To project the long-term cost-effectiveness of treating non-valvular atrial fibrillation (AF) patients for stroke prevention with rivaroxaban compared to warfarin in Portugal.
MethodsA Markov model was used that included health and treatment states describing the management and consequences of AF and its treatment. The model's time horizon was set at a patient's lifetime and each cycle at three months. The analysis was conducted from a societal perspective and a 5% discount rate was applied to both costs and outcomes. Treatment effect data were obtained from the pivotal phase III ROCKET AF trial. The model was also populated with utility values obtained from the literature and with cost data derived from official Portuguese sources. The outcomes of the model included life-years, quality-adjusted life-years (QALYs), incremental costs, and associated incremental cost-effectiveness ratios (ICERs). Extensive sensitivity analyses were undertaken to further assess the findings of the model. As there is evidence indicating underuse and underprescription of warfarin in Portugal, an additional analysis was performed using a mixed comparator composed of no treatment, aspirin, and warfarin, which better reflects real-world prescribing in Portugal.
ResultsThis cost-effectiveness analysis produced an ICER of €3895/QALY for the base-case analysis (vs. warfarin) and of €6697/QALY for the real-world prescribing analysis (vs. mixed comparator). The findings were robust when tested in sensitivity analyses.
ConclusionThe results showed that rivaroxaban may be a cost-effective alternative compared with warfarin or real-world prescribing in Portugal.
Estimar o rácio custo-efetividade a longo-prazo associados à utilização de rivaroxabano na prevenção de acidente vascular cerebral em doentes com fibrilhação auricular (FA) não-valvular relativamente a varfarina em Portugal.
MétodosFoi utilizado um modelo de Markov que representa os estádios representativos da progressão da FA e do seu tratamento. O horizonte temporal modelizado descreve o tempo de vida dos doentes e cada ciclo tem a duração de três meses. A análise foi desenvolvida na perspetiva da sociedade, tendo sido aplicada uma taxa de atualização de cinco por cento para custos e consequências. Os efeitos do tratamento foram obtidos no ensaio clínico de fase III ROCKET AF. Adicionalmente, no modelo foram incluídos valores de utilidade provenientes da literatura e estimativas de custos nacionais. Os outcomes avaliados no modelo incluem anos de vida incrementais, anos de vida ajustados pela qualidade de vida incrementais (AVAQ), custos incrementais e rácio custo-efetividade incremental (RCEI). Foram desenvolvidas análises de sensibilidade com o objetivo de avaliar os resultados do modelo. A evidência existente indica subutilização e subprescrição de varfarina em Portugal e, por esta razão, foi desenvolvida uma análise adicional com um comparador misto, constituído por não tratamento, ácido acetilsalicílico e varfarina, o que reflete melhor o «mundo real de prescrição».
ResultadosRCEI obtido varia entre 3 895€/AVAQ para o cenário-base (relativamente varfarina) e 6 697€/AVAQ para o «mundo real de prescrição» (relativamente comparador misto). As análises de sensibilidade demonstraram que os resultados são robustos.
ConclusãoOs resultados sugerem que rivaroxabano pode constituir uma alternativa custo-efetiva comparativamente a varfarina ou «mundo real de prescrição» em Portugal.
