Chronic kidney disease is related to poor outcomes in patients with heart failure (HF). Few studies have assessed whether renal function influences one-year mortality risk in patients admitted for the first time for acute HF.
MethodsWe reviewed the medical records of all patients aged >50 years admitted within a two-year period for a first episode of decompensated HF. The sample was divided according to the patients’ estimated glomerular filtration rate (eGFR) on admission into three groups (eGFR >60, 30-60 and <30 ml/min/1.73 m2). Index admission and one-year all-cause mortality rates were compared between groups using Cox regression analysis.
ResultsA total of 985 patients were included in the study, mean age 78.4±9 years, and with mean admission eGFR of 60.5±26 ml/min/1.73 m2. Of these, 516 (52.3%) patients had eGFR <60 ml/min/1.73 m2. One-year all-cause mortality was 25.4%, with a significant association between worse eGFR category and mortality (p<0.0001). Cox regression analysis assessing eGFR as a categorical variable confirmed this association (HR 1.378; p=0.030), together with older age (HR 1.066; p<0.001), previous diagnosis of hypertension (HR 0.527; p<0.001), and both lower systolic blood pressure (HR 0.993; p=0.009) and higher serum potassium on admission (HR 1.471; p <0.001).
ConclusionsRenal impairment is common in HF patients, even at the time of first admission. In this group of HF patients the presence of renal impairment was associated with higher mid-term (one-year) mortality risk.
A doença renal crónica (CKD) está relacionada com um pior prognóstico em doentes com insuficiência cardíaca (HF). Poucos estudos avaliaram se a função renal influencia o risco de mortalidade a um ano, em doentes admitidos pela primeira vez por insuficiência cardíaca aguda.
MétodosRevimos os registos médicos de todos os doentes > 50 anos, admitidos num período de dois anos por um primeiro episódio de descompensação de HF. Dividimos a amostra de acordo com a taxa de filtração glomerular estimada dos doentes (eGFR) após a admissão em três grupos (eGFR >60, 30-60 e <30 ml/min/1,73 m2). Comparamos a admissão inicial e as taxas de mortalidade por todas as causas num ano, com análises de regressão de Cox.
ResultadosForam incluídos 985 doentes no estudo, a média foi de 78,4 ± 9 anos e a eGFR média na admissão foi de 60,5 ± 26. Do total, 516 (52,3%) doentes apresentaram eGFR < 60. A um ano, a taxa de mortalidade por todas as causas foi de 25,4%, com uma associação significativa entre a pior categoria eGFR e a taxa de mortalidade (p < 0,0001). A análise de regressão de Cox que avaliou a eGFR como variável categórica confirmou essa associação (HR 1,378; p = 0,030) juntamente com idade avançada (HR 1,066; p < 0,001), diagnóstico prévio de hipertensão (HR 0,527; p < 0,001) e pressão arterial sistólica inferior (HR 0,993; p = 0,009) e maiores valores de potássio sérico após a admissão (HR 1,471; p < 0,001).
ConclusõesA insuficiência renal é comum em doentes com insuficiência cardíaca, mesmo no momento da primeira admissão. Nesse grupo de doentes com insuficiência cardíaca a presença de insuficiência renal está associada a um maior risco de mortalidade em médio prazo (um ano).
The prevalence of heart failure (HF) increases with age, and so does the prevalence of comorbidities, including chronic kidney disease (CKD).1 Worsening renal function is considered to be a sensitive marker of decreased organ perfusion, and is an important independent predictor of increased mortality and hospitalization in patients with chronic or acute HF.2
Glomerular filtration rate is the most commonly used and best overall marker of renal function. Renal dysfunction, defined as an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73 m2, is found in two-thirds of patients admitted to hospital with acute HF,3 with varying rates depending on the type of acute HF patients studied. The presence of renal dysfunction in such patients is a marker of poor prognosis in terms of both mortality and readmission for worsening HF.4–8 Most studies have assessed this relationship only in patients with HF with reduced ejection fraction (HFrEF) or who have previously been hospitalized for acute HF. Fewer studies have focused on patients with preserved ejection fraction (HFpEF) or in the early stages of HF.9,10
To shed more light on the role of renal dysfunction in acute HF, this study aimed to investigate the potential impact of impaired baseline renal function, defined as eGFR <60 ml/min/1.73 m2, on the risk of one-year mortality in the subset of real-world HF patients (regardless of type of ventricular dysfunction) undergoing their first admission for HF decompensation.
