The aim of the study was to compare functional capacity in different types of congenital heart disease (CHD), as assessed by cardiopulmonary exercise testing (CPET).
MethodsA retrospective analysis was performed of adult patients with CHD who had undergone CPET in a single tertiary center. Diagnoses were divided into repaired tetralogy of Fallot, transposition of the great arteries (TGA) after Senning or Mustard procedures or congenitally corrected TGA, complex defects, shunts, left heart valve disease and right ventricular outflow tract obstruction.
ResultsWe analyzed 154 CPET cases. There were significant differences between groups, with the lowest peak oxygen consumption (VO2) values seen in patients with cardiac shunts (39% with Eisenmenger physiology) (17.2±7.1ml/kg/min, compared to 26.2±7.0ml/kg/min in tetralogy of Fallot patients; p<0.001), the lowest percentage of predicted peak VO2 in complex heart defects (50.1±13.0%) and the highest minute ventilation/carbon dioxide production slope in cardiac shunts (38.4±13.4). Chronotropism was impaired in patients with complex defects. Eisenmenger syndrome (n=17) was associated with the lowest peak VO2 (16.9±4.8 vs. 23.6±7.8ml/kg/min; p=0.001) and the highest minute ventilation/carbon dioxide production slope (44.8±14.7 vs. 31.0± 8.5; p=0.002). Age, cyanosis, CPET duration, peak systolic blood pressure, time to anaerobic threshold and heart rate at anaerobic threshold were predictors of the combined outcome of all-cause mortality and hospitalization for cardiac cause.
ConclusionAcross the spectrum of CHD, cardiac shunts (particularly in those with Eisenmenger syndrome) and complex defects were associated with lower functional capacity and attenuated chronotropic response to exercise.
Comparar a capacidade funcional nas cardiopatias congénitas, avaliada por prova de esforço cardiorrespiratória.
MétodosAnálise restrospetiva dos doentes adultos com cardiopatia congénita, submetidos a prova de esforço cardiorrespiratória. Os doentes foram divididos em tetralogia de Fallot operada, transposição de grandes artérias após cirurgia de Senning/Mustard, transposição de grandes artérias congenitamente corrigida, defeitos complexos, shunts, doença valvular esquerda e obstrução do trato de saída do ventrículo direito.
ResultadosForam avaliadas 154 provas cardiorrespiratórias. Os valores mais baixos de consumo de oxigénio no pico foram observados nos doentes com shunt cardíaco (39% apresentavam síndrome de Eisenmenger) (17,2 ± 7,1ml/kg/min, em comparação com 26,2 ± 7,0ml/kg/min na tetralogia de Fallot; p < 0,001); o valor mais baixo da percentagem de consumo de oxigénio no pico relativamente ao previsto foi observado nos defeitos complexos (50,1 ± 13,0%) e o maior valor de rampa ventilação minuto/produção de dióxido de carbono nos shunts cardíacos (38,4 ± 13,4). O cronotropismo foi menos eficaz nos doentes com defeitos complexos. A síndrome de Eisenmenger (n = 17) associou-se ao valor mais baixo de consumo de oxigénio no pico (16,9 ± 4,8 versus 23,6 ± 7,8ml/kg/min; p = 0,001) e ao maior valor de rampa ventilação minuto/produção de dióxido de carbono (44,8 ± 14,7 versus 31,0 ± 8,5; p = 0,002). Idade, cianose, duração da prova, pressão arterial sistólica no pico, tempo para o limiar anaeróbio e frequência cardíaca no limiar anaeróbio foram preditores do outcome combinado com mortalidade de todas as causas e hospitalização de causa cardíaca.
ConclusãoOs shunts cardíacos (particularmente com síndrome de Eisenmenger) e os defeitos complexos associaram-se a menor capacidade funcional e resposta cronotrópica atenuada ao exercício.