atrial fibrillation
American Heart Association/American Stroke Association
cost-effectiveness analysis
congestive heart failure, hypertension, age ≥75, diabetes, stroke (doubled)
congestive heart failure or left ventricular dysfunction, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74, and sex category (female)
confidence interval
clinically relevant non-major
extracranial
European Society of Cardiology
Fibrilhação Auricular em Portugal (Atrial Fibrillation in Portugal)
hazard ratio
intracranial
incremental cost-effectiveness ratio
international normalized ratio
life-years
myocardial infarction
network meta-analysis
oral anticoagulants
one-way sensitivity analysis
probabilistic sensitivity analysis
quality-adjusted life-years
systemic embolism
safety on-treatment
vitamin K antagonists
Atrial fibrillation (AF) is the most common sustained cardiac rhythm disorder and constitutes an important risk factor for stroke,1,2 associated with a five-fold risk3: 15% of all strokes are attributable to AF and around 25% of patients with ischemic stroke present AF.4,5
The prevalence of AF in the general population in developed countries is approximately 1.5–2%. In addition, the mean age of patients with this condition is steadily rising, such that it now averages between 75 and 85 years.3 According to the FAMA (Atrial Fibrillation in Portugal) study, the prevalence of AF in the Portuguese population aged 40 and over is 2.5% (95% confidence interval [CI]: 2.2–2.8%) and increases with age, reaching 6.6% for individuals aged 70–79, and 10.4% for those aged 80 or more.6
Guidelines for stroke prevention in AF have been developed to encourage best practice and a systematic approach to treatment by physicians, with the intention of achieving the best outcome for the AF patient. The clinical guidelines on antithrombotic therapy for AF issued by the Portuguese National Coordinating Body for Cardiovascular Disease,7 the European Society of Cardiology (ESC)3 and the American Heart Association/American Stroke Association (AHA/ASA)8 are generally accepted by the Portuguese medical community. Overall, these guidelines consider oral anticoagulants (OACs) the cornerstone of thromboembolic prevention in AF. Vitamin K antagonists (VKAs), primarily warfarin, are widely regarded as the current standard of care.
A meta-analysis of trials on antithrombotic therapy in the prevention of stroke in non-valvular AF shows that VKAs significantly reduce the risk of stroke by 64% vs. placebo.9 Although effective, VKAs have limitations that make patient management challenging in practice.10 For instance, the anticoagulation response to VKA treatment is unpredictable and is affected by genetic and environmental factors such as drug-drug and food-drug interactions. The high inter- and intra-patient variability in response to therapy means that frequent blood tests for international normalized ratio (INR) monitoring (an INR 2.0–3.0 is recommended) and dose adjustments are necessary.11 The 2012 ESC guidelines state that new OACs such as rivaroxaban are generally preferable to VKAs in patients with non-valvular AF, when used as studied in the clinical trials performed to date.3
Over the past decade, risk factor scoring systems have been widely used to stratify the risk of thromboembolism in non-valvular AF. The simplest risk assessment scheme is the CHADS2 score (congestive heart failure, hypertension, age ≥75, diabetes, stroke [doubled]).12 The CHA2DS2-VASc score (congestive heart failure or left ventricular dysfunction, hypertension, age ≥75 [doubled], diabetes, stroke [doubled], vascular disease, age 65–74, and sex category [female]), which considers additional risk factors, more accurately identifies patients at very low risk for stroke and is recommended in the ESC 2012 guidelines.3
Rivaroxaban (Xarelto®) is a new OAC; it is a highly selective, oral, once daily, direct factor Xa inhibitor. Rivaroxaban has predictable pharmacokinetics and pharmacodynamics, few relevant drug interactions, and does not require monitoring of coagulation parameters.13 The efficacy and tolerability of rivaroxaban for prevention of stroke and systemic embolism (SE) in AF patients has been compared with warfarin in the ROCKET AF (Rivaroxaban Once daily oral direct Factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) trial. ROCKET AF is a multinational, phase III, randomized, double-blind, multicenter, event-driven study, involving 14 264 patients in whom treatment with OACs was recommended. In the primary analysis, rivaroxaban demonstrated a 21% risk reduction in event rate for stroke and SE (hazard ratio [HR] 0.79, 95% CI 0.66–0.96, p<0.001 for non-inferiority in the per-protocol, as-treated population and HR 0.79; 95% CI 0.65–0.95, p=0.02 for superiority in the safety, as-treated population) compared with warfarin. Additionally, rates of intracranial (IC) bleeding, fatal bleeding and bleeding at critical anatomical sites were significantly lower in the rivaroxaban treatment arm compared to the warfarin treatment arm (HR: 0.67, 95% CI [0.47–0.93], p<0.02 for IC bleeding; 0.50 [0.31–0.79], p=0.003 for fatal bleeding and 0.69 [0.53–0.91], p=0.007 for bleeding at critical anatomical sites). 14
The Portuguese pharmaceutical market has been affected by a series of policy measures over the last decade, including the introduction of a reference pricing system, administrative price reductions, and various changes in co-payment regulations.15 In the context of an economic crisis, health economic evaluations such as cost-effectiveness analyses (CEAs) can be useful tools to inform resource allocation decisions in a systematic, transparent and efficient manner. The main outcome of a CEA is an estimate of the costs and effects associated with the new technology compared with alternative treatment(s) such as standard of care, in order to indicate whether the new treatment provides good value for money to the health care payer. Hence, the main aim of this study was to undertake a CEA comparing rivaroxaban with warfarin for stroke prevention in patients with non-valvular AF. An additional analysis in which rivaroxaban was compared with real-world prescribing (no treatment, aspirin and warfarin) was performed, since the existing evidence indicates underuse and underprescription of warfarin in Portugal. This model supported the reimbursement request for rivaroxaban in Portugal in this therapeutic indication.
