Magnetic resonance imaging (MRI) is currently considered an essential complementary method for diagnosis in many conditions. Exponential growth in its use is expected due to the aging population and a broader spectrum of clinical indications. Growth in its use, coupled with an increasing number of pacemaker implants, implantable cardioverter-defibrillators and cardiac resynchronization therapy, has led to a frequent clinical need for this diagnostic modality in patients with cardiac implantable electronic devices (CIED). This clinical need has fueled the development of devices specifically designed and approved for use in a magnetic resonance (MR) environment under certain safety conditions (MR-conditional devices). More than a decade after the introduction of the first MR-conditional pacemaker, there are now several dozen MR-conditional devices with different safety specifications. In recent years, increasing evidence has indicated there is a low risk to MRI use in conventional (so-called non-MR-conditional) CIED patients in the right circumstances. The increasing number, as well as the greater diversity and complexity of implanted devices justify the need to standardize procedures, by establishing institutional agreements that require close collaboration between cardiologists and radiologists. This consensus document, prepared jointly by the Portuguese Society of Cardiology and the Portuguese Society of Radiology and Nuclear Medicine, provides general guidelines for MRI in patients with CIED, ensuring the safety of patients, health professionals and equipment. In addition to briefly reviewing the potential risks of MRI in patients with CIED and major changes to MRI-conditional devices, this article provides specific recommendations regarding risk-benefit analysis, informed consent, scheduling, programming strategies, devices, monitoring and modification of MRI sequences. The ultimate goal of this document is to optimize patient safety and provide legal support to facilitate easy access by CIED patients to a potentially beneficial and irreplaceable diagnostic technique.
A Ressonância Magnética (RM) é atualmente considerada um método complementar de diagnóstico fundamental em inúmeras patologias, sendo expectável um crescimento exponencial da sua utilização em virtude do envelhecimento da população e alargamento do espectro de indicações clínicas. Esta utilização crescente, aliada a um aumento do número de implantes de pacemakers, cardioversores-desfibrilhadores implantáveis e terapêutica de ressincronização cardíaca, conduziu a uma necessidade clínica frequente de realização desta modalidade diagnóstica em doentes portadores de dispositivos cardíacos eletrónicos implantáveis (DCEI). Esta necessidade clínica impulsionou o desenvolvimento de dispositivos especificamente desenhados e aprovados para utilização em ambiente de RM, sob determinadas condições de segurança (dispositivos RM-condicionais). Volvida mais de uma década após a introdução do primeiro pacemaker RM-condicional, existem hoje várias dezenas de dispositivos considerados RM-condicionais, com diferentes especificações de segurança. Paralelamente, nos últimos anos, evidência crescente tem indicado um baixo risco da utilização da RM em doentes com DCEI convencionais (ditos não-RM-condicionais), em circunstâncias apropriadas. O número crescente, assim como a grande diversidade e complexidade de dispositivos implantados justificam a necessidade de normalizar procedimentos, com estabelecimento de protocolos institucionais que implicam uma colaboração estreita entre Cardiologistas e Radiologistas. Este documento de consenso, elaborado em conjunto pelas Sociedades Portuguesas de Cardiologia e de Radiologia e Medicina Nuclear, fornece normas de orientação gerais que visam a realização de RM em doentes portadores de DCEI, assegurando a segurança do doente, dos profissionais de saúde e dos equipamentos. Para além do rever brevemente os potenciais riscos da RM em doentes portadores de DCEI e principais modificações introduzidas nos dispositivos RM-condicionais, o presente artigo fornece recomendações específicas relativamente à análise da relação risco/benefício, consentimento informado do paciente, agendamento, estratégias de programação dos dispositivos, monitorização e modificação das sequências de RM. O objetivo final deste documento é otimizar a segurança do doente e fornecer suporte legal, de forma a promover um acesso facilitado dos doentes portadores de DCEI a uma técnica diagnóstica potencialmente benéfica e insubstituível.
