Transthoracic echocardiography is an important tool after tetralogy of Fallot repair, of which aortic root dilatation is a recognized complication. In this study we aimed to assess its prevalence and potential predictors.
MethodsWe consecutively assessed adult patients by transthoracic echocardiography after tetralogy of Fallot repair, and divided them into two groups based on the maximum internal aortic diameter at the sinuses of Valsalva in parasternal long-axis view: group 1 with aortic root dilatation (≥38 mm) and group 2 without dilatation (<38 mm).
ResultsA total of 53 patients were included, mean age 32±10 years, with a mean time since surgery of 23±7 years. An aortopulmonary shunt had been performed prior to complete repair in 25 patients, and a transannular patch was used in 19 patients. Aortic root measurement was possible in all patients. Aortic root dilatation was identified in eight patients (15%), all male. Male gender (p=0.001), body surface area (1.93±0.10 vs. 1.70±0.20 m2, p=0.003) and increased left ventricular end-diastolic diameter (p=0.005) were predictors of aortic root dilatation. None of the surgical variables studied were predictors of aortic root dilatation.
ConclusionsThe prevalence of aortic root dilatation in this cohort was low and male gender was a predictor of its occurrence. The type of repair and time to surgery did not influence its occurrence.
Quantification of aortic root diameter is possible by transthoracic echocardiography; we suggest indexing it to body surface area in clinical practice.
O ecocardiograma transtorácico é fundamental na avaliação de doentes operados a tetralogia de Fallot. A dilatação da raiz da aorta é uma complicação descrita. Neste estudo, avaliámos a sua prevalência e potenciais preditores.
População e métodosEstudo consecutivo de adultos operados a tetralogia de Fallot. O diâmetro interno máximo da aorta ao nível dos seios de Valsalva (DAo) foi avaliado por ecocardiograma transtorácico, em paraesternal eixo longo. Definimos dois grupos: grupo 1 com dilatação da raiz da aorta (DAo ≥38 mm) e grupo 2 sem dilatação (DAo <38 mm).
ResultadosIncluímos 53 doentes (idade média 32 ± 10 anos); intervalo médio desde a cirurgia 23 ± 7 anos. Vinte e cinco doentes tinham um shunt sistémico pulmonar prévio e 19 tinham patch transanular. Foi possível medir a raiz da aorta em todos os doentes. Em 8 (15%) doentes, todos homens, foi identificada dilatação da raiz da aorta, sendo seus preditores o sexo masculino (p = 0,001), a superfície corporal (1,93 ± 0,10 versus 1,70 ± 0,20 m2, p = 0,003) e um maior diâmetro telediastólico ventricular esquerdo (p = 0,005). Nenhuma das variáveis cirúrgicas estudadas influenciou a dilatação da raiz da aorta.
ConclusõesA prevalência de dilatação da raiz da aorta foi baixa, sendo o sexo masculino um preditor da sua ocorrência. O tipo de cirurgia ou o tempo até à cirurgia não influenciaram o seu aparecimento.
A quantificação do diâmetro da raiz da aorta é possível por ecocardiograma transtorácico, sugerindo-se a indexação à superfície corporal na prática clínica.
aortic root diameter
aortopulmonary
body surface area
congenital heart disease
left ventricular
right atrial
right ventricular
tetralogy of Fallot
transthoracic echocardiography
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease (CHD) with survival to adulthood, and international guidelines recommend regular, long-term follow-up in centers specialized in adult CHD.1
Even after complete surgical repair, these patients present significant residual lesions, and are at risk of developing hemodynamically significant lesions, including dilatation of the right ventricle and pulmonary trunk, pulmonary and/or tricuspid regurgitation, ventricular dysfunction and potentially fatal arrhythmias.2 While less common, progressive aortic root dilatation may also occur,3,4 leading to aortic regurgitation and risk of aneurysmal dilatation and dissection of the thoracic aorta, which can be fatal5,6 and may require surgical intervention.7 Aortic dilatation appears to be due to intrinsic histological abnormalities8–11 that result in elastic and hemodynamic changes in the aorta, and to previous aortic volume overload.12–14
Transthoracic echocardiography (TTE) plays an important role in aortic assessment after TOF repair. Although new imaging techniques are increasingly used, particularly computed tomography and cardiovascular magnetic resonance imaging,15,16 their cost/benefit ratios mean that they should be used sparingly.
