The cardiovascular manifestations of human immunodeficiency virus (HIV) infection have changed significantly following the introduction of highly active antiretroviral therapy (HAART) regimens. On one hand, HAART has altered the course of HIV disease, with longer survival of HIV-infected patients, and cardiovascular complications of HIV infection such as myocarditis have been reduced. On the other hand, HAART is associated with an increase in the prevalence of both peripheral and coronary arterial disease. As longevity increases in HIV-infected individuals, long-term effects, such as cardiovascular disease, are emerging as leading health issues in this population. In the present review article, we discuss HIV-associated cardiovascular disease, focusing on epidemiology, etiopathogenesis, diagnosis, prognosis, management and therapy. Cardiovascular involvement in treatment-naive patients is still important in situations such as non-adherence to treatment, late initiation of treatment, and/or limited access to HAART in developing countries. We therefore describe the cardiovascular consequences in treatment-naive patients and the potential effect of antiretroviral treatment on their regression, as well as the metabolic and cardiovascular implications of HAART regimens in HIV-infected individuals.
As manifestações cardiovasculares da infeção pelo vírus da imunodeficiência humana (VIH) modificaram-se significativamente com a introdução dos regimes de terapêutica antirretroviral de elevada potência (HAART). Por um lado, a HAART modificou o curso da doença VIH, com o prolongamento da sobrevivência dos doentes VIH-infetados. Complicações cardiovasculares da infeção VIH, como a miocardite, foram reduzidas. Por outro lado, a HAART tem sido associada ao aumento da prevalência de doenças arteriais periféricas e coronárias. Com o aumento da longevidade dos indivíduos VIH-infetados, efeitos a longo prazo, como a doença cardiovascular, estão a emergir como questões de saúde proeminentes nesta população. No presente artigo de revisão, discutiremos a patologia cardiovascular associada ao VIH, focando-nos na epidemiologia, etiopatogénese, diagnóstico, prognóstico, abordagem e terapêutica. A importância do envolvimento cardiovascular em doentes não tratados pelas novas terapêuticas é ainda uma realidade em situações como o não cumprimento da terapêutica, o início tardio da terapêutica ou o acesso limitado à HAART nos países em desenvolvimento. Assim, descreveremos as consequências cardiovasculares nos doentes não tratados e o potencial efeito da terapêutica antirretroviral na sua regressão, e as consequências metabólicas e implicações cardiovasculares dos regimes HAART nas pessoas infetadas pelo VIH.
acquired immunodeficiency syndrome
antiretroviral therapy
blood pressure
calcium channel blocker
cardiovascular disease
European AIDS Clinical Society
highly active antiretroviral therapy
human immunodeficiency virus
heart failure
hypertensive nephropathy
infectious endocarditis
ischemic heart disease
inducible nitric oxide synthase
Kaposi sarcoma
left ventricular
left ventricular diastolic dysfunction
myocardial infarction
methicillin-resistant Staphylococcus aureus
non-Hodgkin lymphoma
non-nucleoside reverse-transcriptase inhibitor
nucleoside reverse-transcriptase inhibitor
pulmonary arterial hypertension
pericardial effusion
protease inhibitor
corrected QT
transthoracic echocardiography
Human immunodeficiency virus (HIV) is a retrovirus with tropism for cells expressing CD4. In 2012 the number of HIV-positive individuals was estimated at 35.3 million.1
The introduction of highly active antiretroviral therapy (HAART) has prolonged the survival of HIV-positive individuals, turning acquired immunodeficiency syndrome (AIDS) into a chronic disease.