MethodsPatient selection and study designThis retrospective study was performed in the Hospital Universitari de Bellvitge, a 750-bed tertiary-care public hospital for adults from Barcelona, Spain. Administrative data were retrieved regarding all 1333 admissions to our hospital within a 24-month period (January 2013-December 2014) with HF as the primary discharge diagnosis (identified by the ICD 9-CM codes 398.91, 402.91, 404.01, 404.03, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43 and 428.9). Following this first selection, a thorough review of all these patients’ medical records was undertaken in order to select only those who (a) fulfilled clinical criteria for acute HF and (b) were undergoing their first ever admission due to a first episode of acute HF (those who had already been discharged with a primary or secondary diagnosis of HF were excluded). We also excluded patients younger than 50 years; those in stage 5 CKD undergoing renal replacement therapy; kidney or heart transplant recipients; those already receiving palliative therapy for any cause; patients whose acute HF episode was secondary to an acute coronary syndrome; patients with cirrhosis, ascites or nephrotic syndrome; and patients discharged directly home within 24 hours or transferred to other acute care hospitals from the emergency department. Any doubts regarding a patient's inclusion in the study were discussed by a review panel. The study conformed to the principles outlined in the Declaration of Helsinki and the ethics committee of the University Bellvitge Hospital approved the overall protocol.
The presence of HF was established using the Framingham criteria.11 When echocardiographic data were available, the patient's left ventricular ejection fraction (LVEF) was recorded and HF was coded as HFpEF when LVEF was ≥50% and as HFrEF for lower LVEF values. Demographic data, past medical history diagnoses and all clinical data related to HF history and acute HF signs and symptoms were collected for all patients. A basic blood chemistry panel was obtained, which besides renal function included ionic, lipid and blood glucose profiles and a complete blood count. Admission plasma concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP) were not available in most patients and for this reason this biomarker was not included in the patient database. Patients’ index admission length of stay (LOS) and HF-related medication prescribed on discharge, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), aldosterone blockers, beta-blockers and diuretics, were also recorded.
Assessment of renal functioneGFR on admission to hospital was calculated using the four-variable Modification of Diet in Renal Disease (MDRD-4) equation, using the formula eGFR (ml/min/1.73 m2)=186.3×(serum creatinine)-1.154×(age)-0.203×(1.212 if black race)×(0.742 if female). Patients were divided into three groups according to the eGFR-defined CKD stages of the National Kidney Foundation12: normal or mild (stages 1 and 2, eGFR >60), moderate (stage 3, eGFR 30-60) and severe (stages 4 and 5, eGFR <30). The MDRD-4 formula was chosen because of its usefulness for predicting HF hospitalization.13
Follow-upThe main outcome of the present study was one-year all-cause mortality, defined as death measured as time to event within the year following discharge after the index acute HF admission. Subjects were categorized as alive after 12 months of follow-up, or censored when they died. Secondary outcomes were in-hospital, one- and three-month all-cause mortality. Mortality status was determined by trained physician adjudicators on the basis of medical records from hospitalizations, emergency department visits, death certificates, and autopsy and coroner's reports, when available. No patients were lost to follow-up.
Statistical analysisNormally distributed continuous variables are reported as mean ± standard deviation. Categorical variables are reported as proportions. The Student's t test was used to compare continuous variables, preceded by the Levene test for equality of variances, while either the chi-square statistic or Fisher's exact test was used to compare categorical or dichotomous variables, respectively. For non-normally distributed continuous outcomes we used the Wilcoxon rank sum test for independent variables and the Wilcoxon signed rank test for paired variables.
A multivariate linear regression model was constructed to identify independent associated variables of renal function at baseline, with eGFR as the dependent variable. Kaplan-Meier survival curves were created to identify prognostic differences in terms of mortality between groups. Univariate and multivariate analyses were performed to calculate the hazard ratio (HR) between renal dysfunction and all-cause mortality using forward stepwise Cox proportional hazards models. For multivariate analysis, previously selected covariates considered relevant for prognosis were adjusted and the covariates associated with mortality on univariate analysis (p<0.10) were analyzed using eGFR as a categorical variable (with a cut-off of 60 ml/min/1.73 m2) as a definition of renal dysfunction.