In this study we aimed to assess by TTE the prevalence of aortic root dilatation in adult patients with repaired TOF and to determine the demographic, clinical, surgical and imaging parameters that may be potential predictors.
MethodsThe study included 53 out of a total of 71 consecutive patients with TOF, aged ≥18 years, followed in the adult congenital heart disease clinic at the Centro Hospitalar São João, Porto, between March and December 2011. All patients gave their written informed consent in accordance with the Helsinki Declaration and the study was approved by the hospital's ethics committee.
Patients who had not been operated for anatomical reasons or who had refused surgery were excluded, as were those with moderate to severe valve aortic stenosis or regurgitation (one patient with moderate valve stenosis and two with aortic valve mechanical prostheses) or with genetic syndromes (10 with Down syndrome and one with DiGeorge syndrome); four women were also excluded due to pregnancy.
TTE imaging studies of the 53 patients were performed by a single operator specialized in CHD, using a Vivid 7 ultrasound equipment (GE Healthcare, Milwaukee, Wisconsin), and were post-processed on an EchoPac workstation. Two-dimensional TTE in parasternal long-axis view was used to measure the maximum internal diameters of the thoracic aorta at the sinuses of Valsalva, the sinotubular junction and the ascending aorta (1–2 cm above the plane of the sinotubular junction). The degree of aortic, pulmonary and tricuspid regurgitation was assessed by continuous wave and color Doppler, and was classified as absent, mild, or moderate to severe. Maximum gradients between the right ventricle (RV) and the pulmonary artery, and between the RV and the right atrium, were estimated from the peak velocity by continuous wave Doppler according to the modified Bernoulli equation. Left ventricular (LV) end-diastolic and end-systolic diameters and ejection fraction were assessed in M-mode and two-dimensional TTE using validated techniques. LV systolic function was considered impaired if ejection fraction was <55%. The RV was assessed in 4-chamber apical view in end-diastole; dilatation was considered to be present with a baseline diameter of >40 mm, being classified as normal, mild (<50 mm) or moderate to severe (≥50 mm). RV systolic function was considered impaired if tricuspid annular plane systolic excursion estimated in M-mode was <16 mm or S-wave velocity estimated by tissue Doppler at the lateral tricuspid annulus was <10 cm/s. Intraobserver variation was assessed by repeat measurement of the thoracic aorta in 10 randomly selected patients.
Demographic data and clinical and surgical records were analyzed to determine potential predictors of aortic root dilatation.
The study population was divided into two groups based on the maximum internal aortic diameter (AoD) at the sinuses of Valsalva in parasternal long-axis view: group 1 with aortic root dilatation (≥38 mm) and group 2 without dilatation (<38 mm). The mean and standard deviation of the AoD expressed as the z-score was determined by the Boston Children's Hospital equation17 and indexed to body surface area (BSA) calculated by Haycock's formula18 (Table 1).
Echocardiographic data by group.
| Group 1 (n=8) (AoD ≥38 mm) | Group 2 (n=45) (AoD <38 mm) | p | |
| Sinuses of Valsalva (mm) | 42±4 | 32±3 | <0.0001 |
| Sinuses of Valsalva index (mm/m2) | 22±1 | 19±2 | 0.002 |
| Sinuses of Valsalva z-score | 3.90±0.62 | 2.02±0.93 | <0.0001 |
| Sinotubular junctiona (mm) | 34±7 | 31±3 | 0.35 |
| Ascending aortab (mm) | 39±6 | 32±3 | 0.04 |
| LV end-diastolic diameter (mm) | 52±3 | 46±5 | 0.005 |
| LV ejection fraction (%) | 61±5 | 65±9 | 0.51 |
| Moderate to severe RV dilatation | 4 (50) | 27 (60) | 0.70 |
| RV systolic dysfunction | 2 (25) | 6 (13) | 0.59 |
| Moderate to severe pulmonary regurgitation | 3 (37) | 24 (53) | 0.47 |
| Moderate to severe tricuspid regurgitation | 1 (12) | 3 (7) | 0.49 |
| RV/PA peak gradient | 25±12 | 22±11 | 0.46 |
| RV/RA peak gradient | 36±17 | 32±9 | 0.55 |
AoD: aortic diameter; LV: left ventricular; PA: pulmonary artery; RA: right atrial; RV: right ventricular. Values expressed as mean±standard deviation or n (%).
