Diabetes mellitus and chronic total occlusions are associated with unfavorable outcome after percutaneous coronary intervention. We sought to assess the clinical and angiographic outcomes of diabetic and non-diabetic patients who underwent successful percutaneous revascularization of chronic total occlusions with drug-eluting stents.
MethodsBaseline clinical and angiographic characteristics, procedural details, nine-month angiographic follow-up and clinical events at 12 months were compared between 75 diabetic and 132 non-diabetic patients included in a clinical trial that randomized successful recanalization of chronic total occlusions to receive sirolimus- or everolimus-eluting stents.
ResultsIn both diabetic and non-diabetic groups there was a favorable non-significantly different angiographic result at nine months, with low in-stent late loss (0.14±0.60 mm vs. 0.25±0.68 mm, p=0.305) and rates of binary restenosis (4.0% vs. 10.6%, p=0.180) and reocclusion (0.0% vs. 2.3%, p=0.334). During follow-up similar survival from death (97.3±1.9% vs. 99.2±0.8%, log-rank p=0.273), acute myocardial infarction (100.0±0.0% vs. 97.7±1.3%, log-rank p=0.192), target vessel revascularization (88.7±3.8% vs. 88.2±2.9%, log-rank p=0.899) and stent thrombosis (100.0±0.0% vs. 97.7±1.3%, log-rank p=0.192) was observed. Furthermore, the presence of more diffuse peripheral and coronary artery disease and higher frequency of calcified lesions in diabetic patients did not lead to significant differences in the approach (20.0% vs. 25.0% radial approach, p=0.413), strategy (6.7% vs. 3.8% retrograde strategy, p=0.353), total stent length (48.1±24.6 mm vs. 49.2±23.9 mm, p=0758) or contrast volume (261.3±116.4 ml vs. 297.4±135.9 ml, p=0.109) required for revascularization.
ConclusionsIn the drug-eluting stent era, diabetic and non-diabetic patients have comparable favorable clinical and angiographic outcomes after successful percutaneous revascularization of chronic total occlusions.
A diabetes mellitus e as oclusões crónicas totais estão associadas a resultados desfavoráveis após intervenções coronárias percutâneas. Procurámos avaliar os resultados clínicos e angiográficos dos doentes diabéticos e não diabéticos que se submeteram a revascularização percutânea bem sucedida da oclusão crónica total com stents revestidos.
MétodosCaracterísticas clínicas e angiográficas de base, detalhes técnicos, seguimento angiográfico a 9 meses e eventos clínicos a 12 meses foram comparados entre 75 doentes diabéticos e 132 não diabéticos incluídos num estudo clínico que aleatorizou a recanalização bem sucedida da oclusão crónica total para receber o stent revestido com sirolimus ou com everolimus.
ResultadosEm ambos os grupos diabéticos e não diabéticos houve um resultado angiográfico deiferente favorável e não significativo aos 9 meses com baixa taxa de in-stent loss (0,14±0,60 mm versus 0,25±0,68 mm, p=0,305) e taxas de reestenose binária (4,0% versus 10,6%, p=0,180) e reoclusão (0,0% versus 2,3%, p=0,334). Durante o seguimento foi observada sobrevivência semelhante de morte (97,3±1,9% versus 99,2±0,8%, log-rank p=0,273), enfarte agudo do miocárdio (100,0±0,0% versus 97,7±1,3%, log-rank p=0,192), revascularização do vaso alvo (88,7±3,8% versus 88,2±2,9%, log-rank p=0,899), e trombólise de stent (100,0±0.0% versus 97,7±1,3%, log-rank p=0,192). Além disso, a presença de doença periférica mais difusa, de doença das artérias coronárias e frequência mais elevada de lesões calcificadas nos doentes diabéticos não implicou diferenças significativas na abordagem (abordagem radial 20,0 versus 25,0%, p=0,413), estratégia (estratégia retrógrada 6,7% versus 3,8%, p=0,353), comprimento total do stent (48,1±24,6 mm versus 49,2±23,9 mm, p=0,758) e volume de contraste (261,3±116,4 ml versus 297,4±135,9 ml, p=0,109), necessário para a revascularização.
ConslusõesNa era dos stents revestidos, os doentes diabéticos e não diabéticos podem apresentar resultados clínicos e angiográficos comparáveis e favoráveis, após revascularização precutânea da oclusão crónica total.