The analyses were performed with SPSS version 21.0 (IBM SPSS, Armonk, NY). Tests were two-sided and p-values of <0.05 were considered statistically significant.
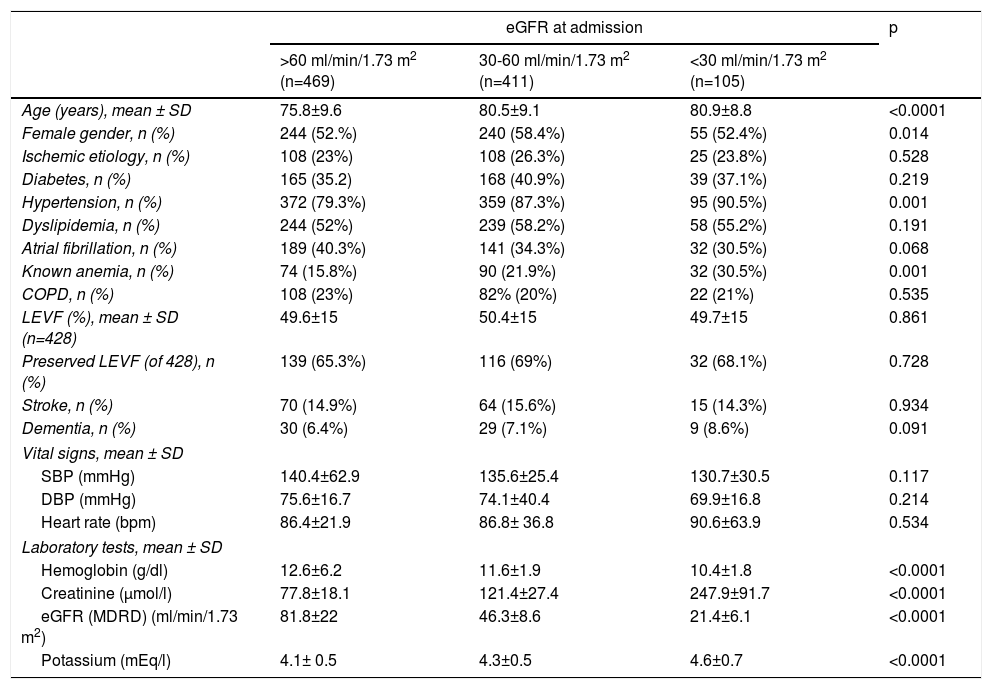
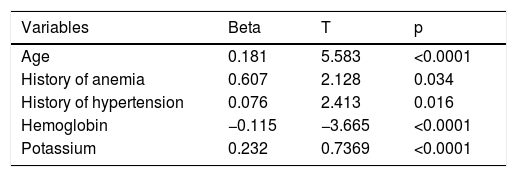
ResultsBaselineA total of 985 patients with a validated diagnosis of first hospital admission for HF within the two-year study period were included in the analysis. Their mean age was 78.36±9 years and 54.7% (n=539) were female. Mean eGFR at admission was 60.5±26 ml/min/1.73 m2; 516 (52.3%) of the patients had stage 3, 4 or 5 CKD (eGFR <60 ml/min/1.73 m2) at the time of admission. When patients were stratified by eGFR, 469 (47.6%) had eGFR >60 ml/min/1.73 m2, 411 (41.7%) 30-60 ml/min/1.73 m2 and 105 (10.6%) <30 ml/min/1.73 m2. Table 1 shows the baseline characteristics of these three patient groups. Some baseline characteristics differed significantly according to eGFR group: patients with lower admission eGFR were older, with a higher prevalence of a previous diagnosis of hypertension or anemia, and presented with higher potassium and lower hemoglobin values on admission. The multivariate linear regression model confirmed the independent association of these variables with worse admission renal function (Table 2).
