.51
The unexpected reduction in HF hospitalizations focused attention on the mechanisms underlying empagliflozin's cardiac action.52 Basic research studies have shown a reduction in the onset and progression of cardiac hypertrophy and cardiomyopathy, explained as a result of alterations in gastrointestinal sodium reabsorption or possibly by optimization of cardiac metabolism.48 Elevated cytoplasmic sodium and calcium and decreased mitochondrial calcium in cardiomyocytes are among the pathophysiological manifestations of HF,46,52 and it has been demonstrated in rabbits and rats that empagliflozin modifies the activity of the cardiac sodium/hydrogen exchanger, one of the most important sodium transporters, reducing cytoplasmic sodium and calcium and increasing mitochondrial calcium. This mechanism has been shown to be independent of empagliflozin’s action on SGLT2,46 suggesting that the drug has additional effects.
Other authors have theorized that the metabolic changes caused by empagliflozin (reduced lipid and glucose oxidation in favor of ketone bodies) result from increased cardiac metabolic effectiveness, myocardial contractility and cardiac efficiency. It is suggested that these changes may also occur in the muscles and kidneys.53
Significantly, empagliflozin’s effect on glycemic control does not explain the differences observed. The small variation in glycated hemoglobin between EMPA-REG OUTCOME groups (only 0.4% less in treatment groups than for placebo), which is similar to the metabolic effects of dipeptidyl peptidase 4 inhibitors,25,27,54 and the early difference between groups in CV endpoints (clearly observable within only a few weeks of the beginning of the study, unlike in previous studies on other drugs in which reduction in CV risk was only seen after several years of follow-up), both weaken the hypothesis that the effects observed are due to the metabolic control induced by the drug.55
Another explanation that has gained support as the main mechanism behind the action of empagliflozin is that of metabolic changes resulting from sustained SGLT2 inhibition, with cardiorenal benefits.53 Constant loss of glucose, due to sustained reabsorption in the proximal convoluted tubule, may result in physiological adaptation of mechanisms including overactivation of SGLT1 downstream, compensating for some glucose loss; increased endogenous glucose production through increased glucagon levels and reduced serum insulin levels; systemic metabolic changes involving increased lipid oxidation and reduced dependence on glucose oxidation; and increased carbohydrate consumption in the context of reduced glycemia, insulin and body mass.53,56,57
It has also been suggested that effects at the mitochondrial level may be behind the benefits of empagliflozin, other than those observed in the EMPA-REG OUTCOME trial itself.58 The hypothesis is that under conditions of mild, persistent hyperketonemia, such as those that prevail during treatment with SGLT2 inhibitors, the resulting increased levels of circulating beta-hydroxybutyrate are responsible for the cardioprotection observed by optimizing metabolism in other organs, particularly the kidneys.58 Empagliflozin thus appears to induce metabolic changes that lead to greater energy efficiency through oxidation of ketone bodies in preference to glucose and fatty acids, enhancing cardiac and renal performance. This could explain the CV benefits observed in EMPA-REG OUTCOME after only three months of treatment.55
All of the above theories, of course, still need to be investigated further before the mechanisms underlying the CV benefit of empagliflozin are fully understood.
Safety and tolerabilityData from the EMPA-REG OUTCOME trial and its subanalyses, as well as phase I, II and III clinical trials on empagliflozin, show that it is well tolerated at both dosages (10 mg and 25 mg daily).30,59
A recent meta-analysis analyzing 15 randomized trials plus four extension studies, including EMPA-REG OUTCOME, and over 15000 patients taking empagliflozin, demonstrated a favorable benefit-risk profile.59 The number of adverse events, whether or not they were severe or led to discontinuation, was no higher in the treatment groups.
Empagliflozin was not associated with an increased risk of hypoglycemia vs. placebo, except in participants on background sulfonylurea or insulin,49,59 and the authors accordingly cautioned that a lower dose of the sulfonylurea or insulin should be considered in patients beginning treatment with empagliflozin.60
Concerning events consistent with volume depletion, safety and tolerability were similar between empagliflozin and placebo, except for a greater incidence with empagliflozin in participants aged 75 years or older and in participants receiving loop diuretics.59
As with other drugs in the SGLT2 inhibitor class, empagliflozin was associated with a higher incidence of genital mycotic infections than placebo. Regarding urinary tract infections, no differences were seen between the treatment and placebo groups in terms of incidence, severity or need fro treatment discontinuation, or in number or prolongation of hospitalizations. The incidence of such events was higher in participants aged over 65 years.59
The incidence of bone fractures, cancer events, renal adverse events, hepatic injury, acute pancreatitis, diabetic ketoacidosis and lower limb amputations in the empagliflozin group was also similar to the placebo group.59
Furthermore, a post-trial analysis of lower limb amputations in EMPA-REG OUTCOME showed that both the incidence and time to first event were similar in the empagliflozin and placebo groups, and that these findings were consistent across subgroups by established risk factors for amputation.61 This demonstrates that lower limb amputation does not result from a class effect of SGLT2 inhibitors.