Diabetes mellitus (DM) and the presence of chronic total occlusions (CTO) have traditionally been considered two of the most powerful predictors for unfavorable outcome after percutaneous coronary intervention (PCI), and so both are still associated with a higher probability of patients being referred for coronary artery bypass grafting (CABG).1,2 Patients with DM have a higher rate of angiographic restenosis and major adverse cardiovascular events (MACE) than non-diabetic patients.3 At the same time, CTO constitutes one of the most challenging scenarios for interventional cardiologists. Apart from the difficulties in achieving recanalization of the vessel, patients with initially successful CTO-PCI still have high rates of restenosis, reocclusion and new revascularization procedures.4,5
Drug-eluting stents (DES) have been shown to dramatically improve the outcome of patients with coronary artery disease, especially in more complex scenarios such as DM or CTO,4,6–8 although both DM and CTO are still independently associated with higher event rates.3–5 Our objective was to compare the clinical and angiographic outcomes of patients with and without DM among those with CTO successfully treated with DES implantation. For this purpose, we used the data from the CIBELES randomized trial, which compared a sirolimus-eluting stent (SES) with an everolimus-eluting stent (EES) in patients with CTO.9
MethodsPatients and methodsThe CIBELES study was a multicenter trial (13 centers in Spain and Portugal) that randomized 207 patients with successfully revascularized CTO to receive SES or EES (clinicaltrials.gov number NCT00793221). The study design has been previously published in detail.10 Briefly, patients aged 18 years or over with a total occlusion (Thrombolysis In Myocardial Infarction [TIMI] flow grade 0 or 1) of more than two weeks duration were included. Patients with acute myocardial infarction in the territory supplied by the target vessel up to two weeks before inclusion in the study, lesions previously treated percutaneously, and patients with contraindication for prolonged dual antiplatelet therapy were excluded. All patients gave written informed consent before the procedure, and the study was approved by the ethics committee of each participating center.
The primary endpoint was in-stent late loss at nine-month angiographic follow-up, and so patients were scheduled for routine angiographic follow-up to demonstrate non-inferiority. Quantitative coronary analysis (QCA) was performed at a central core lab by experienced physicians blinded to the type of stent implanted, and not involved in the treatment of the patients. MEDIS software (MEDIS, Leiden, The Netherlands) was used for QCA.
Secondary endpoints included binary angiographic restenosis (>50% stenosis at angiographic follow-up) and the rate of clinical events at 12 months (death, cardiovascular death, myocardial infarction, target vessel revascularization, stroke, and stent thrombosis). The study featured an independent clinical events committee consisting of three cardiologists not involved in the treatment or follow-up of the patients. The study was monitored by Chiltern International, and sponsored by the Spanish Society of Cardiology (with an unrestricted grant from Abbott Vascular). Episodes of stent thrombosis were classified following the Academic Research Consortium criteria.11
The diagnosis of DM was made according to previous patient records. Of the 207 patients included in the study, 75 (36.2%) had DM (59 on diet and/or oral antidiabetic agents, and 16 under insulin therapy). Baseline clinical and angiographic characteristics, procedural details, and angiographic and clinical follow-up were compared between patients with and without DM.
Statistical analysisThe statistical analysis was performed with the SPSS statistical package, version 12.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared with the Student's t test, and discrete variables with the chi-square test and Fisher's exact test when necessary. The incidence of clinical outcomes was estimated using Kaplan-Meier survival curves, and compared with the log-rank and Breslow tests. Differences were considered statistically significant when p<0.05, although all p values are provided.
