Worsening renal function has an unquestionably negative impact on prognosis in patients with acute heart failure (HF). In Portugal there is little information about the importance of this entity in HF patients admitted to hospital. The objective of this work was to assess the prevalence of cardiorenal syndrome and to identify its key predictors and consequences in patients admitted for acute HF.
MethodsThis was a retrospective study of 155 patients admitted for acute HF. Cardiorenal syndrome was defined as an increase in serum creatinine of ≥26.5 μmol/l. Clinical, laboratory and echocardiographic parameters were analyzed and compared. Mortality was assessed at 30 and 90 days.
ResultsCardiorenal syndrome occurred in 46 patients (29.7%), 5.4±4.4 days after admission; 66.7% (n=24) did not recover baseline creatinine levels. The factors associated with cardiorenal syndrome were older age, chronic renal failure, moderate to severe mitral regurgitation, higher admission blood urea nitrogen, creatinine and troponin I, and lower glomerular filtration rate. Patients who developed cardiorenal syndrome had longer hospital stay, were treated with higher daily doses of intravenous furosemide, and more often required inotropic support and renal replacement therapy. They had higher in‐hospital and 30‐day mortality, and multivariate analysis identified cardiorenal syndrome as an independent predictor of in‐hospital mortality.
ConclusionsRenal dysfunction is common in acute HF patients, with a negative impact on prognosis, which highlights the importance of preventing kidney damage through the use of new therapeutic strategies and identification of novel biomarkers.
O valor prognóstico da deterioração da função renal é indiscutível nos doentes com insuficiência cardíaca aguda. No contexto nacional sabe‐se pouco acerca do peso relativo da síndrome cardiorrenal no internamento destes doentes. Este trabalho pretende avaliar a prevalência, fatores preditores e consequências desta entidade em doentes internados por insuficiência cardíaca aguda.
MétodosAnálise retrospetiva de 155 doentes internados por insuficiência cardíaca aguda. Síndrome cardiorrenal definida como um aumento ≥ a 26,5 umol/L na creatinina sérica relativamente ao valor da admissão. Avaliados e comparados dados clínicos, analíticos e ecocardiográficos. Feito seguimento, referente a mortalidade, aos 30 e 90 dias.
ResultadosA síndrome cardiorrenal ocorreu em 46 (29,7%) doentes, 5,4±4,4 dias após a admissão; 66,7% (n=24) não recuperaram a função renal basal. Associaram‐se ao desenvolvimento desta entidade: idades mais avançadas; antecedentes de insuficiência renal crónica; insuficiência mitral moderada/grave; níveis na admissão mais elevados de ureia, creatinina e troponina I; e mais baixos de taxa de filtração glomerular. Os doentes com síndrome cardiorrenal tiveram internamentos mais longos; necessitaram de doses diárias máximas de furosemida mais elevadas; mais frequentemente necessitaram de inotrópicos e de terapêutica de substituição renal. A sua mortalidade no internamento e aos 30 dias foi superior, sendo a síndrome cardiorrenal um fator preditor independente de mortalidade intra‐hospitalar.
ConclusõesA disfunção renal é comum em doentes com insuficiência cardíaca aguda, com impacto claramente negativo no prognóstico, devendo a prevenção da lesão renal ser um objetivo primário passando por novas estratégias terapêuticas e identificação de novos biomarcadores.
With a prevalence of over five million cases and almost a million hospital discharges a year, treatment of heart failure (HF) represents a growing therapeutic challenge to modern cardiology.1
Renal dysfunction in common in HF patients and is an independent prognostic factor, even in those with only minor alterations in renal function.2–4
Various retrospective studies and prospective registries have investigated the prognostic impact of renal dysfunction in acute HF.5–8 In an analysis of the Acute Decompensated Heart Failure National Registry (ADHERE), only 9% of the 118 465 patients hospitalized for acute HF had normal renal function (defined as a glomerular filtration rate [GFR] of ≥90 ml/min/1.73 m2),7 and moderate to severe dysfunction is reported in 30–35% of cases.3,5,9
In 30–50% of cases, depending on the definition used, admissions for HF are accompanied by worsening renal function, which is associated with longer hospital stay, greater health care costs, and higher rates of in‐hospital mortality, rehospitalization and mortality following discharge.3,5,6,9
Despite the solid evidence on the negative impact of worsening renal function during treatment of acute HF, the pathophysiology of cardiorenal syndrome (CRS) is not fully understood. Furthermore, the lack of an agreed definition has led to uncertainty concerning the diagnosis and treatment of this entity.10
It has been suggested that the definition of CRS needs to be refined, and a new etiological classification has been proposed that divides CRS into five subtypes in an attempt to promote a more consistent and logical approach.11,12 Our study deals with type 1 (acute CRS), characterized by a rapid worsening of cardiac function leading to acute kidney injury.12 The mechanisms by which acute HF causes a worsening of renal function are multiple and complex.13 The causes of renal dysfunction during decongestive therapy can be broadly divided into three categories: pre‐renal (HF with low cardiac output or hypotension); renal (atheroembolism or exposure to contrast agents, nephrotoxic drugs or diuretics); and post‐renal (obstructive nephropathy or intra‐abdominal hypertension).14 The importance of each of these mechanisms will depend on the individual patient and clinical situation.