The role of empagliflozin in the treatment of diabetesThe positive results of the EMPA-REG OUTCOME trial,30 as well as similarly positive results on canagliflozin,31 led to changes in the guidelines on treatment of hyperglycemia in type 2 diabetes, recommending earlier use of an SGLT2 inhibitor in patients with diabetes and CV disease.8,9 In the 2015 update to the position statement of the American Diabetes Association and the European Association for the Study of Diabetes,9 the availability of SGLT2 inhibitors was presented as the major change compared to the 2012 recommendations, representing a new therapeutic option for glucose reduction. Because their action is independent of insulin, SGLT2 inhibitors may be used at any stage of type 2 diabetes, and have additional advantages including modest weight loss and lowering of blood pressure.9
In the latest update to the American Diabetes Association guidelines for treatment of diabetes, published in 2018,62 empagliflozin is the SGLT2 inhibitor recommended as second-line therapy associated with lifestyle management and metformin in patients with type 2 diabetes, given the strong evidence that it reduces major CV events and CV mortality. Empagliflozin is presented as beneficial in patients with atherosclerotic CV disease, chronic HF and diabetic nephropathy.62
Future lines of investigation: the EMPEROR and EMPA-Kidney trialsThe pioneering nature of the EMPA-REG OUTCOME trial resides in the significant reduction of risk for major CV events in patients with type 2 diabetes it demonstrated through treatment with empagliflozin compared with other glucose-lowering drugs, both of the same class – SGLT2 inhibitors – and of other antidiabetic drug classes.50,51,58
At the same time, these results raise questions concerning the mechanisms involved in the effects observed, which undoubtedly go beyond mere glycemic control. We now need to identify which patients will benefit most from pharmacological SGLT2 inhibition, considering both duration of disease and pre-existing comorbidities. It is also necessary to determine whether the benefits of empagliflozin extend to the important area of primary prevention.10
The reduction in HF hospitalizations with empagliflozin appear to indicate that it plays a part in changing the pathophysiology of cardiorenal syndrome. The results of the EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure (EMPEROR) program,63 which will study patients with HF with reduced (EMPEROR-Reduced) and preserved (EMPEROR-Preserved) ejection fraction, with and without diabetes, may be particularly enlightening in this context. In the wake of these trials, Boehringer Ingelheim and Eli Lilly recently announced plans for two functional clinical trials that will evaluate the effect of empagliflozin on exercise ability and HF symptoms in people with chronic HF, again in patients with HF with reduced (EMPERIAL-Reduced) and preserved (EMPERIAL-Preserved) ejection fraction,64 in which Portuguese centers are expected to participate. In addition, the EMPA-KIDNEY study aims to explore the efficacy and safety of empagliflozin in CKD; it will include around 5000 participants with CKD, with and without type 2 diabetes.65
The first trial on CV outcomes with an SGLT2 inhibitor after EMPA-REG OUTCOME, CANVAS (canagliflozin),31 demonstrated similar benefits to empagliflozin, although without the same wide range of effects. The results of two more trials on SGLT2 inhibitors – CREDENCE (canagliflozin)66 and DECLARE TIMI58 (dapagliflozin)67 – are expected soon, and may shed light on some of the current theories. Three additional trials are also recruiting participants to study dapagliflozin, two including patients with HF (Dapa-HF e PRESERVED-HF)68,69 and one on patients with CKD (Dapa-CKD).70 Although it may be premature to speak of a class effect, there are growing expectations that there is a common mechanism underlying the reduction in cardiorenal risk associated with pharmacological SGLT2 inhibition.
ConclusionThere is no doubt that empagliflozin, as demonstrated by the landmark EMPA-REG OUTCOME trial, has led to a paradigm shift in the treatment of patients with type 2 diabetes, and that it has opened a new era in the treatment and management of this condition. As there now exists the possibility of affording CV protection as well as lowering blood glucose in a single drug, the choice of an antidiabetic drug for which there is also evidence of CV protection can help prevent diabetes-associated CV morbidity as well as simply treating the condition. This possibility will have beneficial effects on the epidemiology of the disease.
FundingThis work received an unrestricted grant from Boehringer Ingelheim and Eli Lilly.
Conflicts of interestPedro Monteiro was an investigator in the EMPA-REG OUTCOME trial.
Carlos Aguiar has received consulting fees from AstraZeneca, Bial Portela, Boehringer Ingelheim, Novo Nordisk, and Tecnimede.
Pedro Matos has served on the Advisory Boards of MSD and AstraZeneca and has received speaker honoraria from MSD, AstraZeneca, Novo Nordisk, and Boehringer Ingelheim.
José Silva-Nunes has received training and speaking honoraria from Boehringer Ingelheim/Eli Lilly.
Rita Birne has performed consulting services for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Novartis and Sanofi.
Patrícia Branco has no conflicts of interest with regard to Boehringer Ingelheim.
Joaquim Calado has received honoraria for scientific and medical services from Boehringer Ingelheim, Lilly and AstraZeneca in the last two years.
Miguel Melo has received funding for research projects and consulting or speaking fees from Bial, Boehringer Ingelheim, Lilly, Eisai, Janssen, Novo Nordisk, and Sanofi/Genzyme.
Jorge Polónia has no conflicts of interest to declare.
Please cite this article as: Monteiro P, et al. Efeito da empagliflozina para além do controlo glicémico: benefício cardiovascular em doentes com DMT2 e doenc¸a cardiovascular estabelecida. Rev Port Cardiol. 2019;38:721–735.











![Cardiovascular death, hospitalization for heart failure, all-cause mortality and all-cause hospitalization in patients with and without chronic kidney disease (estimated glomerular filtration rate <60 ml/min/1.73 m2 and/or macroalbuminuria [urine albumin-to-creatinine ratio >300 mg/g]) at baseline in the EMPA-REG OUTCOME trial (adapted from Wanner et al.44). CI: confidence interval; CKD: chronic kidney disease; CV: cardiovascular; HF: heart failure; HR: hazard ratio; p < 0.05 for the interaction between subgroups in the different outcomes.](https://static.elsevier.es/multimedia/21742049/0000003800000010/v2_202002180803/S2174204919302879/v2_202002180803/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)