Baseline patient characteristics of the overall cohort by estimated glomerular filtration rate at admission.
| eGFR at admission | p | |||
|---|---|---|---|---|
| >60 ml/min/1.73 m2 (n=469) | 30-60 ml/min/1.73 m2 (n=411) | <30 ml/min/1.73 m2 (n=105) | ||
| Age (years), mean ± SD | 75.8±9.6 | 80.5±9.1 | 80.9±8.8 | <0.0001 |
| Female gender, n (%) | 244 (52.%) | 240 (58.4%) | 55 (52.4%) | 0.014 |
| Ischemic etiology, n (%) | 108 (23%) | 108 (26.3%) | 25 (23.8%) | 0.528 |
| Diabetes, n (%) | 165 (35.2) | 168 (40.9%) | 39 (37.1%) | 0.219 |
| Hypertension, n (%) | 372 (79.3%) | 359 (87.3%) | 95 (90.5%) | 0.001 |
| Dyslipidemia, n (%) | 244 (52%) | 239 (58.2%) | 58 (55.2%) | 0.191 |
| Atrial fibrillation, n (%) | 189 (40.3%) | 141 (34.3%) | 32 (30.5%) | 0.068 |
| Known anemia, n (%) | 74 (15.8%) | 90 (21.9%) | 32 (30.5%) | 0.001 |
| COPD, n (%) | 108 (23%) | 82% (20%) | 22 (21%) | 0.535 |
| LEVF (%), mean ± SD (n=428) | 49.6±15 | 50.4±15 | 49.7±15 | 0.861 |
| Preserved LEVF (of 428), n (%) | 139 (65.3%) | 116 (69%) | 32 (68.1%) | 0.728 |
| Stroke, n (%) | 70 (14.9%) | 64 (15.6%) | 15 (14.3%) | 0.934 |
| Dementia, n (%) | 30 (6.4%) | 29 (7.1%) | 9 (8.6%) | 0.091 |
| Vital signs, mean ± SD | ||||
| SBP (mmHg) | 140.4±62.9 | 135.6±25.4 | 130.7±30.5 | 0.117 |
| DBP (mmHg) | 75.6±16.7 | 74.1±40.4 | 69.9±16.8 | 0.214 |
| Heart rate (bpm) | 86.4±21.9 | 86.8± 36.8 | 90.6±63.9 | 0.534 |
| Laboratory tests, mean ± SD | ||||
| Hemoglobin (g/dl) | 12.6±6.2 | 11.6±1.9 | 10.4±1.8 | <0.0001 |
| Creatinine (μmol/l) | 77.8±18.1 | 121.4±27.4 | 247.9±91.7 | <0.0001 |
| eGFR (MDRD) (ml/min/1.73 m2) | 81.8±22 | 46.3±8.6 | 21.4±6.1 | <0.0001 |
| Potassium (mEq/l) | 4.1± 0.5 | 4.3±0.5 | 4.6±0.7 | <0.0001 |
COPD: chronic obstructive pulmonary disease; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; MDRD: modification of diet in renal disease; SBP: systolic blood pressure; SD: standard deviation.
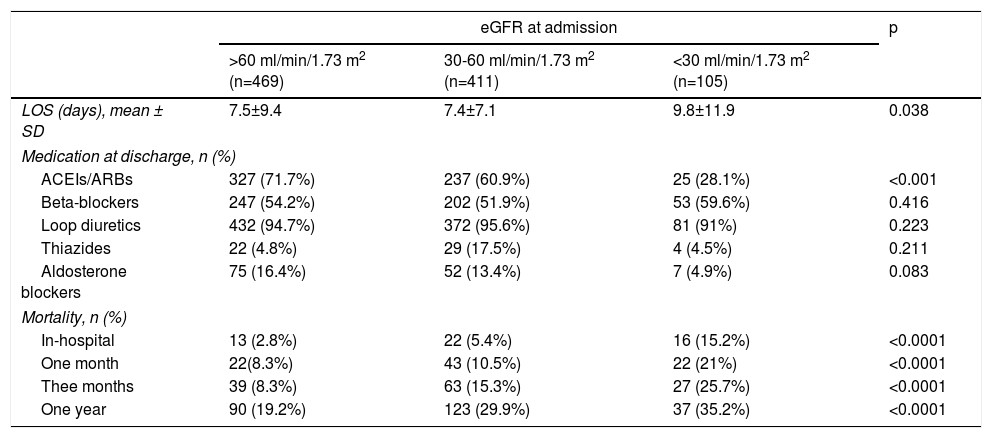
Patients’ mean LOS was 7.7±8.9. Those with lower eGFR at admission had a significantly longer LOS (Table 3).
