Cardiovascular (CV) risk is known to be increased in HIV-infected individuals. Our aim was to assess CV risk in HIV-infected adults.
MethodsCV risk was estimated for each patient using three different risk algorithms: SCORE, the Framingham risk score (FRS), and DAD. Patients were classified as at low, moderate or high CV risk. Clinical and anthropometric data were collected.
ResultsWe included 571 HIV-infected individuals, mostly male (67.1%; n=383). Patients were divided into two groups according to antiretroviral therapy (ART): naïve (7.5%; n=43) or under ART (92.5%; n=528). The mean time since HIV diagnosis was 6.7±6.5 years in the naive group and 13.3±6.1 years in the ART group. Metabolic syndrome (MS) was identified in 33.9% (n=179) and 16.3% (n=7) of participants in the ART and naïve groups, respectively. MS was associated with ART (OR=2.7; p=0.018). Triglycerides ≥150 mg/dl (OR=13.643, p<0.001) was one of the major factors contributing to MS. Overall, high CV risk was found in 4.4% (n=23) of patients when the SCORE tool was used, in 20.5% (n=117) using the FRS, and in 10.3% (n=59) using the DAD score. The observed agreement between the FRS and SCORE was 55.4% (k=0.183, p<0.001), between the FRS and DAD 70.5% (k=0.465, p<0.001), and between SCORE and DAD 72.3% (k=0.347, p<0.001).
ConclusionOn the basis of the three algorithms, we detected a high rate of high CV risk, particularly in patients under ART. The FRS was the algorithm that classified most patients in the high CV risk category (20.5%). In addition, a high prevalence of MS was identified in this patient group.
O risco cardiovascular (RCV) pode estar aumentado em indivíduos com infeção por Vírus Imunodeficiência Humana (VIH). O objetivo deste trabalho foi avaliar o RCV em adultos infetados por VIH.
MétodosO RCV foi estimado utilizando três algoritmos diferentes, Score, Framingham Risk Score (FRSs-CVD) e DAD; os participantes foram classificados apresentando RCV baixo, moderado ou elevado. Recolheram-se dados clínicos e antropométricos.
ResultadosIncluíram-se 571 indivíduos, maioritariamente do género masculino (67,1%; n=383). Dividiram-se os participantes em dois grupos, com e sem terapêutica antirretroviral (cTAR): naïve (7,5%; n=43) versus cTAR (92,5%; n=528). O tempo médio desde o diagnóstico da infeção por VIH foi 6,7±6,5 anos no grupo naïve e 13,3±6,1 anos no grupo cART. A síndrome metabólica (SM) foi identificada em 33,9% (n=179) e em 16,3% (n=7) dos participantes, respetivamente no grupo cART e no grupo naïve. Verificou-se um RCV elevado em 4,4% (n=23) dos participantes, com recurso à ferramenta Score, em 20,5% (n=117) utilizando a FRSs e em 10,3% dos participantes (n=59) utilizando a ferramenta DAD. A concordância observada entre FRSs e Score foi 55,4% (k=0,183; p<0,001), entre FRSs e DAD 70,5% (k=0,465; p<0,001) e entre Score e DAD 72,3% (k=0,347; p<0,001).
ConclusãoCom recurso aos algoritmos utilizados, identificou-se uma presença significativa de elevado RCV, sendo a ferramenta FRSs-CVD a que classificou mais indivíduos na categoria de RCV elevado (20,5%), e simultaneamente verificou-se uma prevalência elevada de SM.
In European countries in recent years, cardiovascular disease (CVD) has been identified as a major cause of mortality in human immunodeficiency virus (HIV)-infected individuals, accounting for 15% of all deaths in this population.1–3 In Portugal, CVD is the major cause of death overall, but mortality from CVD in the general population is still lower than that reported in the HIV population. Also, the mean age of death in patients with HIV in Portugal is significantly lower than in the general population (52 vs. 81 years).4
Cardiovascular (CV) risk in the HIV-infected population has been shown to be high, an estimated 50% higher than in uninfected individuals, although some of the data are still controversial.6–11 Traditional risk factors such as smoking, which are particularly prevalent in this population, contribute to this increased risk.1,2,6,11–16 Other factors include substance abuse6 and changes in lipid profile1,8,12,15,17 and glucose metabolism, with increased insulin resistance and/or impaired insulin secretion.8,18 HIV infection itself, as well as inflammation and antiretroviral therapy (ART), are further contributing factors in this population.11
Overall, obesity and higher waist circumference (WC) are known causes of increased CV risk, and insulin resistance, changes in lipid profile and increased WC19,20 have been associated with HIV infection and treatment.19,20
While increased WC is the key factor for defining the presence of metabolic syndrome (MS) in the non-infected population,19 in HIV-infected individuals MS is better identified by increased triglycerides (TG) and decreased high-density lipoprotein cholesterol (HDL-C). It should be also noted that cardiovascular events occur at much younger ages in the HIV population.20
As in the general European population, obesity is on the rise among HIV-infected individuals.17–24 In Portugal, obesity and CVD are highly prevalent and cardiovascular events are one of the leading causes of death.5
Cardiovascular risk prediction algorithms were developed in non-HIV-infected populations and may not accurately predict risk for HIV-infected individuals, since the etiology of CVD in this population may be different. The Framingham risk score (FRS) calculates CVD risk and predicts future coronary heart disease events at 10 years. However, the FRS may wrongly estimate the risk in populations other than the US population, and the European Society of Cardiology accordingly developed and recommends the use of the Systematic Coronary Risk Evaluation (SCORE) tool25 to assess CVD risk in the general population. The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) group developed a CVD risk equation specifically designed for the HIV population, which includes exposure to HIV treatment (use of indinavir, lopinavir/ritonavir and abacavir), as well as traditional CV risk factors.26