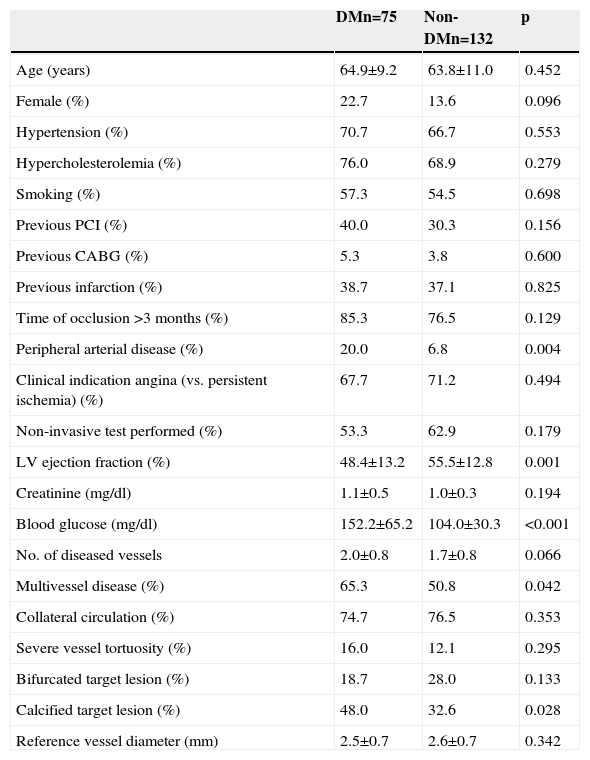
ResultsBaseline profile and procedural details in patients with and without diabetesTable 1 shows clinical characteristics and angiographic features in patients with and without DM. Patients with DM had a higher prevalence of peripheral arterial disease, more extensive coronary artery disease, higher frequency of vessel calcification, lower left ventricular ejection fraction, and a trend to higher frequency of female gender. Other clinical and angiographic characteristics, including estimated time since occlusion and reference vessel diameter, were not found to be significantly different between the groups.
Baseline characteristics of patients included in the CIBELES trial according to diabetes status.
| DMn=75 | Non-DMn=132 | p | |
|---|---|---|---|
| Age (years) | 64.9±9.2 | 63.8±11.0 | 0.452 |
| Female (%) | 22.7 | 13.6 | 0.096 |
| Hypertension (%) | 70.7 | 66.7 | 0.553 |
| Hypercholesterolemia (%) | 76.0 | 68.9 | 0.279 |
| Smoking (%) | 57.3 | 54.5 | 0.698 |
| Previous PCI (%) | 40.0 | 30.3 | 0.156 |
| Previous CABG (%) | 5.3 | 3.8 | 0.600 |
| Previous infarction (%) | 38.7 | 37.1 | 0.825 |
| Time of occlusion >3 months (%) | 85.3 | 76.5 | 0.129 |
| Peripheral arterial disease (%) | 20.0 | 6.8 | 0.004 |
| Clinical indication angina (vs. persistent ischemia) (%) | 67.7 | 71.2 | 0.494 |
| Non-invasive test performed (%) | 53.3 | 62.9 | 0.179 |
| LV ejection fraction (%) | 48.4±13.2 | 55.5±12.8 | 0.001 |
| Creatinine (mg/dl) | 1.1±0.5 | 1.0±0.3 | 0.194 |
| Blood glucose (mg/dl) | 152.2±65.2 | 104.0±30.3 | <0.001 |
| No. of diseased vessels | 2.0±0.8 | 1.7±0.8 | 0.066 |
| Multivessel disease (%) | 65.3 | 50.8 | 0.042 |
| Collateral circulation (%) | 74.7 | 76.5 | 0.353 |
| Severe vessel tortuosity (%) | 16.0 | 12.1 | 0.295 |
| Bifurcated target lesion (%) | 18.7 | 28.0 | 0.133 |
| Calcified target lesion (%) | 48.0 | 32.6 | 0.028 |
| Reference vessel diameter (mm) | 2.5±0.7 | 2.6±0.7 | 0.342 |
CABG: coronary artery bypass grafting; DM: diabetes mellitus; LV: left ventricular; PCI: percutaneous coronary intervention.
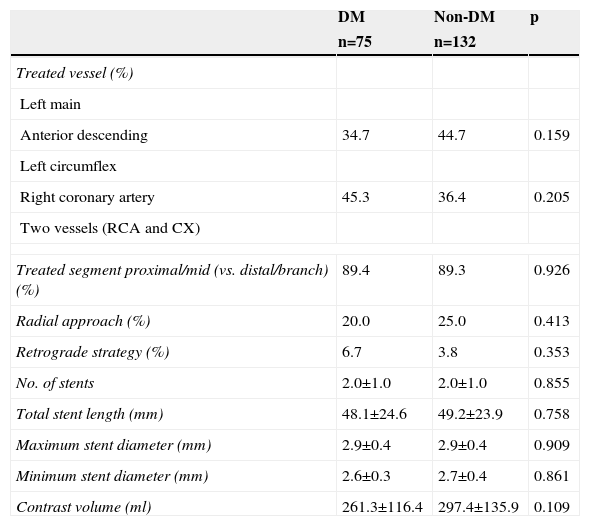
Procedural details are presented in Table 2. The use of retrograde techniques was low, and the number of devices used (guidewires, balloons, stents) was high, in both groups. The total stent length required for successful revascularization was also high in both. The proportion of patients with a final successful angiographic result was similar (97.3% in DM and 98.5% in non-DM; p=0.662) and no significant differences were observed between DM and non-DM patients.