Magnetic resonance imaging (MRI) is a high capacity diagnostic imaging technique. It has excellent spatial resolution and provides clinically relevant information, without the use of ionizing radiation or iodinated contrast agents. MRI is an indispensable imaging method in the diagnosis and monitoring of multiple diseases. Annually, several million MRI studies are performed worldwide, and this number is expected to continue to grow given the increased life expectancy of the population, combined with technical advances and an extension of the clinical applications of this technique. In parallel, and for identical reasons, there has been an increase in the number of patients with pacemakers, implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization devices (CRT). Over the lifetime of the device, the estimated probability of these patients needing an MRI is of 50-75%.1
Until about a decade ago, MRI in patients with implantable electronic cardiac devices (CIED) was contraindicated. However, the clinical need to perform these studies in patients with CIED brought about the development of devices designed especially for use in an MR environment. These are conventionally referred to as MR-conditionals, since the safety of their use is only demonstrated when certain conditions are met. This term is preferable to “MR-compatible”, as it emphasizes the need for certain conditions to be met in order for the study to be safe. At many centers, most CIEDs currently being implemented are already MR-conditional. However, in the general population, older devices are still the most common. Performing MRIs in patients with non-MR-conditional IECDs presents risks that must be recognized and cannot be considered routine, although recent studies have shown that the risk is much lower than initially estimated.2,3
The growing number of patients with electronic devices and the diversity of implanted devices justify the need to standardize procedures. In order for patients with these devices to undergo MRI studies safely, it will be necessary to involve professionals from across the board, including the prescribing clinician, radiologists and cardiologists (including electrophysiologists), radiology and cardiopneumology technicians and administrative staff responsible for scheduling appointments.
The purpose of this article is to briefly review the potential risks of MRI in patients with electronic devices and to provide guidelines for conducting MRIs, ensuring the safety of the patient, professionals and equipment.
Potential risks of magnetic resonance imaging in patients with non-MR-conditional implantable electronic cardiac devicesCIEDs can be classified as either MR-conditional or non-MR-conditional. All devices that have been designed, tested and approved for use in an MRI environment are considered to be conditional when certain conditions are met.
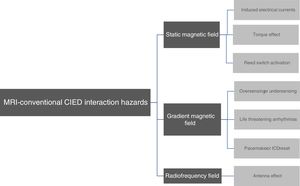
The potential risk of interaction between non-conditional-MR and CIED can be divided into three categories: 1) interaction with the static magnetic field; 2) interaction with the magnetic field gradients and 3) interaction with the radiofrequency (RF) energy (Figure 1).4
The cardiac pacing system consists of leads and an electrical pulse generator, which contains connectors, electronic circuits and a battery may be made from ferromagnetic components. The static magnetic field of most MRI systems used in clinical practice is 1.5 or 3.0 T and affects the ferromagnetic components of cardiac implantable devices in three different ways: 1) by exerting a force of attraction on the pacing system components; 2) producing a torque effect or 3) activating the reed switch on older devices.5,6
The force of attraction and torque effect are maximum at the center of the MRI tunnel and could theoretically result in the vibration or displacement of internal components in the pacing system or ICD. However, studies published so far have not shown any significant risks arising from this effect in conventional devices.6 Older pacemakers and ICDs also have a mechanical network, known as a reed switch. It is activated (closed) in the presence of magnetic fields, even of low intensity, resulting in a change to a fixed stimulation mode (DOO or VOO), with the deactivation of sensing functions on pacemakers and the deactivation of tachycardia detection on ICDs. However, the behavior of the reed switch in the presence of the MRI magnetic field is unpredictable. It can remain disabled when the torque effect is predominant or be activated, leading to the risk of asynchronous stimulation.7
Interaction with magnetic field gradientsDuring MRI, gradient coils create additional magnetic fields used to locate signals, which are repeatedly turned on and off very quickly. This can induce pulses on leads, with the associated risk of oversensing, undersensing or developing potentially fatal arrhythmias.4 Voltages induced by the magnetic field gradient can also result in a reconfiguration of the programming parameters of older conventional devices, with the activation of the emergency mode (typically ventricular demand pacing (VVI)).5