This study aims to estimate the risk of CVD in HIV-infected adults, to assess the agreement between the FRS, SCORE and DAD algorithms, to study the relationship between CV risk and MS in HIV-infected individuals, and to compare the prevalence of traditional cardiovascular factors with the non-infected population. Given the burden of CVD and obesity and the associated health implications, identification of patients at high CV risk could lead to more tailored lifestyle counseling and more aggressive treatment of comorbidities.27–29
MethodsA cross-sectional study was carried out at the Department of Infectious Diseases, Santa Maria University Hospital, Lisbon, Portugal, from December 2013 to May 2014. During the study period, at least five consecutive HIV-infected adults were interviewed per day, randomly selected from the scheduled appointments of that day. We aimed to collect a sample of more than 490 patients to ensure that the study population was representative. Ages ranged from 18 to 65 years, and the interview was performed on the same day as the hospital appointment. Exclusion criteria included pregnancy, hospitalization in the previous three months, presence of opportunistic infections, kidney or liver failure, institutionalization, residence outside Portugal, or inability to understand and sign the informed consent form. Patients with known CVD (coronary artery disease, myocardial infarction, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral arterial disease and/or heart failure) were also excluded. Demographic information (age, gender, race), clinical data (smoking status, family history of cardiovascular events, diagnosis of diabetes and/or hypertension, time of HIV diagnosis), total time and type of ART, antihypertensive or lipid-lowering therapy, laboratory markers (CD4+lymphocyte count and viral load, blood glucose, total cholesterol, HDL-C, LDL cholesterol, TG) and systolic and diastolic blood pressure (SBP and DBP) were collected from medical records.
CV risk was estimated for each patient using three different risk algorithms: SCORE and the FRS30,31 for 10-year risk estimation, and the DAD risk equation for five-year risk estimation.32
SCORE estimates the risk over a 10-year period of a first fatal atherosclerotic event (such as myocardial infarction, cerebrovascular disease, or other occlusive arterial disease, and including sudden cardiac death). The algorithm includes gender, age, total cholesterol, SBP and smoking status. The FRS estimates absolute risk over a 10-year period of cardiovascular events (defined as coronary artery disease, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral arterial disease, or heart failure). The adapted algorithm, developed on the basis of the original Framingham scale to be applicable in HIV, encompasses gender, age, smoking status, presence of diabetes, presence of left ventricular hypertrophy, total cholesterol, HDL-C and SBP. DAD estimates the risk of developing coronary heart disease and myocardial infarction within a five-year period. The algorithm includes the following factors: gender, age, SBP, smoking habits, family history of CVD, presence of diabetes, total cholesterol, HDL-C, and exposure to ART with indinavir, lopinavir and abacavir.
Individuals were classified as presenting low CV risk with SCORE <4%, FRS <10% or DAD <5%; moderate to high CV risk with SCORE 5-10%, FRS 10-20% or DAD 5-10%; or high CV risk with SCORE or DAD ≥10% and FRS ≥20%. The original algorithm for each tool was used and individual risk was determined using IBM SPSS®.
Multivariate logistic regression analysis was performed using all factors significantly (p<0.1) associated with high CV risk (age, gender, smoking status, family history of CVD, blood glucose, total cholesterol, LDL, HDL-C, TG, body mass index [BMI], WC and ART).
Weight and height were measured with participants wearing light clothes and barefoot, using a calibrated scale. These parameters were used to calculate BMI.33 WC was measured halfway between the lowest point of the costal margin and the top of the iliac crest. Male patients with WC ≥94 cm and female patients with WC ≥80 cm were considered to have high WC.34 MS was defined in accordance with the standards of the International Diabetes Federation, by the presence of at least three of the following criteria: increased WC, TG ≥150 mg/dl, HDL-C <40 mg/dl for men and <50 mg/dl for women, SBP ≥130 mmHg and/or DBP ≥85 mmHg and fasting blood glucose ≥100 mg/dl. Pharmacological therapy for any of these conditions was considered an alternative criterion.34
Statistical analysisDemographic and clinical variables were analyzed by overall group and by gender. Continuous variables were summarized as mean and standard deviation and categorical variables as frequency and percentage. The chi-square test was used to determine the independence of categorical variables and the Student's t test or analysis of variance, with multiple comparisons adjusted with the Bonferroni correction, were used for continuous variables. Unadjusted and adjusted odds ratios (OR) and the corresponding 95% confidence intervals (CI) and p-values were reported for each analysis. The forward stepwise method was used for the multivariate logistic regression analysis. The model's goodness of fit was assessed by the Hosmer-Lemeshow test (at least 80% of expected values were ≥5). The significance level was set at 5%. All statistical analyses were performed with IBM SPSS® software, version 22.0.