Nowadays most patients with congenital heart disease (CHD) are expected to reach adulthood. Because exercise intolerance has been documented at all ages of CHD, these patients need close follow-up and an objective assessment of functional capacity.1,2
Due to long-term adaption, the majority of adult patients with CHD self-report their exercise capacity status as satisfactory, even in the presence of significantly depressed functional status. Cardiopulmonary exercise testing (CPET) is an accurate method for quantitative assessment of exercise capacity, including assessment of aerobic capacity, chronotropic response and arrhythmias.1,3–6 Quantifying exercise capacity by measuring parameters such as peak oxygen consumption (VO2) is an established technique in the management of patients with chronic heart failure. However, in adult CHD patients its role has been much less studied, and interpretation of test results remains a challenge. Previous studies have demonstrated that CPET data have an important influence on the treatment approach in CHD, including indication for cardiac transplantation, and on prognosis.2,7,8
The aim of the present study was twofold: to assess and compare functional capacity in different CHD groups, measured objectively by CPET, and to investigate a possible association between CPET parameters and outcome.
MethodsStudy designA retrospective analysis was performed of consecutive adult patients with CHD who underwent CPET for assessment of functional capacity. The data were collected in a single tertiary center between March 2009 and June 2015.
The study population was divided according to diagnosis or pathophysiological status: repaired tetralogy of Fallot, transposition of the great arteries (TGA) after Senning or Mustard procedures and congenitally corrected TGA (both with a right ventricle functioning as a systemic ventricle), complex heart defects (univentricular heart, Fontan surgery, truncus arteriosus), shunts (atrial, ventricular or arterial), left heart valve disease with stenosis or regurgitation (bicuspid aortic valve, subaortic stenosis), and right ventricular outflow tract obstruction (RVOTO).
CPET was performed in patients with some degree of effort intolerance, complex defects or significant residual lesions.
The combined outcome of hospitalization for cardiac cause and all-cause mortality was analyzed.
Cardiopulmonary exercise testingMaximal symptom-limited treadmill CPET was performed using the modified Bruce protocol. CPET and the recovery period were monitored with continuous 12-lead electrocardiogram, blood pressure cuff, saturation probe and a face mask to measure respiratory gases. Blood pressure was measured at rest, at each stage, at peak exercise and at the first, third and sixth minute of the recovery phase. Respiratory gases were analyzed using an Innocor® gas analyzer and VO2, carbon dioxide production and ventilation were measured on a breath-by-breath basis.
Patients were encouraged to perform exercise until the carbon dioxide production/oxygen consumption ratio was 1.15 or higher.
Peak VO2 adjusted for body mass, or for fat-free mass in obese patients (body mass index >30kg/m2), was analyzed, as well as the percentage of predicted peak VO2 for age and gender according to the Wasserman/Hansen equation. The minute ventilation (VE)/carbon dioxide production (VCO2) slope was calculated by automatic linear regression with values obtained during CPET. The ratio between VE/VCO2 slope and peak VO2 was also calculated. Peak circulatory power was determined by multiplying peak VO2 by peak systolic blood pressure. Both baseline and peak oxygen saturations were also collected.
The chronotropic index was calculated as (peak heart rate/resting heart rate)/(220-age/resting heart rate), and considered normal for values between 0.8 and 1.3.9
Statistical analysisThe statistical analysis was performed using SPSS Statistics version 22 (IBM SPSS, Chicago, IL). Continuous variables were expressed as mean ± standard deviation. CPET parameters were compared between study groups using one-way analysis of variance between mean values or the non-parametric Kruskal-Wallis test, and multiple comparisons between the study groups were performed with an appropriate post-hoc test. Pearson's chi-square or Fisher's exact test was applied for categorical variables. The Student's t test or the Wilcoxon-Mann-Whitney test for continuous variables was used for gender comparisons. Pearson's correlation was used to estimate correlation between continuous variables. The association between variables and the combined outcome was assessed with univariate Cox proportional hazards analysis (forward stepwise).
ResultsCPET data were analyzed from 154 patients, mean age 34.8±8.8 years, 55.8% male. The most frequent diagnosis was corrected tetralogy of Fallot (36%), followed by complex defects (21%) (Table 1).