In-hospital outcomes, treatment at discharge and mortality during follow-up according to renal function assessed by estimated glomerular filtration rate at admission.
| eGFR at admission | p | |||
|---|---|---|---|---|
| >60 ml/min/1.73 m2 (n=469) | 30-60 ml/min/1.73 m2 (n=411) | <30 ml/min/1.73 m2 (n=105) | ||
| LOS (days), mean ± SD | 7.5±9.4 | 7.4±7.1 | 9.8±11.9 | 0.038 |
| Medication at discharge, n (%) | ||||
| ACEIs/ARBs | 327 (71.7%) | 237 (60.9%) | 25 (28.1%) | <0.001 |
| Beta-blockers | 247 (54.2%) | 202 (51.9%) | 53 (59.6%) | 0.416 |
| Loop diuretics | 432 (94.7%) | 372 (95.6%) | 81 (91%) | 0.223 |
| Thiazides | 22 (4.8%) | 29 (17.5%) | 4 (4.5%) | 0.211 |
| Aldosterone blockers | 75 (16.4%) | 52 (13.4%) | 7 (4.9%) | 0.083 |
| Mortality, n (%) | ||||
| In-hospital | 13 (2.8%) | 22 (5.4%) | 16 (15.2%) | <0.0001 |
| One month | 22(8.3%) | 43 (10.5%) | 22 (21%) | <0.0001 |
| Thee months | 39 (8.3%) | 63 (15.3%) | 27 (25.7%) | <0.0001 |
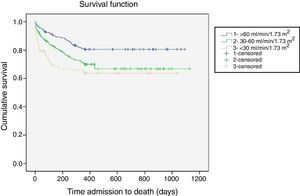
| One year | 90 (19.2%) | 123 (29.9%) | 37 (35.2%) | <0.0001 |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; eGFR: estimated glomerular filtration rate; LOS: length of stay; SD: standard deviation.
Overall in-hospital mortality was 5.2%. Mortality (Table 3) increased linearly and significantly across the different categories of eGFR (eGFR >60 2.8% vs. 5.4% for eGFR 30-60 vs. 15.2% for eGFR <30; p<0.0001) (Table 3).