The study was approved by the Ethics Committee of Santa Maria Hospital and authorized by the hospital's Board of Directors. All subjects provided written informed consent.
ResultsFrom the 3000 HIV-infected adult patients followed in the Department of Infectious Diseases, 719 were recruited for participation during the study period. Thirty-five did not consent to participate and 113 did not meet the eligibility criteria.
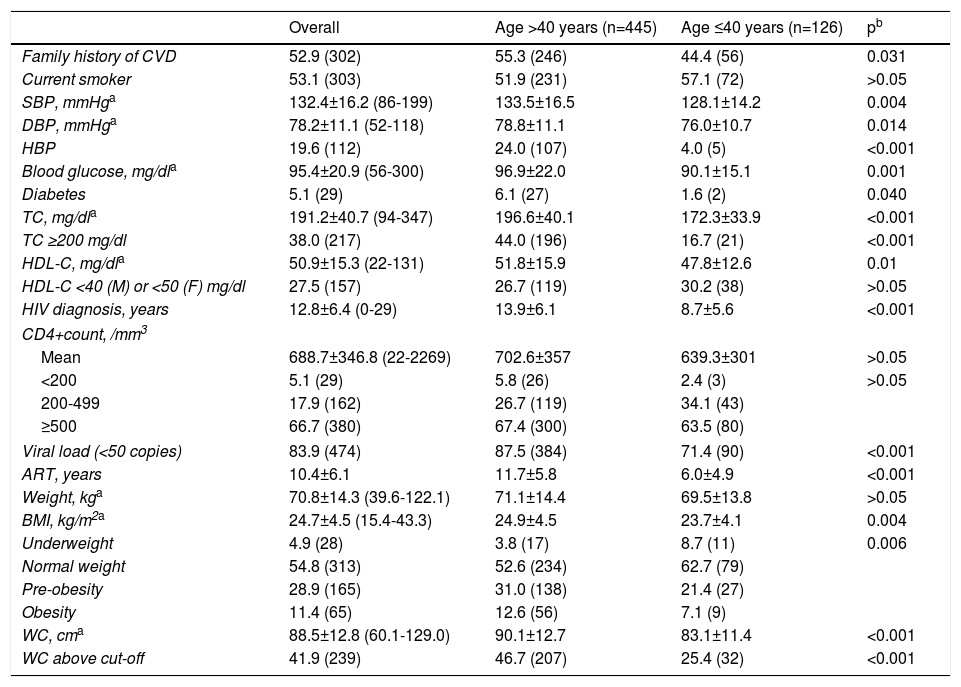
Overall, 571 patients were included. Most were male (n=383; 67.1%) and Caucasian (n=524; 91.3%). Mean age was 46.4±8.9 years; time since HIV diagnosis was 12.8±6.4 years and only 12 patients had been diagnosed in the previous year. Most patients (92.5%) had been on ART for 10.4±6.1 years. Patients with a CD4+count >500/mm3 at the time of the study had a longer mean time since HIV diagnosis than patients with a CD4+count <200/mm3 (13.2±6.2 vs. 10.4±7.0 years; p=0.026) and had been on ART for longer than patients with CD4+count <200/mm3 (11.0±5.9 vs. 8.2±7.0 years; p=0.002). Male participants presented higher blood glucose and SBP levels. Although more than half of the participants had normal weight as defined by BMI (n=313; 54.8%), 40.3% (n=230) presented BMI ≥25 kg/m2, which indicates excess weight. Female patients presented a higher prevalence of WC above the defined cut-off (35.2% vs. 36.3% in males; p<0.001). Regarding traditional CV risk factors, 52.9% (n=302) had a family history of cardiovascular events, 53.1% (n=303) were smokers and 5.1% (n=29) were diabetic. Almost one third had a history of substance abuse (n=166, 29.1%). Table 1 presents patient characteristics and prevalence of CVD risk factors. No significant differences were found between genders, apart from higher HDL-C in female patients (58.0±16.7 mg/dl vs. 47.5±13.3 mg/dl in male patients; p<0.001) and higher SBP in male patients (134.2±15.4 mmHg vs. 128.8±17.1 mmHg in female patients; p<0.001).
Characteristics of the study population, overall and by age.