Procedural details of diabetic compared to non-diabetic patients with successful revascularization of chronic total occlusions.
| DM | Non-DM | p | |
|---|---|---|---|
| n=75 | n=132 | ||
| Treated vessel (%) | |||
| Left main | |||
| Anterior descending | 34.7 | 44.7 | 0.159 |
| Left circumflex | |||
| Right coronary artery | 45.3 | 36.4 | 0.205 |
| Two vessels (RCA and CX) | |||
| Treated segment proximal/mid (vs. distal/branch) (%) | 89.4 | 89.3 | 0.926 |
| Radial approach (%) | 20.0 | 25.0 | 0.413 |
| Retrograde strategy (%) | 6.7 | 3.8 | 0.353 |
| No. of stents | 2.0±1.0 | 2.0±1.0 | 0.855 |
| Total stent length (mm) | 48.1±24.6 | 49.2±23.9 | 0.758 |
| Maximum stent diameter (mm) | 2.9±0.4 | 2.9±0.4 | 0.909 |
| Minimum stent diameter (mm) | 2.6±0.3 | 2.7±0.4 | 0.861 |
| Contrast volume (ml) | 261.3±116.4 | 297.4±135.9 | 0.109 |
DM: diabetes mellitus; CX: circumflex artery; RCA: right coronary artery.
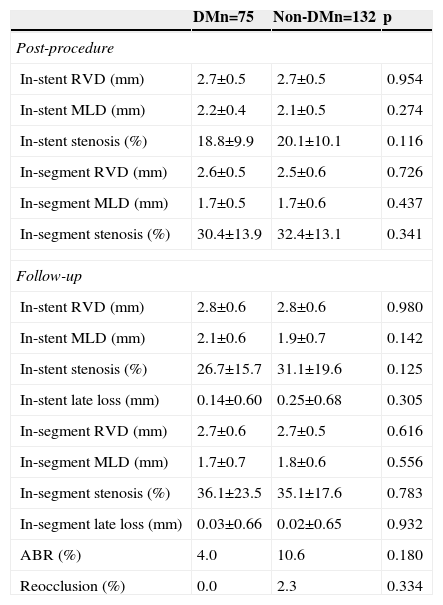
The rate of angiographic follow-up was high in both groups (89.3% and 86.4% in patients with and without DM, respectively; p=0.535). The diameter of the coronary vessel revascularized was relatively small (2.5±0.7 mm in DM and 2.6±0.7 mm in non-DM; p=0.342). Immediately after PCI, no differences were observed between the groups regarding the percentage of in-stent and in-segment stenosis. At nine months, the angiogram showed no differences in these values, and no diabetic patient presented reocclusion during follow-up (Table 3).
Angiographic data (quantitative coronary analysis).
| DMn=75 | Non-DMn=132 | p | |
|---|---|---|---|
| Post-procedure | |||
| In-stent RVD (mm) | 2.7±0.5 | 2.7±0.5 | 0.954 |
| In-stent MLD (mm) | 2.2±0.4 | 2.1±0.5 | 0.274 |
| In-stent stenosis (%) | 18.8±9.9 | 20.1±10.1 | 0.116 |
| In-segment RVD (mm) | 2.6±0.5 | 2.5±0.6 | 0.726 |
| In-segment MLD (mm) | 1.7±0.5 | 1.7±0.6 | 0.437 |
| In-segment stenosis (%) | 30.4±13.9 | 32.4±13.1 | 0.341 |
| Follow-up | |||
| In-stent RVD (mm) | 2.8±0.6 | 2.8±0.6 | 0.980 |
| In-stent MLD (mm) | 2.1±0.6 | 1.9±0.7 | 0.142 |
| In-stent stenosis (%) | 26.7±15.7 | 31.1±19.6 | 0.125 |
| In-stent late loss (mm) | 0.14±0.60 | 0.25±0.68 | 0.305 |
| In-segment RVD (mm) | 2.7±0.6 | 2.7±0.5 | 0.616 |
| In-segment MLD (mm) | 1.7±0.7 | 1.8±0.6 | 0.556 |
| In-segment stenosis (%) | 36.1±23.5 | 35.1±17.6 | 0.783 |
| In-segment late loss (mm) | 0.03±0.66 | 0.02±0.65 | 0.932 |
| ABR (%) | 4.0 | 10.6 | 0.180 |
| Reocclusion (%) | 0.0 | 2.3 | 0.334 |
ABR: angiographic binary restenosis; DM: diabetes mellitus; MLD: minimum lumen diameter; RVD: reference vessel diameter.