A retrospective analysis of causes of death in 13 cohort studies of HIV type 1 (HIV-1)-infected patients who initiated antiretroviral therapy (ART) in Europe and North America from 1996 through 2006 showed lower mortality from AIDS-related causes and higher mortality from causes associated with aging, such as non-AIDS malignancies and cardiovascular disease (CVD). The latter accounted for 7.9% of deaths, of which 40% were from myocardial infarction (MI)/ischemic heart disease (IHD), which suggests that the process of aging will become a dominant factor in HIV-1 mortality in the next decade.2
The cardiovascular manifestations of HIV infection have changed following the introduction of HAART. Cardiovascular involvement in treatment-naive patients is still important in individuals who do not adhere to treatment or start treatment late, and in countries with limited access to ART. We therefore describe the cardiovascular consequences in treatment-naive HIV-positive individuals and the potential effect of treatment on their regression, as well as the implications of HAART.
Cardiovascular manifestations of human immunodeficiency virus infectionCardiomyopathyFour types of cardiomyopathy are associated with HIV infection: myocarditis, hypokinetic cardiomyopathy (particularly in advanced stages of infection), dilated cardiomyopathy, and reduced left ventricular systolic function.3
Pre-HAART studies reported high prevalences of myocarditis, in up to 52% of patients.4 Acute myocarditis can lead to congestive heart failure (HF) and arrhythmias. Myositis is common in this population, and myoglobin is thus less specific as a marker of myocardial damage.5 Clinical features, risk factors such as drugs or antivirals, and complementary exams have a role in diagnosis. The gold standard in the diagnosis of myocarditis is endomyocardial biopsy.6
Dilated cardiomyopathy: With regard to dilated cardiomyopathy, the pre-HAART incidence was reported to range between 8%7 and 35%.8 In the HAART era, a reduction in the prevalence of cardiomyopathy has been reported in developed countries,9 possibly due to reduced viral replication, lower incidence of myocarditis and prevention of opportunistic infections. In developing countries, with less access to ART, cardiomyopathy is a significant problem; a prospective study in Rwanda reported a 17.7% prevalence of dilated cardiomyopathy.10
The pathogenesis of dilated cardiomyopathy is thought to be multifactorial, possibly linked to infection of the myocardium by HIV,7 immunodepression,3,10 nutritional deficiencies,10 diffuse-regressive alterations,11 cardiac autoimmunity,12 infectious endocarditis, coinfection with cardiotropic viruses,7 the action of cytokines,11 and the cardiotoxicity of certain drugs, including zidovudine.13
Clinically, it is often asymptomatic or non-specific and the symptoms of HF may be masked by other conditions. Echocardiography is the method of choice to assess ventricular function.
Cardiomyopathy is associated with increased mortality, with progressive left ventricular (LV) dysfunction leading to HF. The importance of ventricular dysfunction is demonstrated by the reduced survival of patients with cardiomyopathy who died of AIDS in the pre-HAART era compared to those with preserved cardiac function at a similar stage of infection (101 vs. 472 days).14
Barbaro et al. studied the influence of development of encephalopathy on the clinical course of HIV-associated cardiomyopathy and observed that patients with encephalopathy were more likely to die from congestive HF.15 The virus may persist in reservoir cells in the myocardium and cerebral cortex even after ART, and these cells may chronically release cytotoxic cytokines, contributing to progressive tissue damage in both systems. Antagonists of cytokines or inducible nitric oxide synthase (iNOS) or apoptosis inhibitors can reduce cell damage caused by chronic release of cytotoxic cytokines and by activation of iNOS by these reservoir cells.15 However, further studies are needed to assess their therapeutic potential.
In general, standard HF treatment regimens are recommended for HIV-positive individuals with dilated cardiomyopathy and HF.5 Angiotensin-converting enzyme inhibitors may be poorly tolerated because of low systemic vascular resistance from diarrheal disease, infection or dehydration.5 Digoxin may be added for the treatment of patients with persistent symptoms or atrial fibrillation with rapid ventricular response.5 When the patient is euvolemic, a beta-blocker may be started because of its beneficial effects on circulating levels of cytokines.16 There is little evidence that HAART is beneficial in this respect, although it may reduce the incidence of cardiac disease by preventing opportunistic infections.