Interaction with magnetic resonance imaging radiofrequency energyThe most complex and significant interactions occurs between the device and the RF pulses that MRI machines use to produce images. The body absorbs part of this energy resulting in resistive heating (release of heat due to the passage of electrical current through a conductive material). Pacemakers leads and ICDs can act as antennas, receiving the RF signal, amplifying it locally and conducting energy to the lead tip and to the myocardium. Depending on orientation, the RF field can induce significant temperature increases at the lead tips (temperature increases of up to 20 °C).8 The antenna effect can lead to thermal tissue damage with edema or myocardial fibrosis, and also damage to the generator or battery circuit. The main risks associated with this effect are: irreversible increase in the pacing capture threshold, alteration in the sensing function or impedances, resetting of the device and battery depletion.4 Abandoned or fractured leads are especially prone to increases in tip temperature.8
MR-conditional cardiac implantable electronic device technologyThe increasing importance of MRI as a diagnostic method and the limitations imposed by non-MR-conditional CIEDs have led to research into and the development of new devices that are safe for use in an MRI environment.9 In 2008, MR-conditional pacemakers were first introduced to the market. Currently, all major brands offer this technology on some models.5 The MR-conditional CIEDs were tested to perform MRI under certain conditions and were approved by the European Medical Device Directive with CE certification (“in-label” use). The manufacturers of these CIEDs guarantee their safety as long as the specific conditions of use are observed.5
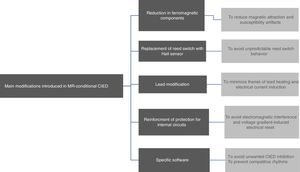
Most of the MRI-associated risks in patients using conventional devices are related to the presence of ferromagnetic components. In MR-conditional CIEDs there are two main forms of avoiding the interaction of magnetic fields: 1) minimizing the ferromagnetic content and the use of methods that prevent it from interacting with the magnetic field (Fig. 2).6
In MR-conditional CIEDs the ferromagnetic content has been reduced to a minimum, in such a way that the effective force has decreased to lower than the gravitational pull and susceptibility artifact production is minimized.10 The use of non-ferromagnetic materials that maintain proper conductivity, durability and biocompatibility results in a reduction in the amount of ferromagnetic materials used. Generators have started to be hermetically sealed with stainless steel or titanium material6 and leads are being manufactured with non-ferromagnetic materials.4
Replacement of the reed switch with a Hall sensorUnlike reed switches on non-MR-conditional devices, which are unpredictable in the presence of a strong magnetic field, the Hall sensor is predictable and can be blocked when performing an MRI.4
Modification of leadsThe main objectives of modifications made to the leads on MR-conditionals CIEDs were: 1) to minimize heating at the lead tip, which could cause myocardial damage and changes in the pacing and/or sensing functions and 2) to reduce the antenna effect and the risk of electric currents induced by electromagnetic energy being conducted by the leads and causing myocardial stimulation and potentially fatal arrhythmias.11 Modifications were made to the lead geometry, including changing the winding pattern of the filaments that make up the internal coil and limiting the RFs that could be transmitted through the leads. The tips of the leads were also coated with a polarization resistant substance and an electromagnetic filter was implanted at the distal end of the lead to prevent it from heating during MRI.4,11
For an CIED to be considered MR-conditional, in addition to the generator, the leads must also be MR-conditional and from the same manufacturer. It is important to note that some leads not designed for this purpose were later tested and considered MR-conditional (retrospective approval).
Strengthening the protection of internal circuitsCIED MR-conditionals are equipped with special filters that limit the transfer of certain frequencies and dissipate energy, reducing the risk of damage to the internal power source and circuits. Generator shielding has also been improved to minimize the transfer of electromagnetic energy.4
Specific softwareA very important feature of MR-conditional CIEDs is that there is a specific “MR mode”. The checklist and specific programming algorithm for MR-conditional CIEDs varies according to model and manufacturer. Usually, the MR mode must be selected before the MRI is performed and deactivated right after the study.4,6 Even with MR-conditional devices, it is necessary to observe a series of parameters so that the MRI can be performed safely. There must be a minimum period of 6 weeks between the implantation of the CIED and the performance of MRI, for adequate healing and fixation, thus reducing the torque effect.6 However, the use of some CIEDs is not recommended in higher MRI fields of 3.0 T. Some models also do not allow MRI when the isocenter is located in the chest (MRI of thoracic structures), a limitation that has been disappearing from more recent devices.6
In the literature, there are several prospective randomized studies that assessed the safety of MRI in patients with MR-conditional CIEDs. No clinically relevant events or complications were reported in any of these studies.12,13 It should be noted, however, that there is still little long-term information on the new MR-conditional CIED technology.