Cardiopulmonary exercise testing data.
| Diagnosis | n | Age (years) | Male (%) | Peak VO2 (ml/kg/min) | % predicted peak VO2 | VE/VCO2 slope | VE/VCO2 slope/VO2 | PCP (mmHg/ml/kg/min) | Baseline OS (%) | Peak OS (%) | CPET duration (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ToF | 55 | 35.0±8.4 | 64.8 | 26.2±7.0a,b | 68.9±15.3a,b | 27.5±5.3a,b | 1.2±0.6 | 4195.6±1484.7a,b | 95.2±3.4a | 91.1±4.5b | 14.0±3.3a |
| TGA | 15 | 30.1±4.5 | 88.5 | 22.0±5.0 | 55.2±11.7 | 34.0±9.5 | 1.7±1.1 | 3388.2±1023.7 | 93.3±3.6 | 88.4±6.0 | 13.3±1.9 |
| ccTGA | 6 | 34.4±8.3 | 83.3 | 24.3±3.1 | 57.0±11.7 | 38.2±13.2 | 1.6±0.7 | 3917.3±540.3 | 93.5±7.8 | No data | 14.8±1.4 |
| Complex defects | 33 | 32.4±7.5 | 50.0 | 20.1±6.2b | 50.1±13.0b | 36.6±11.4b | 1.8±1.0 | 2958.0±1029.7b | 89.5±4.4a,b,c | 83.0±8.9a,b | 12.1±3.1 |
| Shuntsd | 24 | 38.4±11.9 | 43.5 | 17.2±7.1a | 54.4±17.9a | 38.4±13.4a | 1.6±1.3 | 2680.9±1259.9a | 95.1±4.6b | 89.8±11.0a | 10.8±4.3a,b |
| Left heart valve disease | 8 | 31.5±8.9 | 50.0 | 28.1±13.4 | 65.8±12.8 | 26.7±4.2 | 1.2±0.6 | 4612.2±1910.2 | 94.2±4.2 | 92.8±3.9 | 16.1±3.1b |
| RVOTO | 13 | 39.7±7.9 | 30.8 | 22.8±8.8 | 64.3±18.0 | 33.3±8.4 | 1.4±0.7 | 4181.5±1857.5 | 96.0±2.6c | 86.2±8.0 | 12.6±4.7 |
| a p<0.001 b p=0.004 | a p=0.005 b p<0.001 | a p<0.001 b p=0.004 | a p=0.028 b p=0.006 | a p<0.001 b p=0.001 c p=0.005 | a p=0.020 b p=0.015 | a p=0.030 b p=0.046 |
d 39% had Eisenmenger syndrome.
% predicted peak VO2: percentage of predicted peak oxygen consumption; ccTGA: congenitally corrected transposition of the great arteries; CPET: cardiopulmonary exercise testing; OS: oxygen saturation; PCP: peak circulatory power; RVOTO: right ventricular outflow tract obstruction; TGA: transposition of the great arteries after Senning or Mustard procedure; ToF: tetralogy of Fallot; VE/VCO2: minute ventilation/carbon dioxide production; VO2: oxygen consumption.
There were significant differences in CPET parameters between the study groups. As shown in Table 1, the lowest values for peak VO2 were seen in patients with cardiac shunts, related to the fact that 39% of these patients had Eisenmenger syndrome (17.2±7.1ml/kg/min, compared to 26.2±7.0ml/kg/min in tetralogy of Fallot patients; p<0.001). Peak VO2 differed significantly between genders only in the complex heart defect group (males 23.3±6.1ml/kg/min vs. females 16.9±4.6ml/kg/min; p<0.001). Percentage of predicted peak VO2 adjusted for age and gender was lower in the complex heart defect group (50.1±13.0%) compared to the other groups.
Patients with cardiac shunts and congenitally corrected TGA had higher VE/VCO2 slope (38.4±13.4 and 38.2±13.2, respectively), for which the lowest value was in left heart valve disease. A significant difference between genders was observed only in the complex defects group (males 32.3±12.4 vs. females 41.3±8.3; p<0.012). The ratio between VE/VCO2 slope and peak VO2 was higher in complex defects. Peak circulatory power was lower in patients with shunts and complex defects.
Peak oxygen saturation below 90% was seen in patients with RVOTO, TGA and shunts, and it was particularly low in complex defects (83.0±8.9%).
Chronotropism was impaired in patients with complex defects, among whom 71.9% presented chronotropic incompetence, demonstrated by a chronotropic index <0.8 (Table 2).