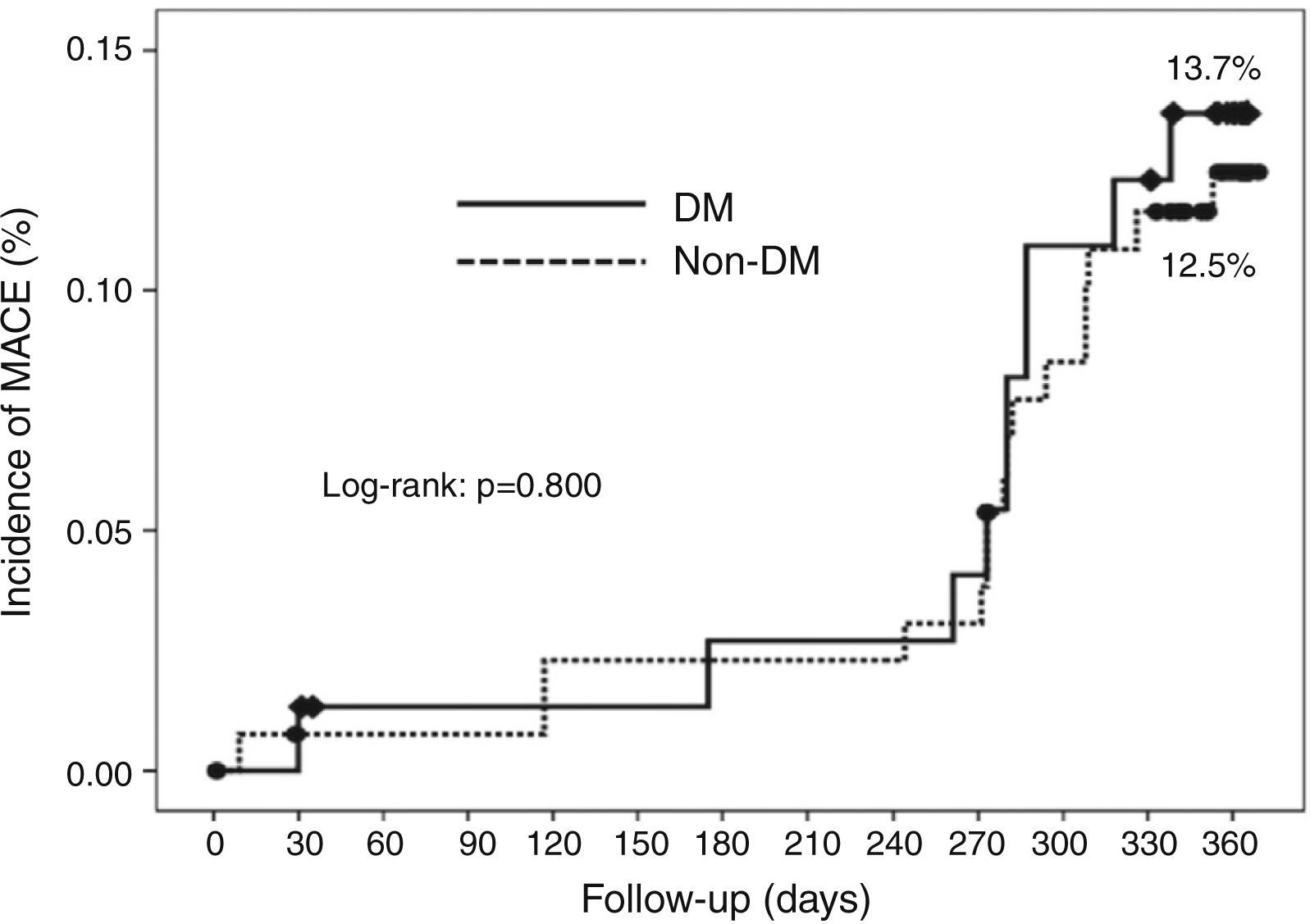
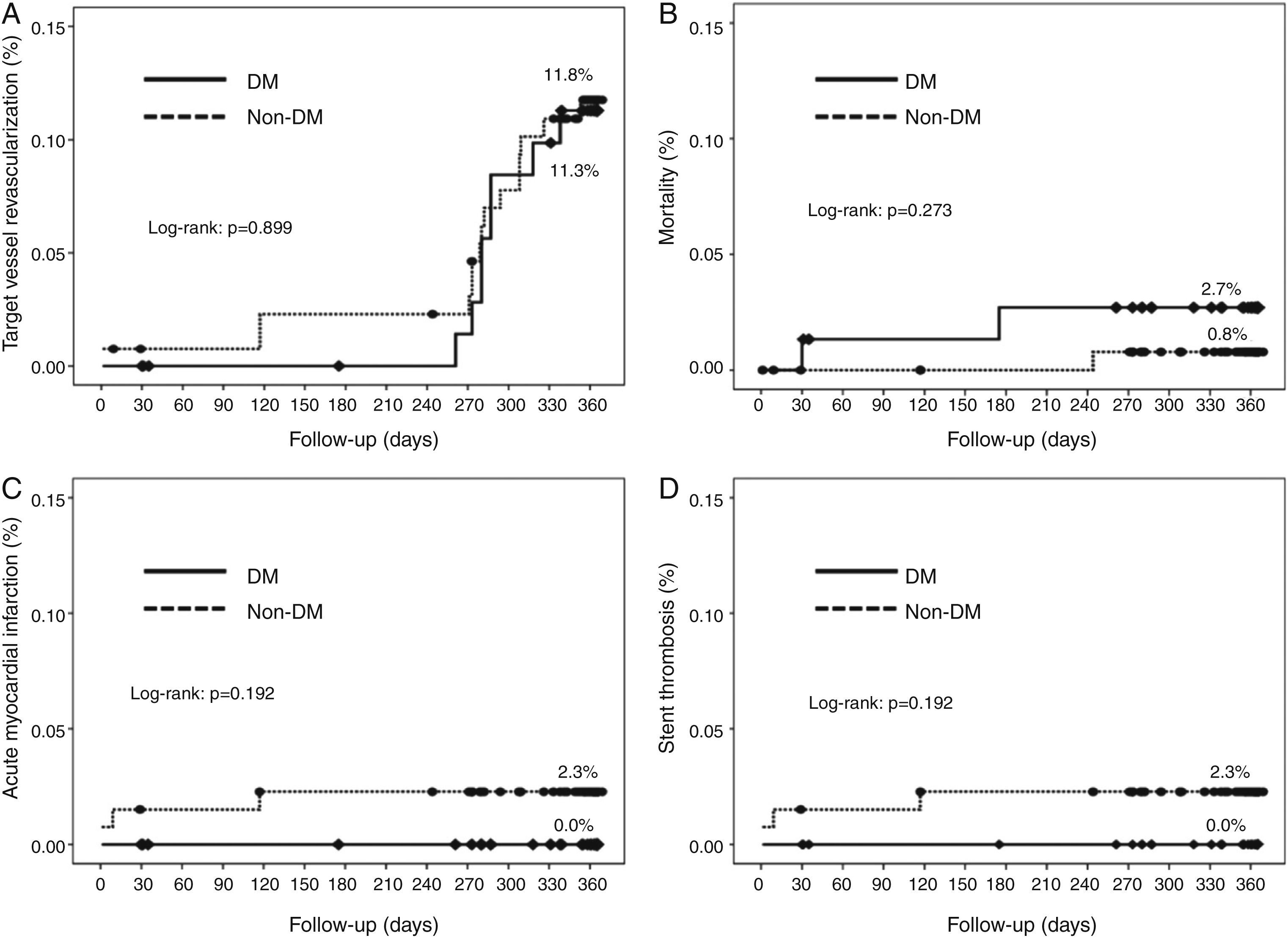
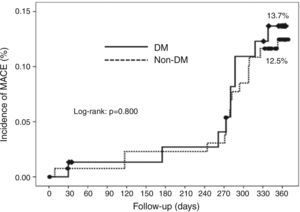
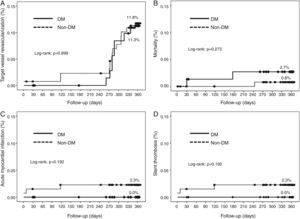
Cumulative survival free from MACE, death, acute myocardial infarction, and target vessel revascularization in patients with and without DM was 86.3±4.0% vs. 87.5±2.9% (log-rank: p=0.800); 97.3±1.9% vs. 99.2±0.8% (log-rank: p=0.273); 100.0±0.0% vs. 97.7±1.3% (log-rank: p=0.192); and 88.7±3.8% vs. 88.2±2.9% (log-rank: p=0.899), respectively. All three episodes of definite or probable stent thrombosis occurred in patients without DM (cumulative survival from stent thrombosis 100.0±0.0% vs. 97.7±1.3% for patients with and without DM, respectively; p=0.192).