The statistical analysis was performed using SPSS version 19.0 (Chicago, Ill, USA). Categorical variables are presented as frequencies and percentages and compared using the chi-square test or Fisher's exact test as appropriate. Continuous variables are presented as mean±standard deviation and compared by the Student's t test. A value of p<0.05 was considered statistically significant.
ResultsThe study included 53 patients, mean age 32±10 years, of whom 30 (57%) were women. Most patients (83%) were in New York Heart Association (NYHA) functional class I, eight (15%) were in class II, and only one was in class III. Mean time since surgery was 23±7 years. An aortopulmonary (AP) shunt had been performed in 25 (47%) patients a median of four years (1–22) prior to complete TOF repair, and a transannular patch was used in 19 (53%). No patient had intracardiac shunt or hemodynamically significant residual pulmonary stenosis on echocardiographic assessment. Thirty-one patients had moderate to severe RV dilatation (end-diastolic diameter ≥50 mm), with RV systolic dysfunction in eight (15%) and LV systolic dysfunction in three (6%). Pulmonary regurgitation was moderate to severe in 27 patients (51%), but only four patients had moderate to severe tricuspid regurgitation. Aortic root measurement was possible in all patients (Table 1). There was an excellent correlation (p<0.0001) between maximum values and those indexed to BSA and z-scores estimated by the Boston Children's Hospital formula (Figures 1 and 2).
Eight patients (15%), all male, had aortic root dilatation (group 1), of whom six were asymptomatic, one was in NYHA class II and one was in class III. Only one of the 10 patients with right aortic arch presented aortic root dilatation. Of the variables analyzed (Table 2), only male gender (p=0.001), BSA (1.93±0.10 vs. 1.70±0.20 m2, p=0.003) and LV end-diastolic diameter (52±3 vs. 46±5 mm, p=0.005) were predictors of aortic root dilatation. None of the surgical variables assessed (previous AP shunt, transannular patching, time between AP shunt and surgical repair, and time to TOF repair) were predictors of aortic root dilatation.
Predictors of aortic root dilatation at the sinuses of Valsalva.
| Group 1 (n=8) (AoD ≥38 mm) | Group 2 (n=45) (AoD <38 mm) | p | |
| Gender (M:F) | 8:0 | 15:30 | 0.001 |
| Age (years) | 34±12 | 32±9 | 0.45 |
| AP shunt (%) | 2 (25) | 23(51) | 0.26 |
| Transannular patcha (%) | 1(12) | 18(51) | 0.08 |
| Right aortic arch | 1(12) | 9 (20) | 1.0 |
| LV end-diastolic diameter (mm)Time shunt to TOF repairb (years) | 52±37 (4–11) | 46±53 (1–22) | 0.0050.29 |
| Time to TOF repair (years)Body surface area (m2) | 8 (3–49)1.93±0.10 | 5 (2–39)1.70±0.20 | 0.100.003 |
| Time since TOF repair (years) | 20±10 | 23±6 | 0.25 |
AoD: aortic diameter; AP: aortopulmonary; F: female; LV: left ventricular. M: male. Values expressed as mean±standard deviation or n (%), or median (minimum-maximum).
Measurement of AoD at the sinuses of Valsalva by TTE in parasternal long-axis view was possible in all patients in this study, confirming that it is an important tool in the assessment of patients after TOF repair.
Aortic dilatation in patients following TOF repair was first described by Capelli et al. in 1982.19 One possible pathophysiological mechanism for its occurrence is LV and aortic overload over a period of years, secondary to the right-to-left shunt resulting from the combination of ventricular septal defect and RV outflow tract obstruction and/or pulmonary stenosis.