| Overall | Age >40 years (n=445) | Age ≤40 years (n=126) | pb | |
|---|---|---|---|---|
| Family history of CVD | 52.9 (302) | 55.3 (246) | 44.4 (56) | 0.031 |
| Current smoker | 53.1 (303) | 51.9 (231) | 57.1 (72) | >0.05 |
| SBP, mmHga | 132.4±16.2 (86-199) | 133.5±16.5 | 128.1±14.2 | 0.004 |
| DBP, mmHga | 78.2±11.1 (52-118) | 78.8±11.1 | 76.0±10.7 | 0.014 |
| HBP | 19.6 (112) | 24.0 (107) | 4.0 (5) | <0.001 |
| Blood glucose, mg/dla | 95.4±20.9 (56-300) | 96.9±22.0 | 90.1±15.1 | 0.001 |
| Diabetes | 5.1 (29) | 6.1 (27) | 1.6 (2) | 0.040 |
| TC, mg/dla | 191.2±40.7 (94-347) | 196.6±40.1 | 172.3±33.9 | <0.001 |
| TC ≥200 mg/dl | 38.0 (217) | 44.0 (196) | 16.7 (21) | <0.001 |
| HDL-C, mg/dla | 50.9±15.3 (22-131) | 51.8±15.9 | 47.8±12.6 | 0.01 |
| HDL-C <40 (M) or <50 (F) mg/dl | 27.5 (157) | 26.7 (119) | 30.2 (38) | >0.05 |
| HIV diagnosis, years | 12.8±6.4 (0-29) | 13.9±6.1 | 8.7±5.6 | <0.001 |
| CD4+count, /mm3 | ||||
| Mean | 688.7±346.8 (22-2269) | 702.6±357 | 639.3±301 | >0.05 |
| <200 | 5.1 (29) | 5.8 (26) | 2.4 (3) | >0.05 |
| 200-499 | 17.9 (162) | 26.7 (119) | 34.1 (43) | |
| ≥500 | 66.7 (380) | 67.4 (300) | 63.5 (80) | |
| Viral load (<50 copies) | 83.9 (474) | 87.5 (384) | 71.4 (90) | <0.001 |
| ART, years | 10.4±6.1 | 11.7±5.8 | 6.0±4.9 | <0.001 |
| Weight, kga | 70.8±14.3 (39.6-122.1) | 71.1±14.4 | 69.5±13.8 | >0.05 |
| BMI, kg/m2a | 24.7±4.5 (15.4-43.3) | 24.9±4.5 | 23.7±4.1 | 0.004 |
| Underweight | 4.9 (28) | 3.8 (17) | 8.7 (11) | 0.006 |
| Normal weight | 54.8 (313) | 52.6 (234) | 62.7 (79) | |
| Pre-obesity | 28.9 (165) | 31.0 (138) | 21.4 (27) | |
| Obesity | 11.4 (65) | 12.6 (56) | 7.1 (9) | |
| WC, cma | 88.5±12.8 (60.1-129.0) | 90.1±12.7 | 83.1±11.4 | <0.001 |
| WC above cut-off | 41.9 (239) | 46.7 (207) | 25.4 (32) | <0.001 |
ART: antiretroviral therapy; BMI: body mass index; DBP: diastolic blood pressure; F: female; HBP: high blood pressure; M: male; SBP: systolic blood pressure; TC: total cholesterol; WC: waist circumference.