Diastolic dysfunctionHigh prevalences of left ventricular diastolic dysfunction (LVDD) have been reported in HIV-positive individuals (36%18–55.7%3),3,17–19 although some studies contradict this.20
Subclinical cardiac abnormalities have been detected at early stages of HIV infection, independently of ART, suggesting that HIV itself may play a part in the genesis of diastolic dysfunction.18 Traditional risk factors are strongly associated with impaired diastolic relaxation.17
LVDD frequently appears in patients with few or no symptoms or in those whose symptoms are related to other conditions. Echocardiography provides a reliable non-invasive assessment of LV systolic and diastolic function and can detect subclinical myocardial involvement in HIV-positive individuals.3 However, in the absence of symptoms, it may be premature to recommend routine screening echocardiograms.19
It is unclear whether HIV-infected patients require any specific therapeutic interventions to manage or prevent cardiac dysfunction. Reduction of HIV-related inflammation with ART would appear to be a reasonable approach, although the benefit of treatment on cardiac function remains unproven.19
Infectious endocarditisStudies in the pre-HAART era reported increased risk of infectious endocarditis (IE) in HIV-positive individuals, but in one study its incidence decreased from 20.5 to 6.6 per 1000 person-years between the pre- and post-HAART eras.21
The risk factors most strongly associated with IE were intravenous drug use21,22 and severe immunodepression.21 In some studies Staphylococcus aureus was the most common agent in HIV-positive individuals,21–23 most often involving the tricuspid valve.23 In HIV-positive individuals with methicillin-resistant S. aureus (MRSA) bacteremia, community-associated MRSA was significantly associated with increased IE prevalence.24
Patients may present fever, weight loss, and concomitant pneumonia and/or meningitis. Transthoracic echocardiography (TTE), complemented by transesophageal echocardiography, is essential to confirm the diagnosis and to guide treatment.
Left heart involvement and severe immunodepression (CD4 <200/mm3) are associated with greater mortality.22,23 Gebo et al. reported higher recurrence and mortality rates within one year of IE infection and recommended more aggressive follow-up, especially in those over 40 years of age.21
Antibiotic therapy is often effective, but surgery is indicated in selected patients.
Pericardial effusionPericardial effusion (PE) is relatively common in this population. Cardiac tamponade is rare.25 In the pre-HAART era, an annual incidence of 11% was reported in AIDS patients.25 In the HAART era, an incidence of 0.25% has been reported in HIV-positive individuals.26
Possible etiologies of PE in these patients include opportunistic infections,27 malignancies such as Kaposi sarcoma (KS) and non-Hodgkin lymphoma (NHL),25 tuberculosis, hypoalbuminemia,25 idiopathic, and end-stage HIV capillary leak syndrome.25
Previous studies of PE in this population reported that pericardial involvement was often an echocardiographic finding that was not clinically suspected, and that since most PEs were small8,25 and rarely progressive25 an exhaustive search for a pericardial diagnosis is usually not indicated.25 Large symptomatic pericardial effusions do occur, however, and may need aggressive evaluation and therapy.25 Dyspnea, exercise intolerance or edema should prompt investigation by TTE.26
PE may be a marker of end-stage HIV infection,25 but it is rarely the cause of death,27 although it has been associated with shorter survival.8,25
Pulmonary hypertensionHIV-related pulmonary arterial hypertension (PAH) has similar clinical, laboratory, imaging and pathological manifestations to those of idiopathic PAH.28
An incidence of 0.5% in HIV-positive individuals was reported in the pre-HAART era,29 while a prospective study in the HAART era reported a prevalence of 0.46%.30 PAH may develop at any stage of HIV infection and all risk groups may be affected.31
The mean age of HIV-positive individuals diagnosed with PAH as reported in a systematic review28 was 35±9.6 years and 59% were male; the main risk factors for contracting HIV infection were injection drug use (49%) and male-to-male sexual activity (21%), and mean CD4 count at the time of diagnosis of PAH was 352±304 cells/μl. AIDS had been diagnosed in 53%, hepatitis B in 12%, and hepatitis C in 14%. The mean time from diagnosis of HIV infection to diagnosis of PAH was 4.3±4.0 years.