The type of surgery, including palliative AP shunt prior to repair, and the time before surgical correction did not influence the occurrence of aortic root dilatation in our study population, which suggests that this complication is dependent on pre-existing factors, such as the degree of pulmonary stenosis and extent of right-to-left shunt, or individual patient characteristics, including genetics. All the patients in group 1 were men, confirming the previously reported relationship between male gender and aortic root dilatation3; this is relevant since there is no difference in the gender distribution of TOF patients. In our study, male gender remained statistically significant even after indexing of AoD to BSA (p=0.002); since greater BSA is associated with larger AoD, it is important that it should be indexed, as this has implications for serial assessments during long-term follow-up.
The z-score of the sinuses of Valsalva, a standard measure of AoD in a population, was used to define groups 1 and 2 (3.90±0.62 vs. 2.02±0.93, p<0.0001), but basing this calculation on the Boston Children's Hospital formula17 may have introduced error in interpreting the data obtained, since it involved extrapolation of AoD in a pediatric population to an adult population.
In a study by Niwa et al. on adult patients with TOF repair and patients with pulmonary atresia, the prevalence of aortic root dilatation was 15%, and male gender, pulmonary atresia, right aortic arch, and a longer shunt-to-repair interval were predictors of its occurrence.3 However, right aortic arch is more prevalent in pulmonary atresia, and aortic dilatation would be expected in these patients. Our study also showed a higher prevalence of aortic root dilatation in men but did not show a statistically significant association with longer shunt-to-repair interval, although only 25 (47%) of our patients had undergone a palliative shunt prior to TOF repair, as opposed to 56% in the above study.
Besides the possible contribution of histological abnormalities in the aorta wall that are present from infancy in patients with TOF,9 the chronic systemic volume overload that has been postulated as the pathophysiological mechanism for aortic dilatation in this context would suggest that longer time to surgical repair, with or without a previous palliative shunt, would predispose to dilatation, although this was not seen in our study. Furthermore, use of a transannular patch in intracardiac TOF repair may indicate a higher degree of pulmonary obstruction, and hence a larger systemic shunt through the ventricular septal defect, which would also predispose to aortic dilatation in the long term. In our study, transannular patching was not statistically significant as a risk factor for aortic root dilatation in adulthood, but detailed information on the type of surgery performed was only available for 36 patients.
Study limitationsThe study analyzed patients followed in specialist consultations for CHD in a tertiary referral hospital in northern Portugal, which may have meant that the cases were more complex.
The AoD threshold of <38 mm used to define the group without aortic root dilatation could be considered arbitrary.
Finally, the small size of the sample and the unavailability of detailed information for some patients on the type of repair could have affected the conclusions.
Despite these limitations, in view of the lack of published studies, we feel the study makes a valid contribution to a better characterization of aortic dilatation after TOF repair.
ConclusionsThe prevalence of aortic root dilatation in this cohort was low, and male gender was a predictor of its occurrence. The risk of developing aneurysmal dilatation or dissection of the aorta years after surgical repair of TOF highlights the importance of regular assessment of AoD in these patients.
Quantification of AoD is possible by TTE, and we suggest indexing it to BSA in clinical practice; prompt diagnosis of this complication will help to prevent a potential cause of death in these patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cruz C, et al. Avaliação ecocardiográfica da dilatação da raiz da aorta em doentes adultos operados a tetralogia de Fallot. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.10.010.








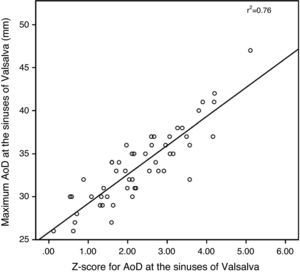 AoD: aortic diameter.'/>
AoD: aortic diameter.'/>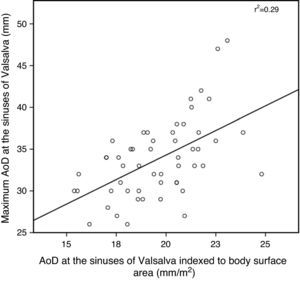 AoD: aortic diameter.'/>
AoD: aortic diameter.'/>