MethodsModel overviewAn analysis was performed to project the long-term cost-effectiveness of treating AF patients with rivaroxaban compared to warfarin for the prevention of stroke in the Portuguese setting. The analysis was conducted using a previously described economic model.16
The Markov model used included health and treatment states describing the management and consequences of AF. The outcomes of the model included incremental life-years (LYs), incremental quality-adjusted life years (QALYs), incremental costs, and incremental cost-effectiveness ratios (ICERs) expressed in terms of €/LY gained and €/QALY gained. The base-case analysis was conducted from a societal perspective using direct costs only. The model's time horizon was set at a patient's lifetime in order to fully incorporate the costs and consequences of AF. The mean age of patients in the model was set to 73 years and the lifetime horizon was therefore set to 20 years. Costs and outcomes were discounted at a 5% annual rate, in accordance with the most recent recommendations for economic evaluations published in Portugal.17
Patient populationThe patient population that was considered in the base-case analysis matched that of the ROCKET AF clinical trial: patients suffering from non-valvular AF with a CHADS2 score ≥2 (mean CHADS2 score 3.5), of whom 62.5% had previously been treated with VKAs. Based on the opinion of an expert panel, management of VKA treatment was assumed to be undertaken primarily in a specialist setting (65% of patients), with the remaining patients (35%) managed in a primary care setting.
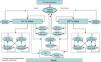
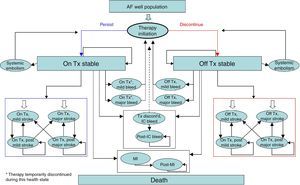
Model structureModel health statesThe model was designed to reflect the progression of AF patients through different health states up to death. A cycle length of three months was deemed short enough to adequately capture the frequency of major events. Patients entered the model with stable uncomplicated AF and received treatment with either rivaroxaban (20 mg oral tablet, once daily) or dose-adjusted warfarin (target INR of 2.0–3.0). Patients who discontinued treatment were assumed to receive aspirin irrespective of initial treatment.
In the model, patients were assumed to be always at risk of major complications unless they were currently experiencing an acute event in the same cycle. Major complications considered in this model included ischemic stroke, either minor or major, SE, myocardial infarction (MI) and bleeding. Bleeding events were categorized as major extracranial (EC), major intracranial (IC) or clinically relevant non-major (CRNM) as defined in the description of the rationale and design of the ROCKET AF trial.18 Major events were classified as transient or with permanent after-effects (Figure 1).
Boxed events were considered permanent while non-boxed events were considered transient. Each health state had a cost and utility weight describing associated quality of life as a pay-off.
Model input dataEfficacy and safety dataIn a Markov model, patients move from one health state to another with particular probabilities referred to as transition probabilities; these are primarily derived from clinical trials. In the base-case analysis, baseline event rates (i.e. the probability of moving from one health state to another) were obtained from the warfarin treatment arm of the ROCKET AF trial. Relative treatment effects describing the efficacy and safety of rivaroxaban vs. warfarin for the prevention of stroke and SE were then applied to these baseline event rates (Table 1).