The underlying vasculopathy is severe angioproliferative disease. Pulmonary veno-occlusive disease is relatively rare29,31,32; pulmonary vascular dysfunction probably results from risk factors such as viral infections, autoimmunity, drugs or toxins, possibly triggering an underlying genetic susceptibility.32 Inflammation appears to play a more active role in the pathogenesis of HIV-related PAH than in idiopathic PAH.32
Diagnosis requires confirmation of pulmonary hypertension and of HIV infection and exclusion of other causes of pulmonary hypertension.33 It should be suspected in cases of unexplained dyspnea.30,31 Symptoms at the time of diagnosis as reported by Janda et al. included dyspnea (93%), pedal edema (18%), syncope (13%), fatigue (11%), cough (8%) and chest pain (6%).28 Echocardiography should be performed in patients with unexplained dyspnea to investigate possible HIV-related cardiovascular complications.34 Right heart catheterization is the gold standard to diagnose PAH and to assess hemodynamic status and response to treatment.35
Development of PAH is associated with worse prognosis, particularly in NYHA functional class III–IV, with 28% survival at three years.36 Patients with HIV-related PAH frequently die from conditions associated with PAH.28,35,36 The most common complication is right-sided HF.28
Since there is no curative treatment, the aim is to improve patients’ functional class. Conventional treatment is directed at controlling its consequences and is similar to that for all forms of pulmonary hypertension.33 In cases of decompensated right heart failure, fluid restriction and diuretics should be used with caution to avoid excessive reduction of intravascular volume. Inotropic agents are used when necessary and home oxygen therapy can be prescribed in patients with chronic hypoxemia.33 Anticoagulation is not routinely recommended because of an increased risk of bleeding, treatment compliance issues, and drug interactions,34 and these individuals should not receive calcium channel blockers (CCBs).34
There have been few studies on PAH treatment in this population and there is a need for controlled randomized trials with large population samples. Administration of sildenafil is the subject of debate, since it interacts with protease inhibitors (PIs); according to Galie et al., if sildenafil is used, the dose should be adjusted if ritonavir and saquinovir are co-administered.34
There are conflicting data on the efficacy of HAART in the treatment of PAH.37–39
HIV infection is generally considered an exclusion criterion for lung transplantation, although in some centers a specific program has been implemented.34
Autonomic dysfunctionCardiovascular autonomic tone has been shown to be involved in advanced HIV disease. Spectral analysis of heart rate variability showed severe global autonomic dysfunction in AIDS patients without clinical or echocardiographic evidence of cardiac disease, and this has been suggested as a possible mechanism of arrhythmogenesis.40 HIV-positive individuals under ART for more than 44 months present increased resting heart rate and reduced short-term heart rate variability, indicative of parasympathetic dysfunction.41 However, a recent prospective study suggests that ART may not contribute to short-term alterations in autonomic function in healthy individuals early in the course of the disease.42
Cardiac malignanciesThe introduction of HAART has led to significant reductions in the incidence of KS and NHL,43 both of which can affect the heart.
