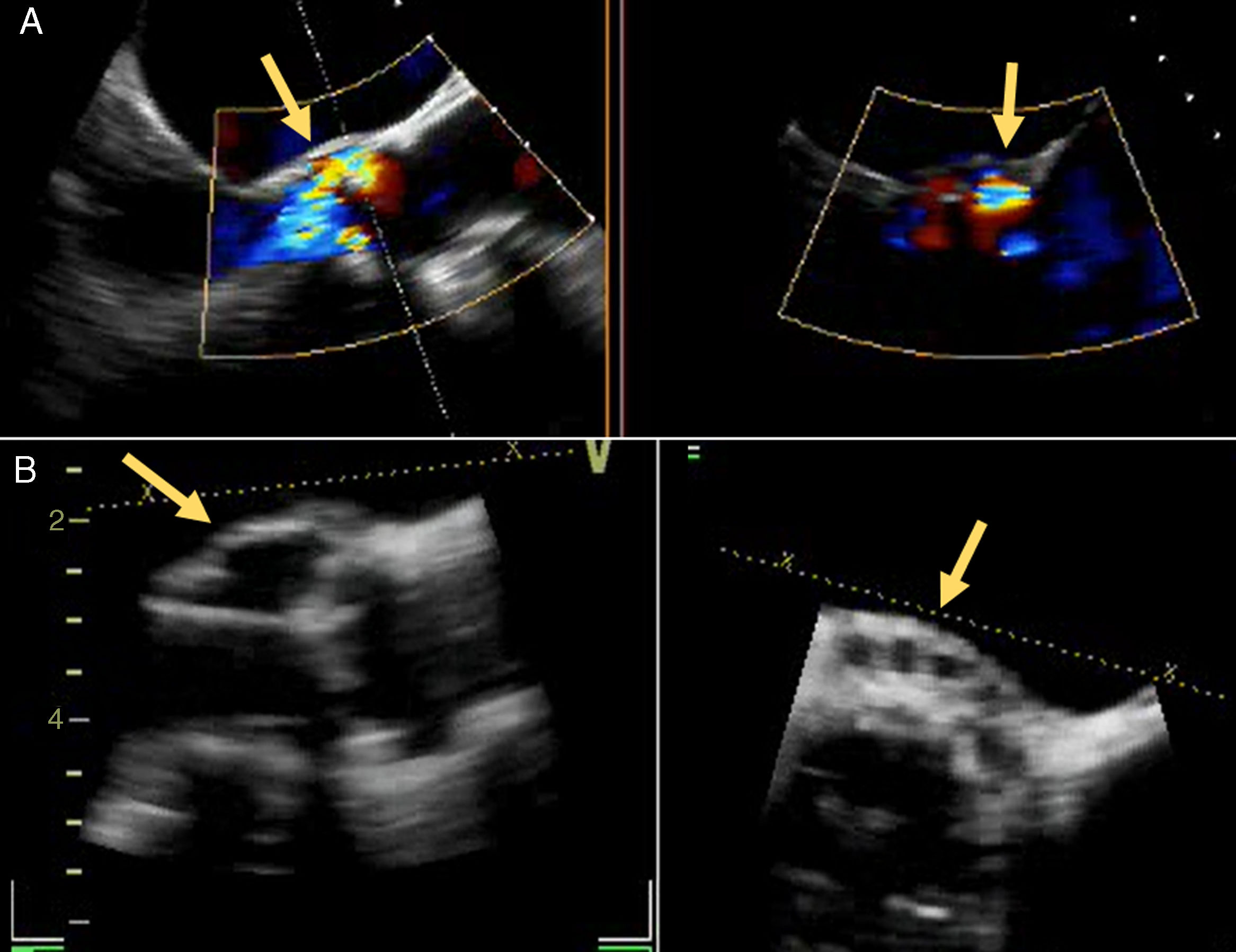
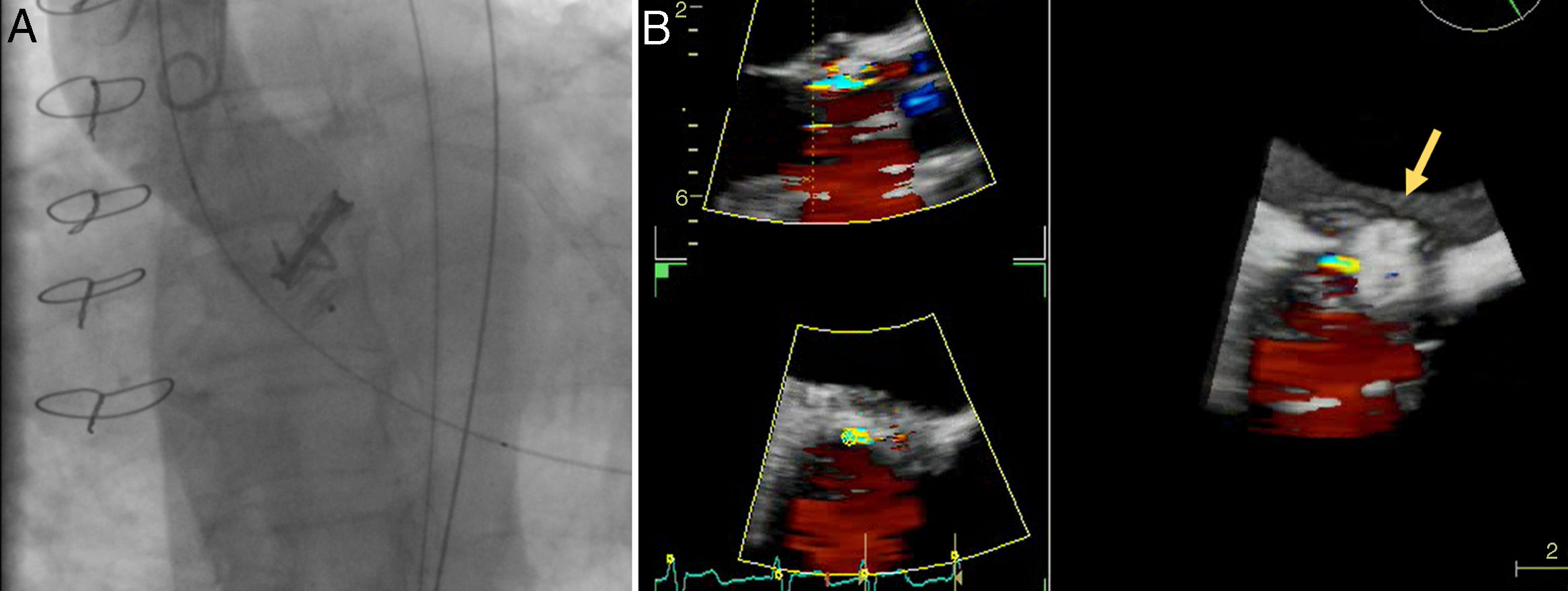
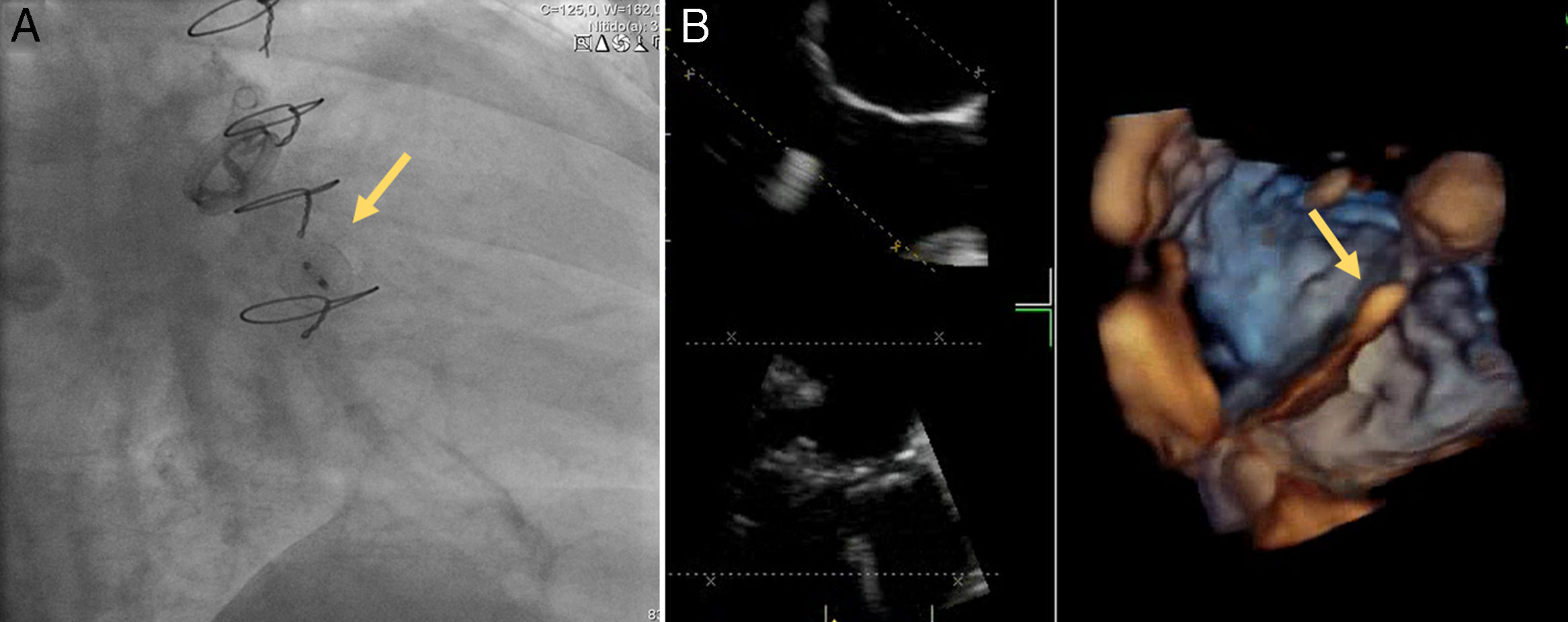
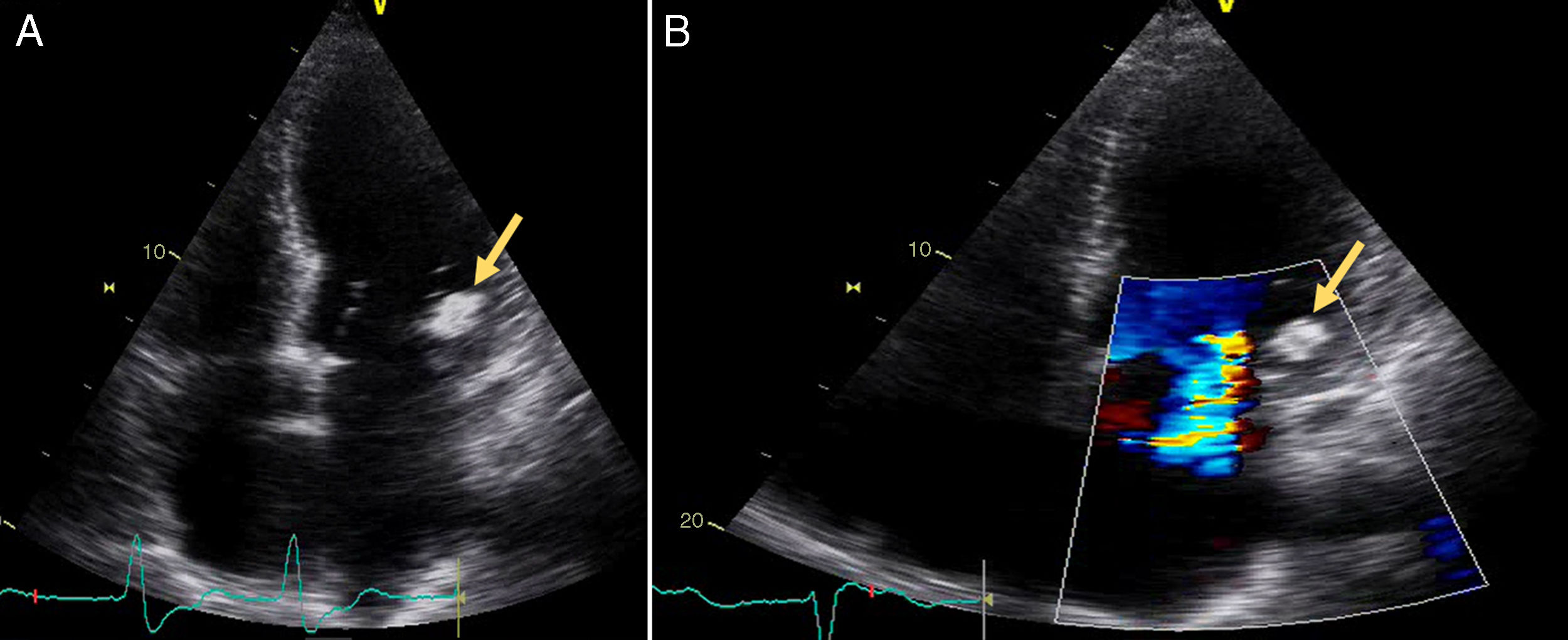
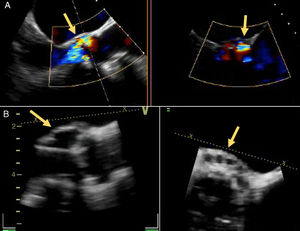
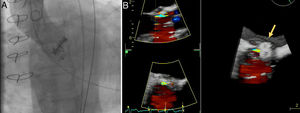
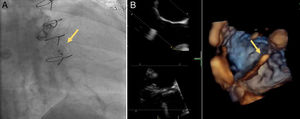
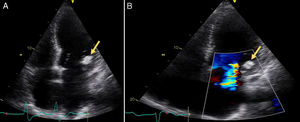
A 72-year old woman with a history of mechanical aortic prosthesis since 2010 and early infective prosthetic endocarditis (medical treatment) was hospitalized for acute heart failure in February 