Overview of assumed clinical parameters and values in the base case.
| Event | Baseline risk (three months) | RR rivaroxaban vs. warfarin | Source |
| Ischemic stroke | 0.36% (0.27–0.45) | 0.94 (0.75–1.17) | ROCKET AFa |
| MI | 0.28% (0.05–1.22) | 0.81 (0.63–1.06) | ROCKET AFa |
| SE | 0.05% (0.00–0.76) | 0.23 (0.09–0.61) | ROCKET AFa |
| Major IC bleeding | 0.19% (0.03–1.04) | 0.67 (0.47–0.93) | ROCKET AFa |
| Major EC bleeding | 0.69% (0.24–1.90) | 1.14 (0.98–1.33) | ROCKET AFa |
| CRNM bleeding | 2.97% (1.79–5.05) | 1.04 (0.96–1.13) | ROCKET AFa |
Persistence is a measure of whether patients continue or discontinue their current treatment. Given that limited data for real-life persistence were available for rivaroxaban, the model used discontinuation rates for the initial cycle of 8.9% and 8.0% for rivaroxaban and warfarin, respectively, from the ROCKET AF trial. In subsequent cycles it was 4.39% and 4.46%, respectively. In the model, patients who had discontinued treatment transitioned into a mirror health state. Patients who discontinued were assumed to switch treatment to aspirin.
MortalityIn the model, mortality was accounted for in two ways: by considering age-adjusted mortality rates from the Portuguese general population and by capturing event-related mortality. For general population mortality rates, life tables published by the Portuguese National Institute of Statistics19 were used. In addition, after experiencing a major ischemic stroke, major bleeding event (EC or IC) or MI, it was assumed that patients may die with a probability equal to the case-fatality rate observed in ROCKET AF. Minor ischemic stroke, SE and CRNM bleeding events were assumed to have no associated case fatality. Patients who had experienced a major ischemic stroke or MI were assumed to have an increased mortality rate, despite recovery from the acute episode, as the evidence shows extended impact on life expectancy. Since post-recovery mortality data were not available from the ROCKET AF trial, these mortality rates were derived from the literature.5,20 For post-IC bleeding, since no mortality rates were identified in the literature, the increase in subsequent mortality rates was assumed to be equal to that following a major ischemic stroke5 (Table 2).
Mortality rates.
| Health state | Three-month mortality (95% CI) | Source |
| Major stroke | 12.60 (9.40–15.70%) | ROCKET AF (SOT) |
| Post-major stroke | 2.63 (0.91–13.50%) | Marini et al.5 |
| Major EC bleed | 1.55 (1.16–1.94%) | ROCKET AF (SOT) |
| IC bleed | 38.80 (29.14–48.56%) | ROCKET AF (SOT) |
| Post-IC bleed | 2.63 (0.91–13.50%) | Marini et al.5 |
| MI | 9.69 (7.27–12.11%) | ROCKET AF (SOT) |
| Post-MI | 2.68 (0.00–6.75%) | Hoit et al.20 |
CI: confidence interval; EC: extracranial; IC: intracranial; MI: myocardial infarction; SOT: safety-on-treatment.
The term utility refers to the preferences individuals or society may have for any particular set of health outcomes.21 Utility analysis is a particularly useful technique in health economic evaluations because it allows for quality of life adjustments to a given set of treatment outcomes while simultaneously providing a generic outcome measure for comparison of costs and outcomes in different programs. The generic outcome is usually expressed using QALYs. A QALY is derived by adjusting the length of time affected through the health outcome by the utility value of the associated health status21 and is calculated by multiplying life-years by utility scores. Due to a lack of Portuguese-specific utility values, this analysis incorporated utility values from a combination of studies in the international literature. A utility value of 0.779 was assigned to stable AF patients.22 Utility values of 0.189, 0.641 and 0.660 were assigned for the health states of major ischemic stroke, minor ischemic stroke23 and SE24 respectively, while the values 0.482 and 0.719 were considered for post major ischemic stroke and post minor ischemic stroke.25 Finally, utility values of 0.776 and 0.598 were assigned for CRNM and major EC bleeding, respectively.24
Data on costs and resource useIdentification and quantification of resource use were estimated by an expert panel. The following direct costs were considered: drug acquisition, drug monitoring costs and event-related costs. All costs were expressed in 2011 euros.
Unit costs were obtained from published Portuguese official sources, namely Orders in Council (Portaria no. 139/200926 and Portaria no. 220/201127) and reports issued by the Central Administration of the National Health System (NHS) (Analytical Accounts of NHS hospitals28).