Of the 571 individuals assessed, 186 (32.6%) had clinical criteria for MS. Those with MS were mostly Caucasian (n=172; 92.5%) and were older (48.7±8.5 vs. 44.4±8.8 years; p<0.001), had HIV infection for longer (13.7±6.3 vs. 12.4±6.4 years; p=0.019) and were exposed to ART for longer (11.6±5.9 vs. 9.8±6.2 years; p<0.001) than participants without MS. An association was found between ART and the presence of MS (OR=2.6 [95% CI: 1.1-6.0]; p=0.018). In the MS group, 96.2% (n=179) were under ART.
TG ≥150 mg/dl and a high WC were found to be the major diagnostic factors of MS (OR=13.6 [95% CI: 8.9-20.7]; p<0.001 and OR=13.1 [95% CI:8.5-20.1]; p<0.001, respectively).
Regarding anthropometric parameters, participants with MS had higher mean weight (79.5±13.3 vs. 66.5±12.8 kg; p<0.001) and a higher BMI (27.5±4.2 vs. 23.3±3.9 kg/m2, p<0.001) than participants without MS.
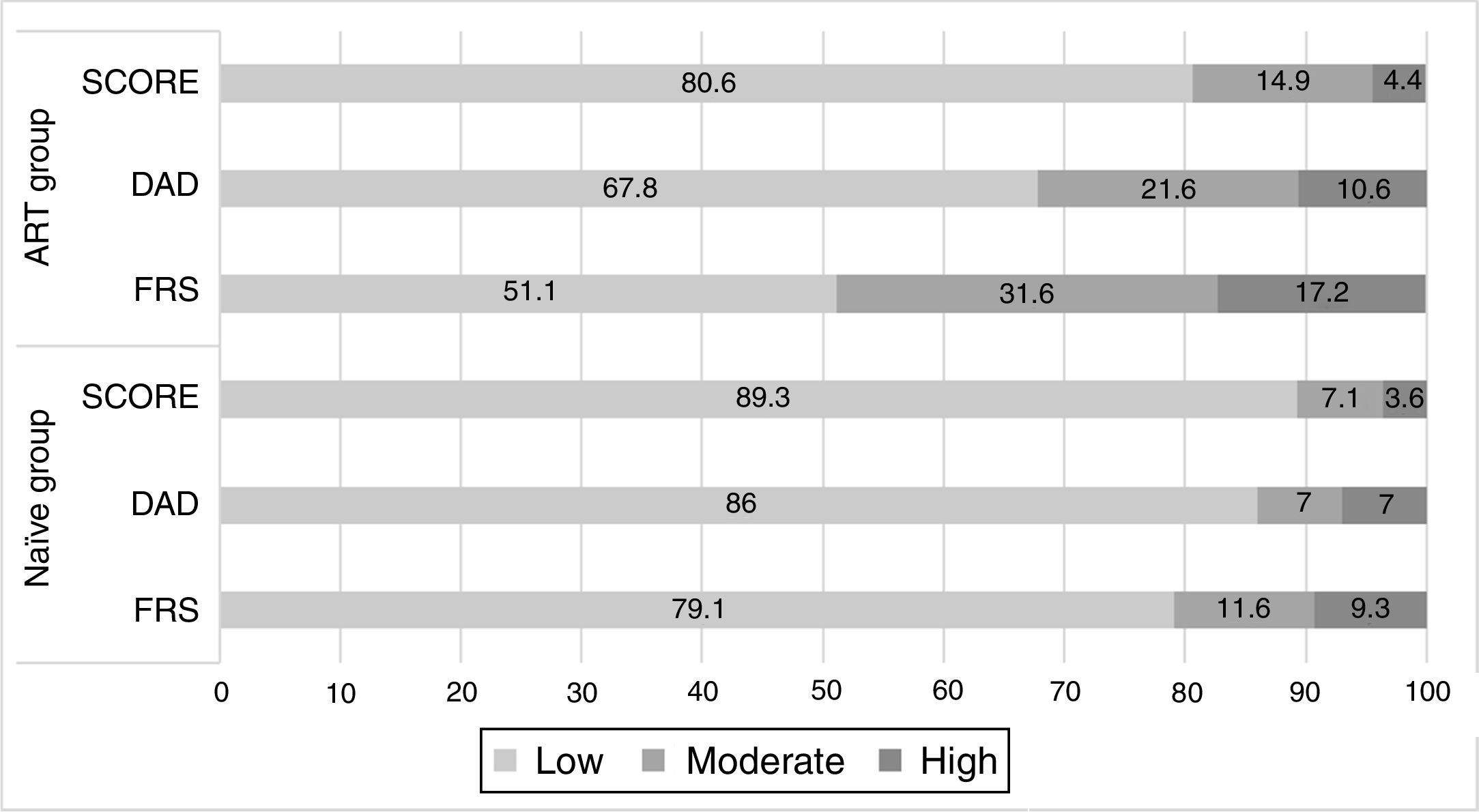
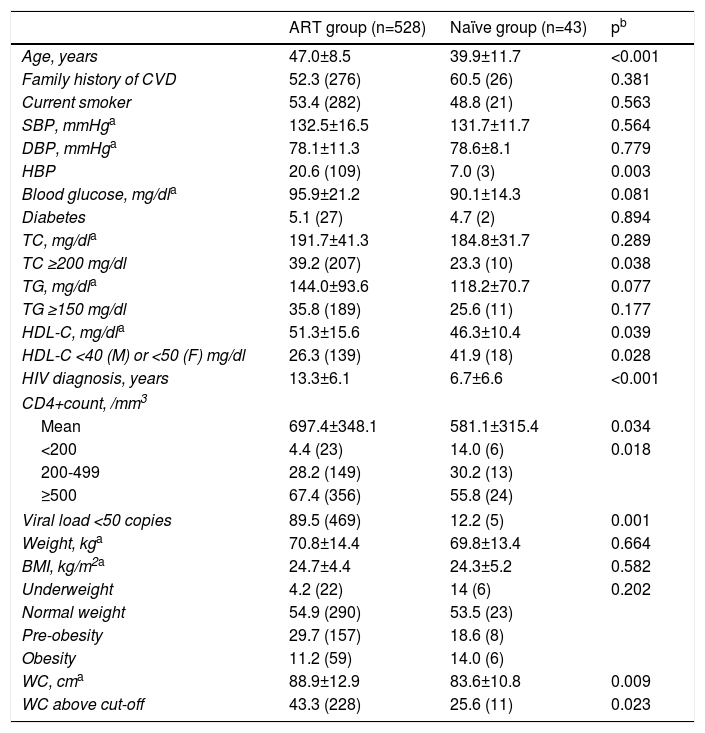
Cardiovascular risk assessmentOverall, CV risk showed wide variation and calculated risk varied according to the algorithm used. A high CV risk was found in 4.4% (n=23) of patients when the SCORE tool was used, in 20.5% (n=117) when FRS was applied and in 10.3% (n=59) of patients using the DAD score. Patients under ART (n=528) had a significantly higher CV risk than ART-naïve patients (n=43) with all three tools used (Figure 1). For this reason, further analysis was performed in two groups: the ART group and the naïve group.
Among the 528 patients on ART, almost half (47.2%, n=249) were on a protease inhibitor-based regimen. Naïve participants (n=43) were younger, had a shorter time since HIV diagnosis, had a lower mean CD4+count than the ART group and were mostly in the category of CD4+count <200/mm3. Table 2 presents patient characteristics and comparison by treatment group.