Performing magnetic resonance imaging in patients with cardiac implantable electronic devicesPerforming MRI in patients with CIEDs must comply with institutional safety protocols prepared and approved jointly by the Radiology and Cardiology Services of each institution. These protocols should be applied in clinical practice using a checklist that summarizes the main steps in the management patients with CIEDs referred for an MRI. The adoption of a structured checklist is supported as a Class I recommendation by the most recent guidelines from the Heart Rhythm Society.11 Adherence to an institutional protocol and adoption of checklists minimizes potential risks and improves access for patients with CIEDs to a potentially beneficial and irreplaceable imaging technique. The following items are essential when creating a checklist: 1) screening of patients with CIED referred for an MRI and identification of the devices, including implantation date; 2) interrogation and reprogramming of the CIED before and after the study; 3) supervision and monitoring during the study; 4) follow-up; 5) inclusion of patients in records for quality control.14 A draft checklist is provided as an appendix to this article (Appendix 1).
The security requirements will vary depending on whether the device is MR-conditional or non-MR-conditional.
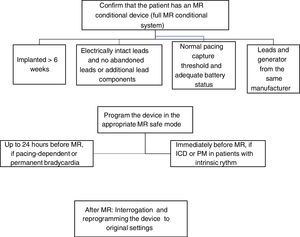
Recommendations for magnetic resonance imaging in patients with conditional-MR cardiac implantable electronic devicesFig. 3 provides a synopsis of the MRI algorithm in patients with MR-conditional CIEDs.
The first step in performing an MRI in patients with conditional-MR CIEDs is to check whether the device is in fact MR-conditional. This means identifying all the installed hardware, since it is only when the entire system meets the criteria that the device is considered MR-conditional.11 Therefore, an MR-conditional generator combined with non-MR-conditional components is non-MR-conditional. Examples of non-MR-conditional components are: abandoned leads; fractured leads; epicardial leads or active non-cardiac devices. Given the combination of components from different manufacturers in the same CIED, the system is also considered non-MR-conditional.11 The location of the device is also important, since a device located in the pectoral area is the only one that is considered MR-compatible in the case of transvenous systems.11 It should also be noted that when an MR-conditional CIED is programmed in any mode other than MR, this results in the device becoming non-MR-conditional.
Identifying the deviceBeing labeled MR-conditional takes into account the whole CIED system. To obtain information on the compatibility of a specific CIED with MRI, the lists provided in the appendixes may be consulted (Appendixes 2 and 3). It is preferable to however consult the specific up-to-date databases provided by the device manufacturers as they are a more reliable alternative, given the constant introduction of new systems to the market and validation of compatibility of older leads with MRI (retrospective approval).11
If the device is MR-conditional, the following must always be checked to ensure that MRI is performed safely, especially:5
- 1
Check that the entire system is MR-conditional (generator and leads)
- 2
Confirm that the device was implanted more than 6 weeks ago
- 3
Check that the leads are electrically intact
- 4
Confirm implantation of the device in the chest region
- 5
Check that the pacing capture thresholds are within the normal range
- 6
Confirm battery longevity
- 7
Exclude the presence of abandoned leads or additional components, such as adapters and lead extensions
- 8
Exclude the presence of other cardiac implants.
After confirmation of the clinical indication, exclusion of contraindications and obtaining informed consent (which can be obtained on the day of the study), the next step is to schedule the study. The need for a prior visit to the electrophysiology laboratory or a pacemaker consultation, to program the device must be flagged. The scheduling of the procedure requires seamless coordination between the cardiology and radiology departments at the institution. Most centers with experience in performing MRI in patients with ICEDs choose to schedule the study on specific dates where they can guarantee minimum staffing requirements for device programming and supervision of the study.