In the pre-HAART era, individuals with AIDS were at increased risk of KS.44 Cardiac involvement usually occurs as a part of disseminated KS.27 Clinical cardiac findings are obscure and pericardiocentesis not only has no diagnostic role but is also a high-risk procedure in this group of patients.27 When there is a high index of suspicion of PE due to KS, a pericardial window should be performed for providing decompression and establishing the pathologic diagnosis.27
An increased incidence of NHL in AIDS patients was also reported in the pre-HAART era.44 Cardiac involvement, usually derived from B cells, is typically high grade and is often disseminated early in patients with AIDS.27,45 It is usually clinically silent, but may present with HF, arrhythmias and/or PE,46,47 and cardiac tamponade.45 Echocardiography may reveal an intracardiac mass or nodular lesions within the three layers of the heart wall, but in infiltrative forms of cardiac NHL, it may underestimate the extent of myocardial involvement.45 Magnetic resonance imaging is useful for assessing the characteristics and potential complications of the malignancy.47
In HIV patients, the occurrence of NHL does not correlate closely with an advanced stage of immunosuppression.45 Although prognosis is poor, systemic chemotherapy may prolong survival.45
VasculopathiesVirtually all types of vasculitides of small, medium and large vessels have been observed in this population.48 They may result from abnormalities induced by HIV and/or other agents. Various inflammatory vascular diseases may develop, including polyarteritis nodosa-like vasculitis,48 Henoch-Schönlein purpura, drug-induced hypersensitivity vasculitis, Kawasaki-like syndromes49 and Takayasu arteritis.50
HIV-related aneurysms have been identified as a distinct entity, characterized by their predilection for young patients, multiplicity, atypical location and distinct histological features. Most patients were asymptomatic and 68% presented advanced HIV disease.51 The pathogenesis of these aneurysms remains unclear.51
A type of occlusive arterial disease apparently unique to HIV-positive individuals has been reported. It is more common in young patients, generally those with advanced disease and significant immunodepression.52 They frequently present with advanced tissue necrosis that precludes limb salvage.52
Human immunodeficiency virus and coronary diseaseElectrocardiographic evidence of asymptomatic IHD has been reported in 10.9% of HIV-infected adults without known IHD, irrespective of type and duration of ART.53
Alterations in lipid metabolism have been described in HIV-positive individuals.54,55
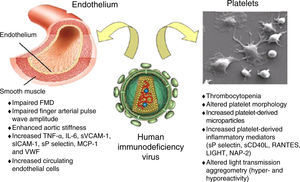
The role of HIV as a risk factor for accelerated atherosclerosis is controversial.56–59 The virus has effects on the endothelium and platelets (Figure 1).60 Endothelial dysfunction occurs early in the process of atherogenesis and contributes to the formation, progression and complications of atherosclerotic plaques.60
Schematic representation of the possible interactions between human immunodeficiency virus and the endothelium and platelets. Adapted from Gresele et al.60
FMD: flow-mediated dilation; LIGHT: TNFSF14 (tumor necrosis factor superfamily member 14); NAP-2: neutrophil activating peptide 2; RANTES: regulated on activation normal T cell expressed and presumably secreted; sCD40L: soluble CD40 ligand; sP selectin: soluble P-selectin.
Atherosclerotic alterations of the arterial wall lead to increased arterial stiffness, which has been reported in untreated HIV-positive individuals in some studies58,61 but not in others.62
Chronic HIV infection leads to immune activation and chronic inflammation, only partially corrected by HAART.60 Immunodepression can have adverse effects on the vasculature.59
Corrected QT interval prolongationAn increased prevalence of corrected QT interval (QTc) prolongation in HIV-positive individuals has been reported.63–66 This may be associated with drugs used to treat other conditions, electrolyte disturbances, longer duration of HIV infection,64,65 cardiomyopathy, autonomic dysfunction or myocardial ischemia.64 Shavadia et al. reported a significantly increased risk for QTc prolongation in HIV-positive individuals under ART compared to untreated individuals,63 but other studies found no significant association.65,66 According to Reinsch et al., factors such as gender, diabetes and hypertension may also be involved in the development of QTc prolongation.66
The use of noncardiac QTc-prolonging drugs has been associated with increased risk for sudden cardiac death in the general population.67 It is important to monitor QTc interval in patients under ART, particular when ART is combined with drugs that can prolong QTc.
Effects of human immunodeficiency virus infection on the cardiovascular system in the highly active antiretroviral therapy eraHighly active antiretroviral therapyThe initial combination regimens recommended by the European AIDS Clinical Society (EACS)68 are presented in Table 1.