Heart failure treatment after dischargeTable 3 shows the discharge HF medication prescribed in the 934 surviving patients, stratified by the three eGFR categories. Patients with poor admission eGFR received significantly less ACEIs/ARBs and there was also a trend towards less prescription of aldosterone blockers. No differences regarding beta-blockers or diuretics were found between the three eGFR groups.
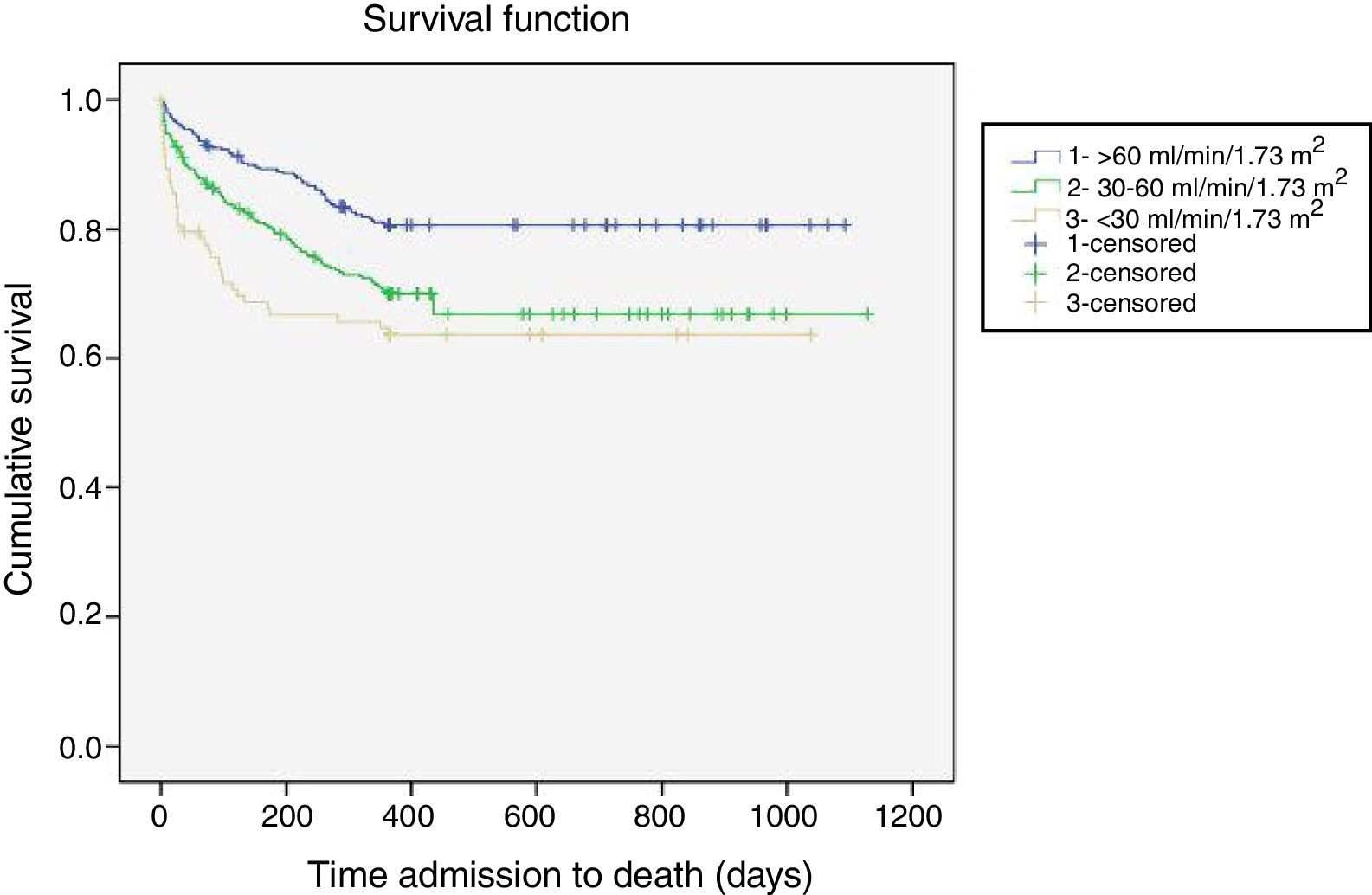
Follow-upAs was the case with in-hospital mortality, all-cause mortality increased linearly and significantly across eGFR categories in all the post-discharge periods analyzed (Table 3). One-year all-cause mortality, the main outcome variable, increased as eGFR worsened (25.4% overall; 19.2% for eGFR >60 vs. 29.9% for eGFR 30-60 and 35.2% for eGFR <30; p<0.0001). Figure 1 shows the one-year survival curves according to the three eGFR categories.
In Cox univariate analysis, worse renal function measured using eGFR as a categorical variable (HR 1.7; p<0.0001) or as a continuous variable (HR 0.990; p<0.0001) was significantly associated with mortality. For continuous variables this was also the case for admission plasma creatinine (HR 1.013; p<0.0001) and BUN (HR 1.021; p<0.001).
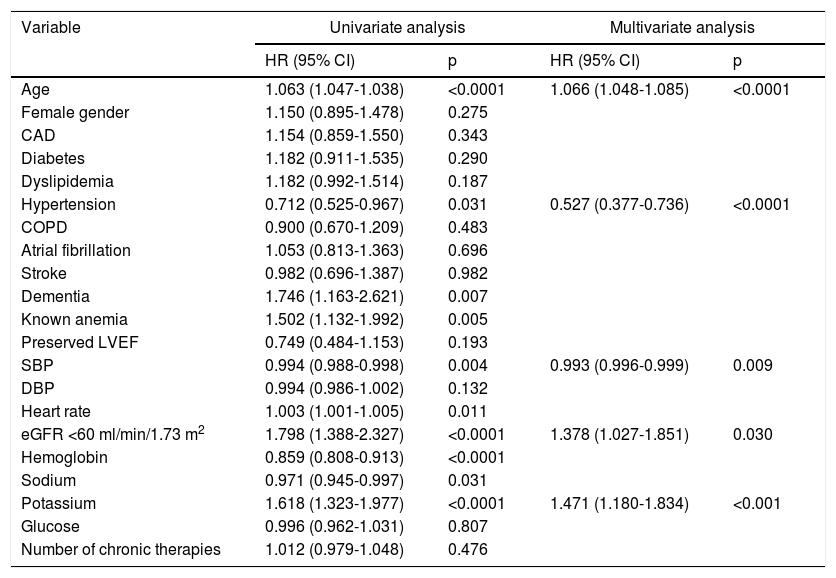
Multivariate Cox regression analysis (Table 4) analyzing eGFR as a categorical variable (<60) confirmed this independent association (HR1.378; p=0.030), and identified other variables as risk factors for increased all-cause mortality: older age (HR 1.066; p<0.001), previous diagnosis of hypertension (HR 0.527; p<0.001), and both lower admission systolic blood pressure (HR 0.993; p=0.009) and higher serum potassium at admission (HR 1.471; p<0.001).