Device programmingUsers of MR-conditional devices must be assessed by the CIED specialized team before and after performing the MRI.5 Before the MRI, the MR-conditional CIED need to be programmed to MR mode, available in the device software options, and includes the following:
- a)
Asynchronous mode or deactivation of the pacing function
- b)
Bipolar configuration of the leads
- c)
Increased stimulation output
- d)
Deactivation of additional stimulation functions such as rate-dependent stimulation, anti-tachycardia pacing and shocks.
Table 1 provides recommendations for programming CIEDs for MRIs according to the patient’s type of pacemaker or ICD and their baseline rhythm.
Recommendations regarding reprogramming of MR-conditional devices to perform MR.
| Type of device | Baseline Rhythm | MR mode | Reprogramming pre-MR | Reprogramming post-MR |
|---|---|---|---|---|
| Pacemaker | Absolute pacemaker dependency | DOO or VOO | Possible at a different time and location than MR study but on the same day | Possible at a different time and location than MR study but on the same day |
| Pacemaker | No pacemaker dependency but stable/permanent bradycardia | DOO or VOO | Possible at a different time and location than MR study but on the same day | Possible at a different time and location than MR study but on the same day |
| Pacemaker | With or without pacemaker dependency; non-permanent bradycardia | ODO or OOO | Immediately pre-MR | Immediately post-MR |
| CRT | With or without pacemaker dependency | DOO or VOO | Immediately pre-MR | Immediately post-MR |
| ICD | Absolute pacemaker dependency | DOO or VOO | Immediately pre-MR | Immediately post-MR |
| ICD | No pacemaker dependency; no history of VT or VF | ODO or DOO* or OVO or VOO* | Immediately pre-MR | Immediately post-MR |
*ODO and *VOO – if high or unstable intrinsic heart rate.
DOO – if low and stable intrinsic heart rate (mode DOO with asynchronous stimulation above the intrinsic heart rate).
CRT: cardiac resynchronization therapy; ICD: implantable cardioverter-defibrillator; MR: magnetic resonance; VF: ventricular fibrillation; VT: ventricular tachycardia.
In the case of MR-conditional devices, the presence of an electrophysiologist or cardiologist with experience in device programming is not considered mandatory. In centers that do not have permanent support from an electrophysiologist, it is necessary to ensure support for the reprogramming of MR-conditional devices by properly trained professionals. However, the decision on the pacing mode is clinical and the responsibility of the cardiologist in charge of the study. Likewise, physical and human resources do not need to be available for the implantation of a provisional pacemaker. As in patients with non-MR-conditional CIEDs, the study must be performed under the supervision of a physician trained in advanced life support (ALS) and with continuous monitoring of the electrocardiogram, pulse oximetry and blood pressure (optional).11
Special precautions regarding the acquisition of magnetic resonance imagesThe image acquisition protocol should be adapted according to the manufacturer's recommendations for each specific device. The main recommendation is to shorten the acquisition time and limit the sequence protocol to the clinical question posed, regardless of the device type.
Other general guidelines to consider when obtaining the images are:
- 1
Not to exceed a slew rate of 200 T/m/s (check manufacturer's recommendations for each specific device)
- 2
Place the patient in the supine position
- 3
Preferably use a body antenna
- 4
Limit the specific absorption rate (SAR) to 2 W/Kg.
Recommendations on specificities of the most suitable MRI mode and ideal timing of the schedule are summarized in Table 1. Unlike non-MR-conditional CIED, reassessment is not recommended 3 months after MRI.
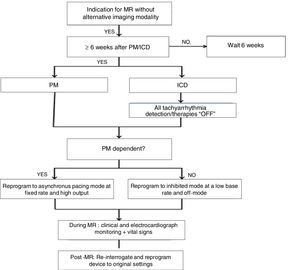
Recommendations for performing MRI in patients with non-MRI-conditional implantable cardiac electronic devicesThe algorithm for performing MRI in patients with non-MR-conditional CIEDs is summarized in Fig. 4.