Drug acquisition costs were estimated on the basis of the daily dose estimated by the expert panel and costs were obtained from the Portuguese Ministry of Health's INFOMED database.29 Monitoring costs related to warfarin treatment included costs of physician monitoring and INR testing. Monitoring cost differed according to setting of care (general physician or specialist). In clinical practice, when patients begin warfarin for the first time, or after a period without taking it, regular INR monitoring by a physician is recommended. Based on the expert panel's opinion, during the first three months of warfarin treatment it was assumed that patients required seven monitoring visits. Thereafter, patients required 3.5 monitoring visits per cycle, based on the findings of a Portuguese study.30
Although treatment with rivaroxaban or aspirin does not require blood monitoring, it was conservatively assumed that two physician visits per year were necessary based on the European Heart Rhythm Association Practical Guide31 and the opinion of the expert panel.
Costs were assigned to acute events and to those with long-term sequelae. The most severe events, such as ischemic stroke and major IC bleeding, were modeled using multiple components for calculating the acute costs. Besides the cost of acute treatment, long-term costs associated with an acute event, such as its impact on functional status, were also considered by including a cost for rehabilitation. The latter was assumed to be incurred by the patient until the end of the first three-month cycle after a major ischemic stroke or IC bleed (Table 3).
Overview of drug acquisition, drug monitoring and event costs from a societal perspective.
| Event | Total cost (€) | ||
| Acquisition | |||
| Rivaroxaban – 20 mg or 15 mg once daily | 2.65 | ||
| Warfarin | – loading dose: 5 mg once daily | 0.06 | |
| – maintenance dose: 3.75 mg once daily | 0.05 | ||
| Aspirin – 100–150 mg once daily | 0.10 | ||
| Monitoring visits | |||
| Rivaroxaban | 105 | ||
| Warfarin | First visit | 148 | |
| Subsequent visits | General physician | 33 | |
| Specialist | 90 | ||
| Ischemic stroke (cost per cycle) | |||
| Minor ischemic stroke | 1270 | ||
| Major ischemic stroke | Acute | 1989 | |
| Rehabilitation (per day) | 37 | ||
| Long-term follow-up | 392 | ||
| Bleedings | |||
| CRNM bleeding | 150 | ||
| Major EC bleeding | 2425 | ||
| IC bleeding | Acute | 2628 | |
| Rehabilitation (per day) | 57 | ||
| Long-term follow-up | 462 | ||
| SE (per cycle) | |||
| Acute | 2603 | ||
| MI (per cycle) | |||
| Acute event | 7270 | ||
| Long-term follow-up | 802 | ||
CRNM: clinically relevant non-major; EC: extracranial; IC: intracranial; MI: myocardial infarction; SE: systemic embolism.
In the base-case analysis, an ICER was derived by dividing the incremental costs by the incremental LYs or QALYs gained with rivaroxaban compared to warfarin. Unlike several countries, such as the United Kingdom, the Portuguese Health Authority (INFARMED) does not use an explicit willingness-to-pay threshold when assessing ICERs.
Sensitivity analysesSensitivity analyses assess the uncertainty around outputs from economic models and represent an important element of a sound economic evaluation.21 Extensive sensitivity analyses were carried out in order to further evaluate the findings of the model: a one-way sensitivity analysis (OWSA) was carried out in order to identify the key drivers of cost-effectiveness and a probabilistic sensitivity analysis (PSA) was performed to test the overall robustness of the model.
In the OWSA, the base-case value of parameters was varied using low and high values within plausible ranges. For parameters such as clinical efficacy values, the reported 95% CIs from the ROCKET AF trial were used. The base-case value of cost inputs were varied by ±25%, which was considered sufficient variation to capture the relevant uncertainty. PSA is a stochastic analysis conducted to test second-order uncertainty in the model. In this analysis 500 simulations were run, each time varying all model parameters carrying second-order uncertainty within logical ranges.