2015. Transesophageal echocardiography showed a severe paravalvular leak in the posterior portion of the aortic prosthesis (Figure 1A, arrow) and a pseudoaneurysm with fistulization to the left ventricle (LV) and aorta (Figure 1B, arrow), moderate mitral regurgitation and mild LV systolic dysfunction. Surgery was considered high risk and percutaneous closure of the paravalvular leak was attempted. The procedure was guided by fluoroscopy and transesophageal echocardiography. After confirming the stability of the device (Amplatzer® Vascular Plug II 12 mm/9 mm) and reduction of the paravalvular leak without functional compromise of the mechanical prosthesis (Figures 2A and 2B, arrow), the device was released. Minutes after deployment, the device migrated into the LV (Figure 3A, arrow and Supplementary data online, Movie S1). The device could not be retrieved (Figure 3B, arrow). Echocardiography images showed the device lodged beneath the posterior mitral leaflet, entrapped in the subvalvular mitral apparatus, with no significant compromise of mitral valve function (Figures 4A, arrow and 4B, arrow and Supplementary data online, Movie S2). Follow-up echocardiographies showed the device still in the same position. The patient died of sepsis due to a Clostridium difficile infection 58 days after admission (one month after the procedure). This case illustrates a rare and dramatic complication of percutaneous leak closure, but without significant hemodynamic impact on mitral valve and LV function.
The authors have no conflicts of interest to declare.