Before requesting an MRI from a patient with a non-MR-conditional CIED, the risk-benefit ratio of the procedure should be considered. In terms of benefit, it is important that the study has a clear and relevant indication, and that the clinical question cannot be satisfactorily clarified using alternative imaging methods. With regard to risk, it is essential to identify the patient's baseline rhythm to assess the risk of developing arrhythmias and to determine the most appropriate device program for performing an MRI.5
Exclusion of contraindicationsOnce a request for an MRI study has been formalized in a patient with a MR-conditional device, a clinical questionnaire (ideally associated with the MRI request) that addresses several safety issues that may contraindicate or limit the performance of the study should be completed.15 This questionnaire should screen for circumstances that contraindicate exposure to magnetic fields (absolute contraindications) or that have implications for the study methodology, including the presence of CIEDs. In this case, the patient's medical records should be consulted to obtain, the device model and year of implantation, as well as the existence of earlier devices, even if already removed. In the presence of a non-MR-conditional device, there are some circumstances that usually contraindicate MRIs:
- 1
Year of implantation of the device prior to 1998 (pacemakers) or 2000 (ICD);
- 2
Device implantation under 6 weeks ago;
- 3
Presence of epicardial leads and fractured or abandoned leads.11,16
Performing MRI in patients with non-MR-conditional devices is not without risks, which means that a discussion between doctor and patient about the potential risks and clinical benefits of the procedure is required. To perform an MRI in patients with non-MR-conditional CIEDs, specific informed consent must be obtained, using a template that complies with the recommendations of the Portuguese General Health Directorate. An informed consent template is provided for this purpose as an attachment to this article (Appendix 4).
SchedulingThe scheduling of the MRI study in patients with non-MR-conditional CIEDs should follow the same principles as in patients with MR-conditional devices, particularly the need for coordination with the electrophysiology and pacing laboratory for device interrogation and programming.
Device programmingThe optimal programming of the non-MR-conditional device depends on the type of device and the patient's intrinsic heart rhythm. The first step is to check whether the patient is pacing-dependent or not.11
A) In pacing-dependent patients and in non-dependent but with symptomatic permanent bradycardia (intrinsic heart rhythm <50 bpm), the pacemaker must be programmed in asynchronous mode (DOO or FLIGHT or AOO) with a stimulation frequency significantly above the intrinsic heart rate of the patient. This is to avoid competition with the native rhythm and the development of pacemaker-mediated arrhythmias (Table 2). Pre- and post-MR programming should be carried out as close as possible to the study. In pacing-dependent patients with atrial and ventricular leads, the indicated programming mode is DOO (Fig. 3). The reprogramming of the original mode and verification of the normal functioning of the device must take place immediately after performing the MRI.5
Recommendations for reprogramming non-MR-conditional devices to perform magnetic resonance imaging.
| Type of device | Baseline patient rhythm | MR mode | Reprogramming pre-MR | Reprogramming post-MR | |
|---|---|---|---|---|---|
| Pacemaker | Absolute pacemaker dependency | DOO or VOO or AOO | Possible at a different time and location than MR study but on the same day | Immediately post-MR | |
| No pacemaker dependency with stable/permanent bradycardia | DOO or VOO or AOO | Possible at a different time and location than MR study but on the same day | Immediately post-MR | ||
| No pacemaker dependency and non-stable/permanent bradycardia | ODO or OVO or OAO | Immediately pre-MR | Immediately post-MR | ||
| VVI or DDI | Possible at a different time and location than MR study but within 48 h | Immediately post-MR | |||
| CRT | With or without pacemaker dependency | VOO or DOO | Immediately pre-MR | Immediately post-MR | |
| ICD | No pacemaker dependency and no history of VT/VF | High or unstable intrinsic HR | ODO or OVO | Immediately pre-MR | Immediately post-MR |
| Low, stable, intrinsic HR | DOO or VOO | ||||
| ICD | No pacemaker dependency but history of VT/VF | High or unstable intrinsic HR | ODO or OVO | Immediately pre-MR | Immediately post-MR |
| Low, stable, intrinsic HR | DOO or VOO | ||||
| ICD | Absolute pacemaker dependency | DOO or VOO | Immediately pre-MR | Immediately post-MR | |
CRT: cardiac resynchronization therapy; ICD: implantable cardioverter-defibrillator; HR: heart rate; MR: magnetic resonance; VF: ventricular fibrillation; VT: ventricular tachycardia.