Univariate and multivariate analyses by one-year overall mortality.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.063 (1.047-1.038) | <0.0001 | 1.066 (1.048-1.085) | <0.0001 |
| Female gender | 1.150 (0.895-1.478) | 0.275 | ||
| CAD | 1.154 (0.859-1.550) | 0.343 | ||
| Diabetes | 1.182 (0.911-1.535) | 0.290 | ||
| Dyslipidemia | 1.182 (0.992-1.514) | 0.187 | ||
| Hypertension | 0.712 (0.525-0.967) | 0.031 | 0.527 (0.377-0.736) | <0.0001 |
| COPD | 0.900 (0.670-1.209) | 0.483 | ||
| Atrial fibrillation | 1.053 (0.813-1.363) | 0.696 | ||
| Stroke | 0.982 (0.696-1.387) | 0.982 | ||
| Dementia | 1.746 (1.163-2.621) | 0.007 | ||
| Known anemia | 1.502 (1.132-1.992) | 0.005 | ||
| Preserved LVEF | 0.749 (0.484-1.153) | 0.193 | ||
| SBP | 0.994 (0.988-0.998) | 0.004 | 0.993 (0.996-0.999) | 0.009 |
| DBP | 0.994 (0.986-1.002) | 0.132 | ||
| Heart rate | 1.003 (1.001-1.005) | 0.011 | ||
| eGFR <60 ml/min/1.73 m2 | 1.798 (1.388-2.327) | <0.0001 | 1.378 (1.027-1.851) | 0.030 |
| Hemoglobin | 0.859 (0.808-0.913) | <0.0001 | ||
| Sodium | 0.971 (0.945-0.997) | 0.031 | ||
| Potassium | 1.618 (1.323-1.977) | <0.0001 | 1.471 (1.180-1.834) | <0.001 |
| Glucose | 0.996 (0.962-1.031) | 0.807 | ||
| Number of chronic therapies | 1.012 (0.979-1.048) | 0.476 | ||
CAD: coronary artery disease; CI: confidence interval; COPD: chronic obstructive pulmonary disease; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HR: hazard ratio; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure.
Our study sheds more light on the role of renal function impairment in HF, which has previously been assessed in only a few groups of patients. It shows that reduced eGFR is not only already present in more than half of patients presenting with a first clinical episode of decompensation serious enough to warrant hospitalization, but also acts as a marker of poor short- and mid-term prognosis in such patients.
Impaired renal function commonly accompanies both acute and chronic HF.8 Results from the ADHERE database in 118465 hospitalization episodes show that the majority of patients admitted with acute decompensated HF have significant renal impairment, which influences treatment and outcomes.14 Our percentage (52.3%) of abnormal eGFR (<60 ml/min/1.73 m2) on admission is striking, and is almost identical to the 53% reported in patients admitted for the first time because of acute HF, all of them however with HFpEF.8 It should be noted that the mean age of our patients is much older than in other studies.8 The prevalence of CKD in outpatients with HF has also been reported at around 50%.15
Our patients with impaired renal function were characterized by older age, higher prevalence of a known risk factor (hypertension) and a well-known complication (anemia) of CKD, and laboratory findings that were also consistent with this diagnosis (high potassium and low hemoglobin).9,16 As has been described in other cohorts of patients with acute HF,9 our patients’ discharge prescriptions are similar for those with mild or moderate CKD, but show significantly lower use of ACEIs and ARBs in the presence of eGFR <30 ml/min/1.73 m2 and almost significantly lower use of aldosterone blockers. The guidelines do indeed suggest caution in the use of these drug classes in patients with severely impaired renal function, and therefore their underprescription is probably justified for reasons of safety.