Scenario analysisGiven the underuse of OAC in Portugal,6,32 an additional analysis was conducted using a mixed comparator composed of no treatment (6%), aspirin (44%) and warfarin (50%), which better reflects real-world prescribing in the Portuguese setting. The composition of this mixed comparator was based on the literature33–36 and expert opinion. The patient population for this additional scenario was defined by the expert panel to be the stroke risk group with a distribution among patients set to 30% CHADS2 risk score of 2 and 70% CHADS2 risk score of 3 or higher. Among warfarin patients, 25% were considered warfarin-naïve. Relative treatment effects describing the efficacy of rivaroxaban vs. the mixed comparator for the prevention of stroke, SE, bleeding and MI were based on results from a network meta-analysis (NMA). This NMA was conducted to allow comparison of rivaroxaban with non-VKA comparators, which were not included in the ROCKET AF trial. Baseline event rates were set as the placebo arms of clinical trials used in the NMA.37
ResultsBase-case analysisOver a patient's lifetime horizon, and compared with warfarin, rivaroxaban was projected to be associated with an additional cost of €81 as well as with a gain of 0.023 LYs and 0.021 QALYs, resulting in an ICER of €3494/LY and €3895/QALY. Assuming a €30 000/QALY willingness-to-pay threshold, rivaroxaban is deemed a cost-effective alternative compared to warfarin (Table 4).
Cost-effectiveness results of base-case analysis.
| Rivaroxaban | Warfarin | Difference | |
| Total costs | €6142 | €6061 | €81 |
| Drug acquisition | €3267 | €114 | €3152 |
| Drug administration | €695 | €3484 | −€2789 |
| Event treatment | €2425 | €2585 | −€160 |
| Total LYs | 5.00 | 4.98 | 0.023 |
| Total QALYs | 3.83 | 3.81 | 0.021 |
| ICER (€/LY) | – | – | €3494 |
| ICER (€/QALY) | – | – | €3895 |
ICER: incremental cost-effectiveness ratio; LY: life-year; QALY: quality-adjusted life-year. All values have been rounded up.
The OWSA showed that the key drivers of cost-effectiveness were the clinical and economic inputs, particularly discontinuation and subsequent discontinuation rate for rivaroxaban and relative risk for rivaroxaban vs. warfarin for stroke. Additional analyses were run assuming shorter time horizons (10 and 15 years) and, as expected, marginally higher ICERs were obtained: €4286/QALY for a 10-year time horizon and €3910/QALY for a 15-year time horizon.
The results of the PSA confirmed the overall robustness of the base-case analysis. The cost-effectiveness acceptability curve showed that at a willingness-to-pay threshold of €30 000/QALY, the likelihood of rivaroxaban being cost-effective compared to warfarin was 72% (Figure 2).
Scenario analysisAn additional scenario comparing rivaroxaban with a mixed comparator was conducted. For a lifetime horizon, this analysis yielded an ICER of €9492/LY and €6697/QALY (Table 5).
Cost-effectiveness results of additional scenario analysis.
| Rivaroxaban | Mixed comparator | Difference | |
| Total costs | €6835 | €6278 | €557 |
| Drug acquisition | €3267 | €109 | €3158 |
| Drug administration | €695 | €2279 | −€1585 |
| Event treatment | €2425 | €2935 | −€510 |
| Total LYs | 4.97 | 4.91 | 0.059 |
| Total QALYs | 3.76 | 3.68 | 0.083 |
| ICER (€/LY) | – | – | €9433 |
| ICER (€/QALY) | – | – | €6697 |
ICER: incremental cost-effectiveness ratio; LY: life-year; QALY: quality-adjusted life-year.
In the present study, an economic evaluation was undertaken to compare rivaroxaban to existing treatments for the prevention of stroke in patients with AF in Portugal from a societal perspective. For this purpose, a Markov model describing the management and consequences of AF and treatment was used. The base-case analysis was based on ROCKET AF trial data. Rivaroxaban was compared to warfarin, which is the most widely used OAC and is recommended for AF patients with any additional risk factor for stroke in guidelines including those of the ESC and AHA/ASA. Patient population settings (e.g. split by CHADS2 risk score and split between warfarin-naïve and warfarin-maintenance patients) were based on the baseline characteristics of the ROCKET AF trial population. Due to the fact that not all patients receive warfarin, an additional analysis focused on real-world prescribing including a weighted average of warfarin, aspirin and no treatment, which better reflects Portuguese clinical practice.
Results from the base-case and scenario analyses, using a societal perspective, indicate that the ICER of rivaroxaban against the comparator varies between €3895/QALY and €6697/QALY respectively. In the base-case scenario, the drug acquisition costs of rivaroxaban were substantially higher than those of warfarin. However, this difference was largely compensated by the reduction in costs related to monitoring and events. In the scenario analysis, treatment costs are not offset, but the efficacy of rivaroxaban leads to a substantial improvement in outcomes.