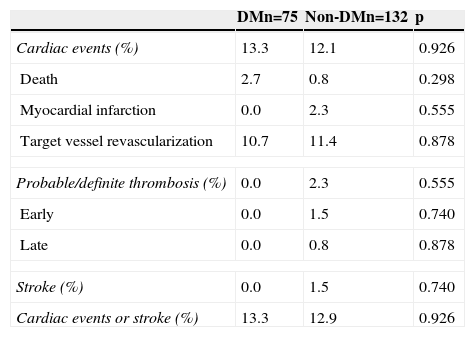
Figure 1 shows the Kaplan-Meier curves for the cumulative incidence of MACE (death, myocardial infarction, and target vessel revascularization) during 12-month follow-up. Figure 2 shows the cumulative incidence of death, acute myocardial infarction and target vessel revascularization separately as well as of stent thrombosis. As can be seen, there were no significant differences between the groups (Table 4).
Clinical events at 12 months.
| DMn=75 | Non-DMn=132 | p | |
|---|---|---|---|
| Cardiac events (%) | 13.3 | 12.1 | 0.926 |
| Death | 2.7 | 0.8 | 0.298 |
| Myocardial infarction | 0.0 | 2.3 | 0.555 |
| Target vessel revascularization | 10.7 | 11.4 | 0.878 |
| Probable/definite thrombosis (%) | 0.0 | 2.3 | 0.555 |
| Early | 0.0 | 1.5 | 0.740 |
| Late | 0.0 | 0.8 | 0.878 |
| Stroke (%) | 0.0 | 1.5 | 0.740 |
| Cardiac events or stroke (%) | 13.3 | 12.9 | 0.926 |
DM: diabetes mellitus.
Our study constitutes the first available analysis of data from a randomized trial that compares the clinical and angiographic outcomes of successful CTO-PCI with DES between diabetic and non-diabetic patients. The most important findings of the present subanalysis from the CIBELES trial are that, among diabetic patients with a successful CTO-PCI with DES: (1) angiographic results at nine months were favorable, with low in-stent late loss and rates of binary in-stent restenosis and vessel reocclusion, which did not differ between patients with and without DM; (2) during the 12-month follow-up we found a comparable low incidence of MACE in both groups; (3) the presence of more diffuse peripheral and coronary artery disease, lower left ventricular ejection fraction and higher frequency of calcified target CTO lesions in DM patients did not lead to significant differences in the approach, strategy, total stent length and contrast volume required to perform successful CTO-PCI compared with patients without DM.