The relationship between poor renal function and worse outcomes in HF has been studied mainly in patients with HFrEF, but also in HFpEF.4–10 In our study, mean LOS was significantly greater in patients with worse eGFR, and mortality increased linearly and significantly with decreasing eGFR in all assessments.
The main finding of our study is the confirmation that impaired renal function, defined by eGFR <60 ml/min/1.73 m2, in patients first admitted due to HF, is associated with higher one-year mortality. This risk is observed not only in univariate analysis but also when controlling for other well-known causes of one-year mortality in acute HF patients. These findings are in line with other reports,2 but with the distinguishing feature that in our study only first hospital admissions were analyzed, which in most cases was the time of diagnosis of HF. This risk probably persists over time, since some studies have identified increases in mortality after a first hospitalization for HF at as many as seven years of follow-up when baseline renal function was impaired.9
Other factors associated with increased mortality have been previously reported in patients with first admission or new-onset HF, such as age17 and lower systolic blood pressure.18 In our study patients diagnosed with hypertension had a better prognosis, which may be related to the fact that HF of ischemic etiology has a worse prognosis.19 Potassium levels were also associated with higher mortality in our study; abnormal potassium levels are associated with arrhythmogenic processes, especially ventricular arrhythmias and consequent cardiac arrest, and previous studies report that both hypokalemia and hyperkalemia are associated with poor prognosis among HF patients.20,21
Some strengths of this study should be mentioned. It is based on a sizable number of real-world patients with all types of HF, whose medical records were thoroughly reviewed to confirm that they truly fulfilled the diagnostic criteria for HF and that no previous episodes of acute HF requiring admission had ever taken place. eGFR was assessed using the same technique at a single laboratory. Moreover, the MDRD-4 formula is a simple and standardized method requiring only four clinical parameters, and is not influenced by the patient's weight, which may vary significantly in an acute HF scenario.22 However, it should be pointed out that some studies favor the use of other formulas in this clinical scenario.23,24 Plischke et al. reported that the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was marginally better in predicting all-cause mortality than MDRD-4,25 and a recent study that compared five different formulas (Cockcroft-Gault, MDRD-4, MDRD-6, CKD-EPI in patients <70 years and Berlin Initiative Study-1 (BIS-1) in older patients) applied to 1104 unselected acute HF patients reported that the Cockcroft-Gault formula showed the highest prognostic accuracy to predict death.26
Study limitationsSeveral limitations should be taken into account when interpreting our results. First, and probably most important, we calculated our patients’ eGFR using a spot determination of plasma creatinine obtained at the time of hospital admission. Beyond the presence of a previous diagnosis of CKD, we could not determine whether this value truly reflected the patient's usual renal function or reflected a transient worsening associated with the acute HF episode. Similarly, data were not collected on changes in patients’ eGFR during and after hospitalization, so the effect of worsening or improving renal function (beyond the spot admission data) on prognosis cannot be ascertained. We excluded patients in stage 5 CKD (eGFR <15 ml/min/1.73 m2) even if they were not undergoing renal replacement therapy; however, the number of such patients was low and unlikely to alter the prognostic role of eGFR found for stage 4 patients. Although our study includes some patients over 50-65 years of age, the CKD-EPI equation might have been a better estimator of eGFR given the mean age of our study sample.27 Finally, we did not have access to a basic prognostic HF biomarker (NT-proBNP) for most patients, or to other markers of renal function such as albuminuria or cystatin C, which have been found to provide valuable prognostic information in HF patients regardless of their eGFR – in the latter case, however, our study follows standard clinical practice, in which most HF patients are managed without access to plasma cystatin C levels.
ConclusionsOur study confirms a high prevalence of renal impairment on first admission of HF patients. Renal dysfunction was shown to be a marker of higher short- and mid-term mortality even in this patient population of HF patients presumed to be in the earlier stages of the disease, free from previous acute HF episodes that could have triggered prior kidney damage. Further prospective studies focusing on this population, with a more thorough assessment of renal function before, during and after the acute episode, may shed more light on this subject.
FundingThis research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.