As expected, the OWSA indicated that the clinical efficacy of rivaroxaban is a strong driver of cost-effectiveness, particularly the relative risk of major IC bleeding and ischemic stroke compared to warfarin. Economic inputs describing the cost of warfarin monitoring were also important drivers. Assuming a willingness-to-pay threshold of €30000 per QALY, the cost-effectiveness acceptability curve demonstrated that rivaroxaban had a high (72%) probability of being cost-effective compared with warfarin only, which is in line with results from previous analyses. The results of both the OWSA and the PSA support the conclusion that the model is robust and that rivaroxaban is a cost-effective alternative to existing therapies for the prevention of stroke in non-valvular AF patients.
Markov models such as the one used to conduct the analyses presented in this study offer an opportunity to evaluate the impact of a health technology with respect to economics and health outcomes. Nonetheless, there were several limitations associated with this analysis. The base-case analysis was based on data reported from an international randomized controlled clinical trial (ROCKET AF),14 which is not necessarily representative of clinical practice in Portugal. Additionally, due to unavailability of Portuguese-specific data in terms of resource use, an expert panel was consulted to provide estimates, which introduces some uncertainty. Lastly, in the absence of Portuguese-specific utility values, utilities from other European countries and the US were used in the analyses and were consequently less representative of the Portuguese population.
This analysis can furthermore be deemed conservative since no disutility associated with warfarin treatment was applied. Several studies have suggested that such a disutility exists. Protheroe et al.38 showed that many AF patients would prefer not to receive warfarin therapy even if they meet the criteria for receiving it according to clinical guidelines.
An economic evaluation of dabigatran for stroke prevention in patients with non-valvular AF in Portugal was recently published.39 A comparison between this evaluation and the study described in this paper is neither possible nor appropriate for several reasons, including differences in modeling methods and in the composition of the comparators as well as the lack of head-to-head trials comparing rivaroxaban and dabigatran. Additionally, while an indirect comparison between rivaroxaban and dabigatran is possible, the findings may not be robust given significant differences in the study populations and in study design.40
As previously mentioned, there is evidence of extensive underprescribing of OACs even in patients with a high risk of stroke.41,42 In Portugal, the challenge is two-fold: firstly to increase the number of patients treated with OACs, and secondly to improve health outcomes of patients on OACs. The FAMA study concluded that 25% of patients with AF in Portugal were not being adequately treated for this condition, and that this proportion was even greater in patients aged 60 years or less.6 OACs were prescribed in slightly over a third of patients with AF, a figure in line with published studies that have highlighted the underuse of these drugs in AF patients, with 21.8% taking antiplatelets only.6 The results obtained show that the use of rivaroxaban offers opportunities to manage an unmet need for untreated patients and could reduce the stroke burden of an aging population.
ConclusionThis cost-effectiveness analysis demonstrated that rivaroxaban is cost-effective compared with VKA therapy in a trial-based setting, and also compared to real-world antithrombotic prescribing in Portugal for stroke prophylaxis in patients with AF. The drug acquisition costs of rivaroxaban were partially offset by savings in warfarin administration costs and savings in costs due to stroke and bleeding events, such as IC bleeding. The use of rivaroxaban in this therapeutic indication would help to decrease the disease burden for patients and for the Portuguese healthcare system.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Please cite this article as: Morais J, Aguiar C, McLeod E, Chatzitheofilou I, Fonseca Santos I, Pereira S. Estudo de custo-efectividade de rivaroxabano para prevenção de acidente vascular cerebral em doentes com fibrilhação auricular em Portugal 2014. Rev Port Cardiol. 2014;33:535–544.










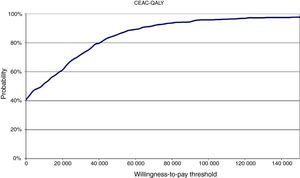 CEAC: cost-effectiveness acceptability curve; QALY: quality-adjusted life-years.'/>
CEAC: cost-effectiveness acceptability curve; QALY: quality-adjusted life-years.'/>