Characteristics of the study population by treatment group.
| ART group (n=528) | Naïve group (n=43) | pb | |
|---|---|---|---|
| Age, years | 47.0±8.5 | 39.9±11.7 | <0.001 |
| Family history of CVD | 52.3 (276) | 60.5 (26) | 0.381 |
| Current smoker | 53.4 (282) | 48.8 (21) | 0.563 |
| SBP, mmHga | 132.5±16.5 | 131.7±11.7 | 0.564 |
| DBP, mmHga | 78.1±11.3 | 78.6±8.1 | 0.779 |
| HBP | 20.6 (109) | 7.0 (3) | 0.003 |
| Blood glucose, mg/dla | 95.9±21.2 | 90.1±14.3 | 0.081 |
| Diabetes | 5.1 (27) | 4.7 (2) | 0.894 |
| TC, mg/dla | 191.7±41.3 | 184.8±31.7 | 0.289 |
| TC ≥200 mg/dl | 39.2 (207) | 23.3 (10) | 0.038 |
| TG, mg/dla | 144.0±93.6 | 118.2±70.7 | 0.077 |
| TG ≥150 mg/dl | 35.8 (189) | 25.6 (11) | 0.177 |
| HDL-C, mg/dla | 51.3±15.6 | 46.3±10.4 | 0.039 |
| HDL-C <40 (M) or <50 (F) mg/dl | 26.3 (139) | 41.9 (18) | 0.028 |
| HIV diagnosis, years | 13.3±6.1 | 6.7±6.6 | <0.001 |
| CD4+count, /mm3 | |||
| Mean | 697.4±348.1 | 581.1±315.4 | 0.034 |
| <200 | 4.4 (23) | 14.0 (6) | 0.018 |
| 200-499 | 28.2 (149) | 30.2 (13) | |
| ≥500 | 67.4 (356) | 55.8 (24) | |
| Viral load <50 copies | 89.5 (469) | 12.2 (5) | 0.001 |
| Weight, kga | 70.8±14.4 | 69.8±13.4 | 0.664 |
| BMI, kg/m2a | 24.7±4.4 | 24.3±5.2 | 0.582 |
| Underweight | 4.2 (22) | 14 (6) | 0.202 |
| Normal weight | 54.9 (290) | 53.5 (23) | |
| Pre-obesity | 29.7 (157) | 18.6 (8) | |
| Obesity | 11.2 (59) | 14.0 (6) | |
| WC, cma | 88.9±12.9 | 83.6±10.8 | 0.009 |
| WC above cut-off | 43.3 (228) | 25.6 (11) | 0.023 |
ART: antiretroviral therapy; BMI: body mass index; DBP: diastolic blood pressure; F: female; HBP: high blood pressure; M: male; SBP: systolic blood pressure; TC: total cholesterol; WC: waist circumference.
Regarding CV risk factors, there were no significant differences in cholesterol (total or LDL) or smoking status between the ART group and the naïve group. A trend was found for naïve patients to present lower TG and blood glucose levels, although this was not statistically significant. Naïve patients had a smaller mean WC than the ART group (p=0.009).
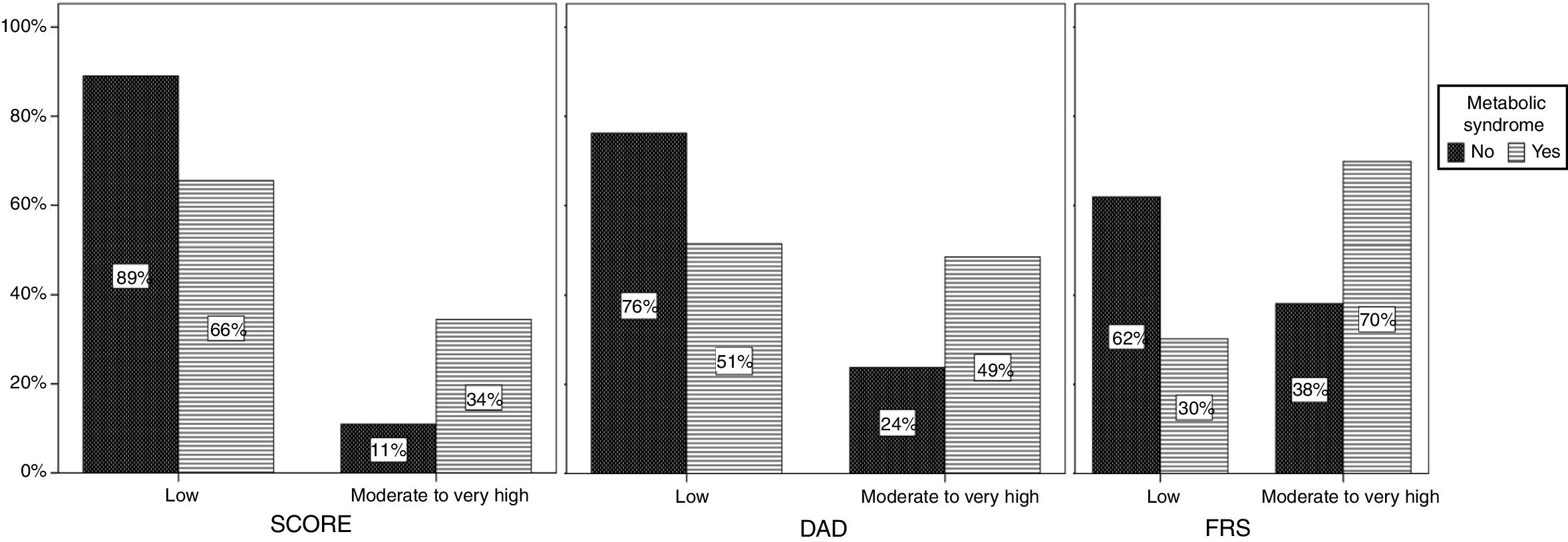
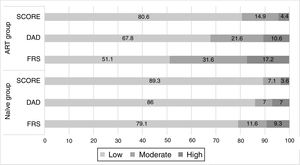
The presence of MS was associated with increased CV risk in the ART group when all three tools were used (p<0.001) (Figure 2). In the naïve group the same association was found, although without statistical significance (data not shown).