B) In patients not-dependent on pacing with high or unstable intrinsic heart rates, there are two programming possibilities5:
B.1) Deactivation of the stimulation function by programming in ODO, OVO or OAO or, when none of the options is possible, as in the case of older pacemakers, reprogramming the stimulation energy below the threshold. This procedure requires the device to be reprogrammed immediately before and after the MRI.
B.2) Reprogramming in VVI/DDI mode, with the advantage that pre-MR programming can be performed at a different time and place than an MRI setting (since these modes prevent spontaneous episodes of symptomatic bradycardia). A maximum 24-h interval pre-MR is acceptable for programming. It is recommended that immediately after the MRI the original mode is restored and it is checked that the pacemaker works normally. The disadvantage of the latter is that, despite programming in VVI mode, asynchronous stimulation can occur during the study (the activation of the reed switch by the static magnetic field), with the potential risk of potentially fatal ventricular arrhythmias (Table 2).
C) In patients with cardiomyopathies with cardiac resynchronization therapy systems, disabling the pacing function or temporary programming in a non-biventricular pacing mode can have significant hemodynamic consequences.11 For these patients, it is justifiable to program the CRT device in an asynchronous pacing (FLIGHT or DOO), with a pacing frequency that avoids competition with the intrinsic rhythm.
Regardless of the type of device, there are other aspects of pre-MR programming that should be considered:
- 1
Pacemaker/ICD output must be increased to 4 times the value of the pacing capture threshold or to 5.0 V/1.0 ms before MRI, to compensate for a possible increase in the pacing capture threshold due to lead heating induced by RF energy
- 2
If possible, the sensing and stimulation polarity of the leads should be programmed to bipolar.
- 3
Disable additional stimulation functions5,11:
- a)
Frequency response mode
- b)
Pacemaker-mediated anti-tachycardia pacing (PMT algorithms)
- c)
Left ventricular pacing triggered by sensing response to intrinsic right ventricular activity (triggered pacing) - CRT systems only
- d)
Anti-tachycardia therapies (including ATPs and shocks) - in ICDs
- e)
PVC-triggered pacing response (in response to ventricular extrasystoles sensing)
- f)
PAC-triggered pacing response (in response to atrial extrasystole sensing)
- g)
Atrial fibrillation therapies (such as rate smoothing and overdrive pacing)
- h)
Hysteresis
- i)
Magnet response (when option exists).
- a)
Performing MRI studies in patients with CIED requires appropriate supervision and monitoring during the study. In the particular case of non-MR-conditional devices, prior evaluation by an electrophysiologist or cardiologist with experience in device programming is essential. All MRI studies in non-MR-conditional devices should therefore be performed in centers with electrophysiology back-up support and with means immediately available if there is an urgent need for a temporary pacemaker implantation.
The following safety requirements must be ensured when performing MRI for patients with CIED:
- 1
Immediate availability of drugs and equipment necessary for ALS, including an emergency car with defibrillator and capacity for external pacing
- 2
Supervision of the study by a physician with recognized training in ALS
- 3
Clinical surveillance through verbal contact (intercom) and, if possible, visual (camera).
- 4
Electrocardiographic, blood pressure (optional) and pulse oximetry monitoring.
On the day of the study, after admission to the radiology department, the patient should then be referred to the Electrophysiology Unit or a Pacemaker Consultation for identification, interrogation and programming of the device. These procedures should take place in the MRI Unit, dependent upon the logistics and organization of the department. Only after a prior assessment by electrophysiology can the patient access the Security Zone 2 of the MRI Unit to prepare and plan for the study.
Special precautions regarding the acquisition of MR imagesWhen performing MRI on patients with MR-conditional devices, the same safety precautions apply as on non-MR-conditional devices.
Post-study evaluationNon-MR-conditional CIEDs should be interrogated immediately after an MRI to detect possible changes (such as electrical reset, increase in the pacing capture threshold, battery depletion) and allow the original programming settings to be restored. It is also recommended to perform a reassessment three months after the MRI to exclude long-term changes (such as, for example, a chronic increase in the pacing capture threshold due to scar tissue formation).5 Follow-up is recommended in the first week, if one of the following conditions are met: 1) Any increase in the capture threshold >1.0 V; 2) Reduction in the amplitude of P wave or R wave >50%; 3) Change in pacing impedance ≥50 Ω or 4) Change in shock impedance ≥5 Ω.11.