Initial combination regimens recommended by the European AIDS Clinical Society.
| A | B | Remarks |
|---|---|---|
| NNRTI | NRTI | |
| EFVRPV | ABC/3TC or TDF/FTC | ABC/3TC co-formulatedTDF/FTC co-formulatedEFV/TDF/FTC co-formulatedRPV/TDF/FTC co-formulated |
| PI/r | ||
| ATV/rDRV/r | ABC/3TC or TDF/FTC | ATV/r: 300/100 mg qdDRV/r: 800/100 mg qd |
| INSTI | ||
| EVG+COBI | TDF/FTC | TDF/FTC/EVG/COBIco-formulated |
| DTG | ABC/3TC or TDF/FTC | DTG 50 mg qdTDF/FTC co-formulatedABC/3TC/DTG co-formulated |
| RAL | ABC/3TC or TDF/FTC | RAL: 400 mg bd |
A drug from column A should be combined with the drugs listed in column B.
/r: ritonavir used as booster; 3TC: lamivudine; ABC: abacavir; ATV: atazanavir; COBI: cobicistat; DRV: darunavir; DTG: dolutegravir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; INSTI: integrase strand transfer inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitors; NRTI: nucleoside reverse transcriptase inhibitors; PI: protease inhibitors; RAL: raltegravir; RPV: rilpivirine; TDF: tenofovir.
Adapted from the European AIDS Clinical Society Guidelines68.
The 2014 Recommendations of the International Antiviral Society–USA Panel propose that ART should be initiated in all individuals who are willing and ready to start treatment after confirmed diagnosis of HIV infection.69 ART is recommended for the treatment of HIV infection and the prevention of transmission of HIV regardless of CD4 cell count (strength of recommendation AIa-BIII).69 These measures will subject HIV-positive individuals to earlier exposure to HAART and its adverse effects. The recommendations of the 2014 EACS guidelines for initiation of ART in HIV-positive persons without prior ART exposure are graded taking into account both the degree of progression of HIV disease and the presence of, or high risk for developing, various types of (comorbid) conditions.68 ART is always recommended in any HIV-positive person with a current CD4 count <350 cells/μl.68 For persons with CD4 counts above this level, the decision to start ART should be considered on an individual basis.68
Effects of highly active antiretroviral therapyVarious cardiovascular risk factors can be induced or strengthened by HAART. Traditional risk factors have been significantly associated with increased risk for MI in HIV-positive individuals.70,71
The incidence of diabetes in HIV-positive men under HAART has been reported as over four times higher than in HIV-negative individuals.72 Traditional risk factors (age, male gender, obesity, low HDL cholesterol and high total cholesterol) play an important role in the increased risk of diabetes in this population,73–75 while lipodystrophy73,76 and immunodepression76 have also been associated with increased incidence of diabetes in HIV-positive individuals. The impact of coinfection with hepatitis C virus is the subject of debate.74–76 ART is not unanimously recognized as a risk factor for diabetes, but several studies have reported increased prevalence of diabetes with certain antiretrovirals73,74,77 and with longer exposure to ART.73,78 The drugs most often associated with diabetes are PIs74,77 and some nucleoside reverse-transcriptase inhibitors (NRTIs).73,74,77
Certain PIs have been associated with a dyslipidemic profile.55,79,80 Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) generally result in a more favorable lipid profile than PIs.80 However, studies have shown a significant risk of dyslipidemia induced by efavirenz.54,81 Of the NRTIs, tenofovir appears to be associated with less unfavorable lipid profiles.82,83 It has been suggested that integrase strand transfer inhibitor-based regimens may be a good option for patients with pre-existing dyslipidemia.69
HIV-related lipodystrophy, mainly considered an adverse effect of HAART, has a reported mean prevalence of 42% in HIV-positive individuals treated with PI-containing HAART.84 The term ‘lipodystrophy syndrome’ is used by some authors to include morphological and metabolic phenomena, but it is not clear that they result from the same mechanism. Not all patients present all the characteristics of the syndrome; dyslipidemia has been reported in 70% and diabetes in 8–10% of these patients.85