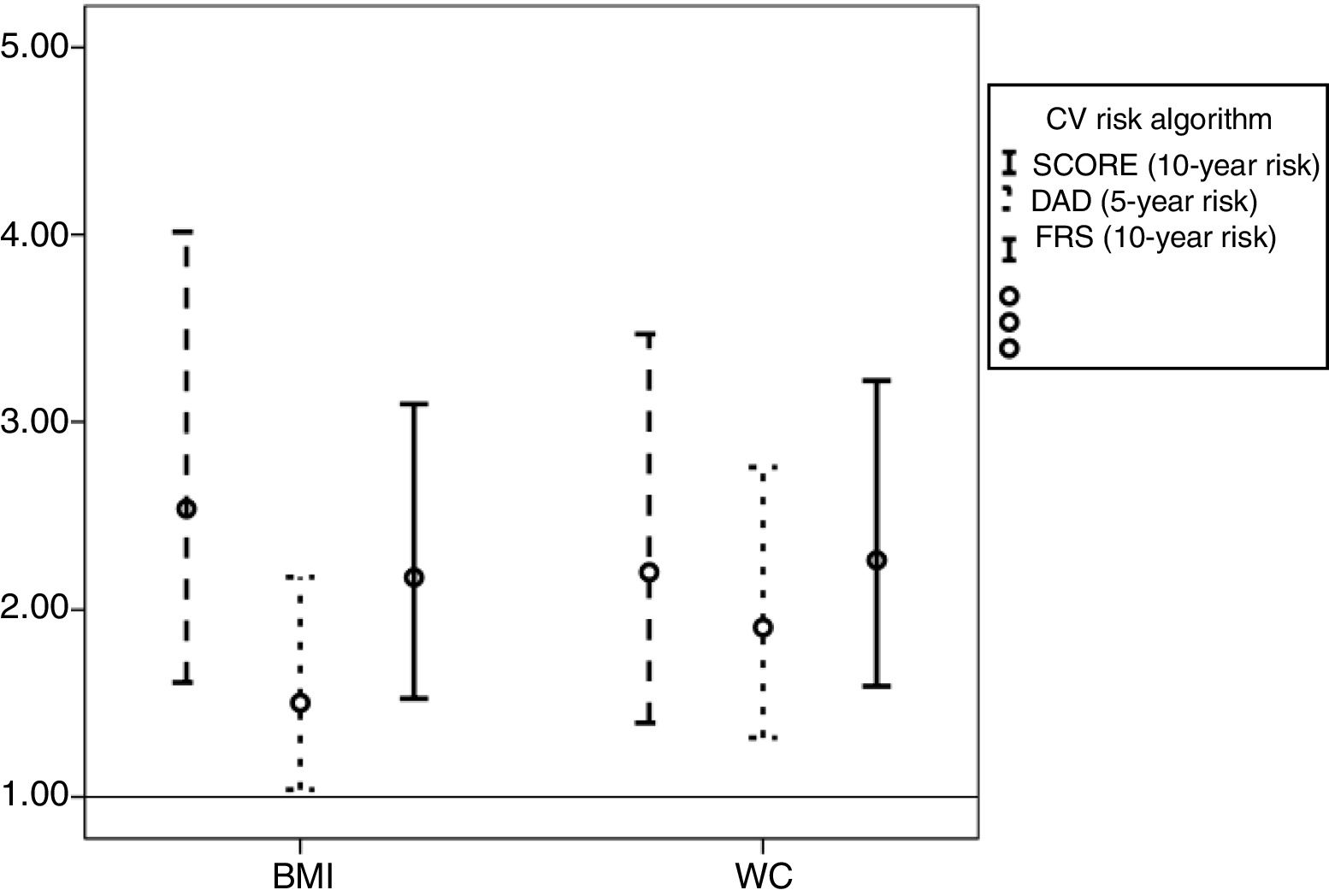
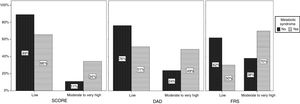
Independently of the tool used, increased mean BMI and WC were strongly associated with higher CV risk (Figure 3).
Odds ratios and 95% confidence intervals associated with increased body mass index (BMI) and waist circumference (WC) according to cardiovascular risk score (Systematic Coronary Risk Evaluation (SCORE), the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) risk equation and the Framingham risk score (FRS).
The observed agreement between the FRS and SCORE was 55.4% (k=0.183, p<0.001), while between FRS and DAD it was 70.5% (k=0.465, p<0.001) and between SCORE and DAD, 72.3% (k=0.347, p<0.001), which represents fair to moderate agreement.35 The observed agreement between the algorithms did not improve significantly when only patients aged over 40 years were analyzed (data not shown).