If these precautions are taken before, during and after MRI, complications are expected to be rare. In a total of about 1000 patients with non-MR-conditional pacemakers included in several recent studies, in which MRI was performed under controlled conditions, no clinically relevant complications were reported.5 There have been no reports in the literature of bradyarrhythmias or tachyarrhythmias requiring therapeutic intervention, no lead dysfunction in need of revision, no damage to CIED components or dysfunction of devices requiring replacement, nor cardiac perforation or death. Similarly, in a very recent prospective study of more than 1,500 patients with non-MR-conditional CIEDs undergoing a 1.5 T MRI, with due safety precautions, there were no clinically significant adverse events in long-term follow-up.3
Magnetic resonance image quality in patients with cardiac implanted electronic devicesOnce the safety of the MRI study has been guaranteed in patients with a CIED, it is important to consider the challenge of obtaining quality diagnostic images in this context. Although an extensive review of this subject is outside the scope of this Consensus Document, brief technical recommendations for optimizing image quality are summarized here.
In general, the most frequent artifacts are the “black bands” caused by the inhomogeneity of the field. This occurs due to the presence of the CIED, especially the battery. The extent of these artifacts depends on several factors, espeically the strength of the magnetic field, the location and size of the CIED, the anatomical zone under study and the sequences used.17 In the specific case of cardiac MRI, the strategies currently available for minimizing these artifacts include:
- 1
Focusing on fast gradient echo sequences in detriment to the usual steady-state free precession (SSFP) for the acquisition of cine images18
- 2
Acquiring cine images after administration of paramagnetic contrast agent
- 3
Using compressed sensing techniques, when available.
- 4
If using SSFP sequences:
- -
using frequency scout to choose where the artifacts are smaller
- -
increasing bandwidth and slightly decreasing the resolution,to reduce the repetition time to the minimum possible19
- 5
In late enhancement sequences, the inversion-recovery pulse bandwidth should be increased. If available, wideband sequences, developed specifically for this purpose, should be used.20
MRI has a major potential for impact on diagnosis, monitoring and decisions in all clinical areas. Its non-invasive and non-ionizing character and advances in the technology used mean it is key to diagnostic algorithms and indispensable in clinical practice.
The presence of CIEDs has traditionally been a contraindication for MRI due to the magnetic effects of the static field, magnetic gradients and RF on the devices and interaction-associated risk. The advent of MR-conditional devices and also recent studies assessing non-MR-conditional devices, have revealed that as long as appropriate safety measures are adopted, this risk is minimized and the use of diagnostic MRI is feasible in most situations. However, cardiologists and radiologists must be familiar with the type of device and its technical limitations (absolute and relative), the programming protocols appropriate to the devices, and the monitoring measures necessary for patient safety. Working as a team means that MRI can be made available to the vast majority of patients, preventing them from being deprived of this powerful diagnostic tool.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Prof. Dudley Pennell and Prof. Francisco Alpendurada of the Royal Brompton & Harefield NHS Foundation Trust, who allowed the adaptation of original documents to prepare the attached checklist.
They also thank Prof. Nuno Bettencourt and Dr. Leonor Parreira for the reviewing of the article.
Please cite this article as: Almeida AG, António N, Saraiva C, Ferreira AM, Reis AH, Marques H, et al. Documento de Consenso sobre a realização de ressonância magnética em doentes com dispositivos cardíacos electrónicos implantados. Rev Port Cardiol. 2021;40:41–52.
Endorsed by: Portuguese Society of Cardiology and Portuguese Society of Radiology and Nuclear Medicine. Prepared by the Portuguese Association of Arrhythmology, Pacing and Electrophysiology (APAPE) and the Nuclear Cardiology, Magnetic Resonance and Cardiac CT Study Group (GECNRMTC) of the Portuguese Society of Cardiology; Cardiac Imaging Center of the Portuguese Society of Radiology and Nuclear Medicine.