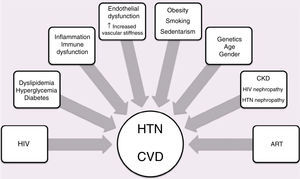
HypertensionSome studies suggest that the prevalence of hypertension is increased in HIV-positive individuals under ART,86,87 but this is not confirmed by others.88
The role of HIV and ART in the pathogenesis of hypertension is not clear. Possible mechanisms are presented in Figure 2. A prospective study in HIV-infected patients starting HAART found an increase in blood pressure (BP) after 48 weeks.86 In HIV-positive individuals starting their first HAART regimen, treatment with lopinavir/ritonavir was associated with increased BP,89 while patients taking atazanavir, efavirenz, nelfinavir or indinavir were less likely to develop high BP.89
Highly active antiretroviral therapy and cardiovascular diseaseMendes et al. detected abnormalities in myocardial deformation through assessment of strain and strain rate in a population of relatively healthy HIV-infected patients without established CVD or risk factors.20
It has been demonstrated that HIV infection and HAART are independent risk factors for early carotid atherosclerosis,56 but Kaplan et al. reported that ART was not consistently associated with atherosclerosis59 and there is conflicting evidence on the effects of ART on arterial stiffness.61,62
HIV-positive patients, especially those under ART, are at increased risk of CVD, particularly MI and coronary disease, compared to HIV-negative individuals.57,70,90,91 Several studies have shown a higher frequency of vascular events in HIV-infected adults under ART compared to untreated individuals,90,92,93 although other studies disagree.94 It has been suggested that immune reconstitution may be partly responsible for the increased risk of IHD.90
Nevertheless, the benefits of ART continue to outweigh the increased cardiovascular risk associated with this treatment, and concerns about coronary risk should not prevent HIV-positive individuals from receiving ART.
Several studies have shown an increased frequency of MI with longer exposure to certain antiretrovirals.70,71,95 However, Obel et al. did not observe any increase up to eight years after treatment initiation.90 In a shorter study, ART was independently associated with a 26% increase in the rate of MI per year of exposure in the first 4–6 years of treatment.70 Another study showed higher relative risk of MI for every year of exposure to PIs, but no significant association was seen with NNRTIs.71 Treatment with indinavir, lopinavir/ritonavir, didanosine and abacavir was associated with an increased risk of MI,95 but other authors found no association between exposure to abacavir and increased risk of MI.96 Consideration should be given to avoiding use of abacavir, ritonavir/lopinavir, and ritonavir/fosamprenavir in persons at high risk for CVD because these regimens have been associated with increased risk of cardiovascular events in some studies.69
Assessment of cardiovascular riskCardiovascular risk should be assessed and monitored in order to identify those at high risk and to implement preventive measures (Figure 3).
Assessment of cardiovascular risk in HIV-positive individuals. Adapted from European AIDS Clinical Society Guidelines Version 7.1 – November 2014.68
a The Framingham equation can be used. This assessment and the associated considerations outlined in this figure should be repeated annually in all persons under care.
b Of the modifiable risk factors outlined, drug treatment is reserved for certain subgroups where benefits are considered to outweigh potential harm.
c Target levels are to be used as guidance and are not definitive – expressed as mmol/l with mg/dl in parentheses.
d Evidence for benefit when used in persons without a history of CVD (including diabetics) is less compelling. Blood pressure should be reasonably controlled before aspirin use in such a setting.
HIV-positive individuals have various risk factors for CVD, both traditional factors and those related to their HIV status, including the infection itself, duration of infection, viral load, therapy, and altered immune response.