There are few studies specifically assessing the effect of DM on CTO-PCI outcomes. Old registry data, with stents being used in only 25% of cases, reported significant lower acute angiographic success rates in diabetic patients undergoing PCI-CTO compared with propensity-matched diabetic patients undergoing non-CTO-PCI. However, more interestingly, there were no differences in the rates of mortality, MACE and urgent or in-hospital CABG between the groups, implying that in diabetic patients CTO-PCI might be considered at least as safe as non-CTO-PCI.12 Bare-metal stents (BMS) have been shown to improve outcomes compared with balloon angioplasty in CTO,13 but DM was still associated with lower minimal lumen diameter at nine months13 and with higher rates of reintervention.14
Benefits of drug-eluting stents in chronic total occlusionsDES have constituted a revolution in CTO-PCI, in patients both with and without DM.4,7,8 CTO lesions of diabetic patients treated with first-generation DES already presented significant lower in-stent restenosis risk6 and lower incidence of MACE at one year15 than those treated with BMS. In agreement with current studies that do not show differences in DM prevalence between successful and failed CTO-PCI,4,16,17 a multinational CTO registry18 has found similar procedural success rates in patients with and without DM, in spite of the higher clinical risk profile of the DM group. The beneficial effect of DES compared with BMS in reducing the rate of target vessel revascularization is even more evident in patients with DM, without an increase in the risk of definite or probable stent thrombosis.18 In fact, DM no longer appears as a predictor of in-stent restenosis following CTO-PCI with DES in a recent multivariate regression analysis.5 Our findings are consistent with previous reports and support the idea that DES may be considered as safe in DM as in non-DM patients when undergoing CTO-PCI. Besides, in routine angiographic follow-up we documented similar favorable outcomes of DES in DM and non-DM patients, and consequently consider DES the first choice for CTO-PCI in both groups.
The definition of the best strategy for CTO revascularization in patients with DM deserves special attention due to the magnitude of the problem and the absence of evidence-based recommendations. It is known that approximately one-fifth (34% of them diabetic)1 to one-half (30% diabetic)19 of patients with significant coronary artery disease have at least one CTO, and that the prevalence of CTO lesions is higher in non-infarct-related arteries of diabetic patients undergoing primary PCI than in non-diabetic patients (21% vs. 12%).20 Moreover, DM is significantly more prevalent among patients presenting with a CTO on nonemergent angiography than among those presenting with no CTO (34% vs. 26%).1 However, taking into account the results of previous studies and the present analysis, and given the lack of randomized trials comparing the outcomes of CTO-PCI vs. CABG in diabetic patients (the recently published FREEDOM trial only included 5.8% of CTO lesions and found 11.8% MACE and cerebrovascular events at 12 months in diabetic patients undergoing CABG21), what seems to us more striking is the greater tendency observed to avoid PCI in these patients19 and to refer them for CABG,1 especially considering the current good acute22 and long-term outcomes of CTO-PCI.17
When coronary arteries become stenotic or occluded, collateral circulation development is known to be less in DM than in non-DM,23 which might explain the worse outcome of CTO lesions in DM. In fact, the presence of a CTO lesion in non-infarct-related arteries of diabetic patients with ST-elevation myocardial infarction is an independent predictor of five-year mortality, but not the presence of multivessel coronary artery disease without CTO.20 Thus, as observed in the general population,17 successful CTO-PCI is associated with significant reductions in mortality and in need for CABG in patients with DM.18 Our analysis also suggests that CTO-PCI in DM may be not more technically demanding than CTO-PCI in non-DM. Considering all these data, we hypothesize that in DM patients with multivessel disease percutaneous revascularization should perhaps focus on CTO lesions rather than on other instances of coronary stenosis.
LimitationsThe standard definition of CTO is those with an estimated time since occlusion >3 months, but we included patients with an estimated time since occlusion of >2 weeks. However, we do not believe that this influenced our results, as 85% of lesions in DM had a time of occlusion >3 months and in CTO-PCI the estimated time since occlusion has more impact on the probability of achieving successful recanalization than on the risk of subsequent restenosis/reocclusion.24 Furthermore, the number of patients under insulin treatment in our study was low (21.3% of DM patients), so our results may only reflect the improvements in outcomes already observed in non-insulin dependent diabetic patients undergoing non-CTO-PCI but not seen in insulin-dependent patients.25 Finally, the analysis is limited to patients included in a randomized trial and followed for 12 months after undergoing successful CTO-PCI with DES, so further studies with longer follow-up are required to confirm our findings.
ConclusionIn the DES era, diabetic and non-diabetic patients have similar favorable clinical and angiographic outcomes after successful percutaneous revascularization of CTO lesions. PCI with DES may constitute a valid and safe alternative to CABG for diabetic patients requiring CTO revascularization.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis work was sponsored by the Spanish Society of Cardiology, with an unrestricted grant from Abbott Vascular.
Conflicts of interestThe authors have no conflicts of interest to declare.