In the final logistic regression model, the factors that were still associated with higher CV risk differed depending on the tool used. When SCORE was used, the factors associated with higher CV risk were older age (OR=14.4 [95% CI: 6.8-30.7]; p<0.001) and increased TG (OR=2.5 [95% CI: 1.5-4.1]; p<0.001). Using the DAD estimator, the associated factors were age (OR=21.4 [95% CI: 12.3-37.1]; p<0.001), increased TG (OR=3.8 [95% CI: 2.4-6.0]; p<0.001), and smoking (OR=3.8 [95% CI: 2.2-6.2]; p<0.001). Using the FRS, the factors were age (OR=20.6 [95% CI: 12.2-34.8]; p<0.001), increased TG (OR=3.9 [95% CI: 2.5-6.2]; p<0.001) and smoking (OR=5.5 [95% CI: 3.3-9.4]; p<0.001). Interestingly, after applying logistic regression to our data, ART was only associated with increased CV risk when the FRS was used (OR=3.2 [95% CI: 1.2-8.5]; p=0.002).
DiscussionThe present study was conducted in a population of HIV-infected individuals in Lisbon, mainly composed of adult Caucasian males. In this sample, traditional CV risk factors15,26,36–40 were associated with higher CV risk, in agreement with previous studies. However, we found a much higher prevalence of smoking (53.1%) compared to the general population in Portugal (20%).41 Interestingly, the prevalence of low HDL-C (27.5%) found in our study was less than in other studies (37.2-36.3%), probably because of a large proportion of black patients in our study (8.7%).
As expected, older patients had higher CV risk regardless of ART status.43 The inclusion of younger patients (<30 years) in this study means caution should be exercised when interpreting the data on CV risk, since for some algorithms, the risk is mostly relative. However, data on mortality from CVD in the HIV population indicate that even at younger ages, these patients present non-negligible cardiovascular mortality, and this was the primary reason to include these patients in the analysis.44 In addition, previous studies have reported the application of these algorithms to younger HIV patients.38
Increased TG was the major factor associated with MS. Low HDL-C, identified in previous studies as the second most common factor associated with MS, was less strongly associated than increased WC.36 Unlike Maloberti et al.,45 in this study we found that patients with MS presented a longer mean time since HIV diagnosis and longer exposure to ART. The prevalence of MS was lower in this study than in the general non-infected Portuguese population (43.1%).46
The use and clinical benefits of CV risk algorithms have been much discussed in recent years and debate continues as to which algorithm is the most accurate for the HIV-infected population. In our study, the prevalence of patients classified as moderate to high CV risk using the SCORE tool was lower than that found by others39 in which patients with a known history of CVD were also included. However, the prevalence in this study was higher than that found in other studies, which could be due to differences in patients’ age.42 Using the DAD algorithm, the prevalence of infected individuals on ART with high CV risk was much higher in our study (10.6 vs. 2.1%),38 which could be explained by the fact that our patients were on average 10 years older and were more often smokers. Finally, using the FRS, the prevalence of moderate to high CV risk (46.8%) was much higher than that reported in other studies (6-23.4%).14,38,39,42 Again, the differences found could be due to the higher prevalence of hypertriglyceridemia and smoking and older age in our study. Comparing moderate to high CV risk by gender, the prevalence found was similar to other studies,10,16,47 highlighting the increased CV risk seen in males, which is consistent with data on uninfected patients. Apart from SCORE, the algorithms showed that higher CV risk was associated with duration of ART exposure, as reported by others.48 Although some studies48,49 describe a higher incidence of CVD in HIV-infected patients with lower CD4+lymphocyte counts, in this study there were more patients in the higher CV risk categories with a CD4+count over 500/mm3 than <200/mm3, which may be due to longer ART exposure and hence more alterations in lipid profile.50
Overall, our results indicate that the DAD and FRS tools appear to be more sensitive in detecting high CV risk patients. Of note, we found that anthropometric parameters, although not contributing directly to the CV risk algorithms, were positively associated with cardiovascular risk, suggesting that any new tool developed to assess CV risk should include anthropometric parameters. Given the results of the different tools used in this study, a longitudinal study is necessary to determine the accuracy of these estimations.
Regardless of the algorithm used, measuring CV risk in HIV-infected patients should be considered a priority, since our results demonstrate that there is a high prevalence of at-risk patients. Determination of CV risk, using tools tailored to this population, should be seen as routine for all patients. Portugal has a well-established HIV control program, and prevention has been the primary focus. However, with the increased survival rate witnessed in the HIV-infected population with ART, guidelines should also incorporate recommendations to tackle unhealthy lifestyles, as already seen for smoking, particularly promotion of healthier dietary habits and exercise to ameliorate the burden of obesity and CVD.
DisclosuresNo financial support.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the patients participating in our study, as well as the hospital staff at the Department of Infectious Diseases, for their support during the investigation.
Partially presented at the 12th International Congress on Drug Therapy in HIV Infection, November 2014, Glasgow, Scotland (PP 195) and at the 36th Congress of the European Society for Clinical Nutrition and Metabolism, September 2014, Geneva, Switzerland (SUN-PP219).