The Framingham risk score may underestimate risk in this population.58 Two other risk scores have recently been developed: a risk equation developed from a population of HIV-infected patients, incorporating routinely collected cardiovascular risk parameters and exposure to antiretrovirals97; and a model to predict the short-term risk of new-onset diabetes in HIV-positive populations during follow-up.76
RecommendationsBehavioral and therapeutic interventions to reduce cardiovascular risk are recommended in HIV-positive individuals under ART. They should be advised regarding diet, weight loss, smoking cessation and exercise. An independent association has been reported between high salt consumption and increased arterial stiffness.98
Lima et al. analyzed the effect of a prevention program (non-pharmacological and, when appropriate, pharmacological therapy) on cardiovascular risk in HIV-positive patients.99 After a six-month follow-up, significant changes were seen in triglycerides and total and LDL cholesterol and a significant reduction in the number of individuals at high cardiovascular risk.99
The EACS has published recommendations on the treatment of dyslipidemia, diabetes and hypertension in HIV-positive individuals.68
If lifestyle modification and change of ART are not effective, lipid-lowering medication should be considered.68 Of the drugs used to lower LDL cholesterol, statins are the first-line treatment, and should be prescribed in patients with established vascular disease and in those with type 2 diabetes or at high risk of CVD, irrespective of lipid levels.68 However, interactions between statins and antiretrovirals are common; PIs can interact with statin metabolism via cytochrome CYP3A4, increasing overall exposure to statins. Simvastatin is contraindicated with concurrent PI use.54 In a small pilot study, rosuvastatin for 24 weeks was effective against hyperlipidemia in patients taking PIs, with a favorable tolerability profile.100
The goals and characteristics of treatment of type 2 diabetes recommended by the EACS68 are shown in Figure 4.
Management of type 2 diabetes in HIV-positive individuals. Adapted from European AIDS Clinical Society Guidelines Version 7.1 – November 2014.68
a Very limited data for any oral antidiabetic agents in terms of CVD prevention, and no data in HIV-positive persons.
The aim of treatment for hypertension is to achieve BP <140/90 mmHg. The EACS recommendations for drug treatment of hypertension68 are presented in Figure 5. CCBs should be used with caution, since they may interact with PIs. Comorbidities should be borne in mind when selecting drug therapy.
Choosing drugs for HIV-infected persons newly diagnosed with hypertension. Adapted from European AIDS Clinical Society Guidelines Version 7.1 – November 2014.68
ACEi: angiotensin-converting enzyme inhibitor (e.g. perindopril, lisinopril or ramipril); ARB: low-cost angiotensin receptor blocker (e.g. losartan, candesartan); CCB: calcium-channel blocker (e.g. amlodipine). Thiazide-type diuretic includes e.g. indapamide or chlorthalidone but excludes thiazides (e.g. hydrochlorothiazide, bendroflumethiazide, etc.)
The authors have no conflicts of interest to declare.
Please cite this article as: Amado Costa L, Almeida AG. Patologia cardiovascular associada ao vírus da imunodeficiência humana. Rev Port Cardiol. 2015;34:479–491.








 ART: antiretroviral therapy; CKD: chronic kidney disease;
ART: antiretroviral therapy; CKD: chronic kidney disease; 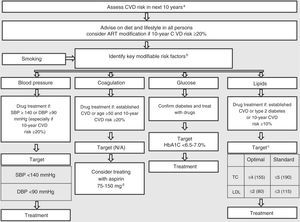 HIV-positive individuals. Adapted from European
HIV-positive individuals. Adapted from European 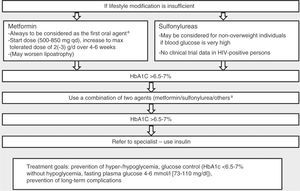 HIV-positive individuals. Adapted from European
HIV-positive individuals. Adapted from European 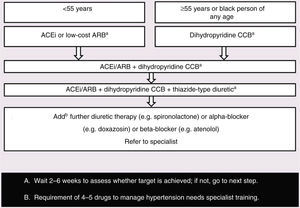 HIV-infected persons newly diagnosed with hypertension. Adapted from European
HIV-infected persons newly diagnosed with hypertension. Adapted from European 