Assessment of chronostropism.
| Diagnosis | Peak heart rate (bpm) | Chronotropic index <0.8 (%) | Patients under Beta-blockers (%) |
|---|---|---|---|
| ToF | 156.7 ± 23.5 | 46.3 | 18.5 |
| TGA | 151.5 ± 34.8 | 26.9 | 3.8 |
| ccTGA | 147.0 ± 17.0 | 66.7 | 50.0 |
| Complex defects | 143.8 ± 29.1 | 71.9 | 40.6 |
| Shunts | 138.7 ± 23.7 | 69.9 | 13.0 |
| Left heart valve disease | 168.5 ± 27.5 | 37.5 | 12.5 |
| RVOTO | 157.7 ± 23.7 | 46.2 | 30.8 |
ccTGA: congenitally corrected transposition of the great arteries; HR: heart rate; RVOTO: right ventricular outflow tract obstruction; TGA: transposition of the great arteries after Senning or Mustard procedure; ToF: tetralogy of Fallot.
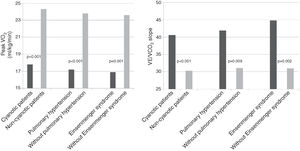
Analyzing the overall population, cyanotic patients (n=33) presented significantly lower peak VO2 and higher VE/VCO2 slope than non-cyanotic patients (17.8±5.4 vs. 24.3±7.9ml/kg/min; p<0.001 and 40.6±13.0 vs. 30.3±8.0; p<0.001, respectively). Patients with pulmonary hypertension (n=23) also presented lower peak VO2 (17.2±5.9 vs. 23.8±7.8ml/kg/min; p<0.001) and higher VE/VCO2 slope (41.9±14.9 vs. 31.1±8.5; p=0.009). Eisenmenger syndrome (n=17) was associated with even lower peak VO2 (16.9±4.8 vs. 23.6±7.8ml/kg/min; p=0.001) and higher VE/VCO2 slope (44.8±14.7 vs. 31.0± 8.5; p=0.002) (Figure 1).
TGA patients (after Mustard or Senning surgery) presented a negative linear correlation between time from intervention to CPET and peak VO2 (r=-0.564; p=0.070) and peak circulatory power (r=-0.632; p=0.037), and a positive linear Pearson correlation between time from intervention to CPET and VE/VCO2 slope (r=0.554; p=0.122) (Figure 2).
Transposition of the great arteries. (A) Negative linear Pearson correlation between time from Mustard or Senning surgery to CPET and peak oxygen consumption (VO2) (r=-0.564); (B) positive linear Pearson correlation between time from Mustard or Senning surgery to CPET and VE/VCO2 slope (r=0.554).
In the overall population, 18.2% of patients (n=28) reached a carbon dioxide production/oxygen consumption ratio of 1.15 or higher.
During a mean follow-up of 31.9 months (minimum six months; maximum 79 months), the combined outcome of all-cause mortality and hospitalization for cardiac cause was documented in 34 patients. In univariate Cox regression, age, cyanosis, CPET duration, peak systolic blood pressure, time to anaerobic threshold and heart rate at anaerobic threshold were predictors of the combined outcome (Table 3). Peak VO2 was not a predictor of the combined outcome (hazard ratio 0.995; confidence interval 0.949-1.043; p=0.829).
Predictors of the combined outcome (hospitalization for cardiac cause and all-cause mortality) by univariate Cox regression.
| Predictors | HR | 95% CI | p |
|---|---|---|---|
| Age | 1.065 | 1.019-1.113 | 0.005 |
| Cyanosis | 3.584 | 1.094-11.737 | 0.035 |
| CPET duration | 0.907 | 0.826-0.995 | 0.040 |
| Peak systolic blood pressure | 0.982 | 0.967-0.998 | 0.029 |
| Time to anaerobic threshold | 0.819 | 0.717-0.935 | 0.003 |
| Heart rate at anaerobic threshold | 0.973 | 0.955-0.993 | 0.007 |
CI: confidence interval; CPET: cardiopulmonary exercise testing; HR: hazard ratio.
Although an increasing number of CHD patients reach adulthood, their exercise capacity is often significantly impaired. CPET enables more detailed assessment of functional capacity by measuring respiratory gases. Peak VO2, which reflects VO2 in tissues and depends on cardiac output, arterial oxygen and the oxygen extraction capacity of muscle tissue, is an accurate measure of exercise capacity.10
In line with previous research,1,10–13 our study showed markedly depressed functional capacity in CHD patients, as shown by the fact that all study groups presented peak VO2 values below 70% of those predicted. Regardless of the type of CHD, the peak VO2 values reached by the study population were substantially lower than those expected for healthy subjects of the same age and gender.
Another useful parameter is VE/VCO2 slope, which expresses ventilatory efficiency and has been shown to be an independent predictor of outcome.14 The ratio between VE/VCO2 slope and peak VO2 integrates these parameters and also has prognostic value.15 By integrating the hemodynamic values monitored in this test it is possible to compute peak circulatory power. All these parameters were consistently abnormal in all groups.
Across the CHD spectrum, patients with left heart valve disease and repaired tetralogy of Fallot presented better exercise capacity. On the other hand, TGA (after Mustard or Senning surgery, or congenitally corrected), complex heart defects and shunts were associated with more severely impaired functional capacity.
It should also be noted that 39% of patients with shunts presented Eisenmenger physiology, which explains the severe impairment of exercise tolerance in this group.
Irrespective of the baseline defect, cyanosis and pulmonary hypertension were associated with poor exercise tolerance, particularly when the two were combined, which was associated with lower peak VO2 and higher VE/VCO2 slope. According to Diller et al.,2 patients with Eisenmenger physiology have the most severe impairment in exercise capacity, reflected in the lowest peak VO2 and the highest VE/VCO2 slope.
Our data demonstrated a negative correlation between time from Mustard or Senning surgery to CPET and peak VO2 and peak circulatory power, and a positive correlation between time from surgery to exercise testing and VE/VCO2 slope. This deleterious evolution and ongoing morbidity could be explained by progressive failure of a systemic right ventricle and also by chronotropic incompetence.16
Furthermore, chronotropic response to exercise is an important determinant of functional capacity, and diminished heart rate during exercise may contribute to reductions in peak VO2.12,17
Exercise intolerance has been associated with increased risk of hospitalization and mortality.2,7,18,19 However, unlike in previous studies, we found no correlation between peak VO2 or VE/VCO2 slope and the combined outcome of hospitalization for cardiac cause and all-cause mortality. This could be due to the small sample size and the heterogeneity of the study population. Instead, our data showed lower values of CPET duration, peak systolic blood pressure, time to anaerobic threshold and heart rate at anaerobic threshold as benchmarks of lower functional capacity correlated with poor prognosis. Our results are in line with Diller et al.’s study of 321 Fontan patients, in whom chronotropic capacity was strongly related to survival, contrasting with a lack of association between peak VO2 or ratio between VE/VCO2 slope and survival or cardiac transplantation.8 The relation between chronotropic incompetence and all-cause mortality in CHD patients could be secondary to underlying autonomic dysfunction, neurohormonal activation and arrhythmias.4,7
We also established an association between cyanosis and poor outcome, with a 3.6-fold increase in mortality and hospitalization compared to non-cyanotic patients. Similar data were reported by Dimopoulos et al., who also interestingly demonstrated that in adult CHD the VE/VCO2 slope is a strong predictor of mortality only in patients without cyanosis, suggesting that cyanotic patients differ substantially in pathophysiological processes and in these patients the prognostic value of the VE/VCO2 slope is weaker.19
Age was also related to worse prognosis in our study, reflecting progressive impairment in left and/or right ventricular function, increasing prevalence of pulmonary hypertension and occurrence of arrhythmias in older CHD patients.
To the best of our knowledge, this is the first Portuguese publication on CPET in adult CHD patients. We consider that the report of our initial experience is of interest, highlighting the importance of CPET in this population.
Study limitationsThis was a retrospective study at a tertiary adult CHD center that reflects daily clinical practice. There was thus certainly a bias in patient selection, favoring more symptomatic patients and those in which it is important not to rely only on self-reported functional capacity. This could explain the severity seen in the shunt subgroup, in which there was a large proportion of Eisenmenger syndrome.
The study population size inevitably reflects the limitations of a single-center experience. Considering that CPET is extremely important in the assessment of CHD patients and should be performed routinely, in future we intend to study more patients, with different clinical situations.
ConclusionOur sample of adult CHD patients who underwent CPET presented exercise intolerance, which differed significantly across the spectrum of CHD. These data illustrate the more severe impairment in functional capacity and attenuated chronotropic response to exercise in Eisenmenger syndrome and complex defects.
In this study, age, cyanosis and worse functional capacity were, as expected, associated with the combined outcome of all-cause mortality and hospitalization for cardiac cause.
Increasing the number of patients and the variety of defects and performing serial assessments in each patient will enable us to obtain more robust results in the future.
Conflicts of interestThe authors have no conflicts of interest to declare.