Patients with acute HF and renal dysfunction represent a particularly demanding challenge for clinicians.
The objective of this study was to assess the incidence of CRS (defined as an absolute increase in serum creatinine levels), the time it occurred, and its risk factors and prognostic impact, in consecutive patients hospitalized for acute HF. We also performed a detailed descriptive analysis of the evolution of renal function during hospitalization.
MethodsPopulationWe performed a retrospective analysis of 155 consecutive patients (50.3% male; mean age 74.1±10.7 years) admitted to the cardiology department of a central hospital who met the diagnostic criteria for acute HF in the European Society of Cardiology (ESC) guidelines.15 Patients with end‐stage chronic renal disease (under regular hemodialysis or with GFR <15 ml/min/1.73 m2) were excluded.
Clinical characteristics and laboratory parametersThe following data were obtained from patient medical records: urea and creatinine levels at admission and discharge and peak urea and creatinine levels during hospital stay, as well as the day on which these peaks occurred. GFR (at admission and discharge and at peak creatinine) was calculated using the Modification of Diet in Renal Disease (MDRD) formula, which has been shown to be the best method to assess renal function indirectly in HF patients.16
Besides standard clinical characteristics, the following data were also collected at admission: New York Heart Association (NYHA) class, classification of clinical presentation in accordance with current ESC guidelines,15 cardiac rhythm and heart rate, systolic blood pressure (SBP), and HF etiology and triggering factor. Besides renal function parameters, the following laboratory values were also obtained at admission: hemoglobin, NT‐proBNP, troponin I, sodium, potassium, uric acid, albumin, glutamic oxalacetic transaminase, glutamic pyruvic transaminase and total bilirubin. In patients who underwent transthoracic echocardiography, we assessed global systolic function and ejection fraction, left ventricular dimensions, pulmonary artery systolic pressure (PASP) and presence of mitral regurgitation. The following treatment parameters were recorded: maximum daily furosemide dose, use of intravenous vasodilators, inotropes (dopamine and/or dobutamine), levosimendan and noninvasive ventilation and type of renal replacement therapy (ultrafiltration or hemodialysis). Medication at discharge and length of hospital stay were also recorded.
Telephone follow‐up was used to assess 30‐day and 90‐day survival.
Definition of cardiorenal syndromeIn accordance with previous studies,3,5,6 we defined CRS as an increase of ≥26.5 μmol/l in serum creatinine compared to admission values, since this has been shown to have the greatest sensitivity and specificity to predict in‐hospital mortality and length of hospital stay.5 Worsening renal function was defined as transient when creatinine levels returned to baseline values before discharge.
Statistical analysisThe statistical analysis was performed using SPSS for Windows version 17.0. Nominal variables were expressed as counts and percentages and compared by the chi‐square test (combinations of frequencies). Continuous variables were expressed as means ± standard deviation; the Student's t test was used to compare those with normal distribution and the Mann‐Whitney U test for those with non‐normal distribution. Binary logistic regression analysis was used to construct a predictive model of CRS which included all variables showing an association with CRS on univariate analysis. Two endpoints were considered: length of hospital stay and mortality (in‐hospital, 30‐day and 90‐day). A value of p<0.05 was taken to be statistically significant.
ResultsCharacteristics of the study populationThe study population consisted of 155 patients, 50.3% male, mean age 74.1±10.7 years. There was a history of hypertension in 67.1%, diabetes in 30.3%, coronary artery disease (CAD) in 29.7%, chronic obstructive pulmonary disease in 37.4%, and chronic renal disease in 34.2%. Systolic function was preserved (ejection fraction ≥50%) in 48.4%.
The most common cause of HF was valve disease (42.6%), followed by CAD (27.1%) and hypertension (18.7%). The most frequent triggering factors were supraventricular tachycardia (23.9%) and respiratory infection (18.7%); no trigger was identified in 29.7%. At admission, most patients (66.5%) were in NYHA class III, and decompensated chronic HF was the most common form of clinical presentation (81.9%).
At the time of hospitalization, mean SBP was 127±28 mmHg and heart rate 93±20 bpm; 43.9% of patients were in sinus rhythm and 56.1% in atrial fibrillation.
Forty‐six patients (29.7%) developed CRS, 5.4±4.4 days after admission, with a mean increase in creatinine of 62.1±50.8 μmol/l.
Mean hospital stay was 11.6±7.3 days. In‐hospital mortality was 9% (n=14) and 14 patients died during follow‐up. Four patients (2.6%) were lost to follow‐up.
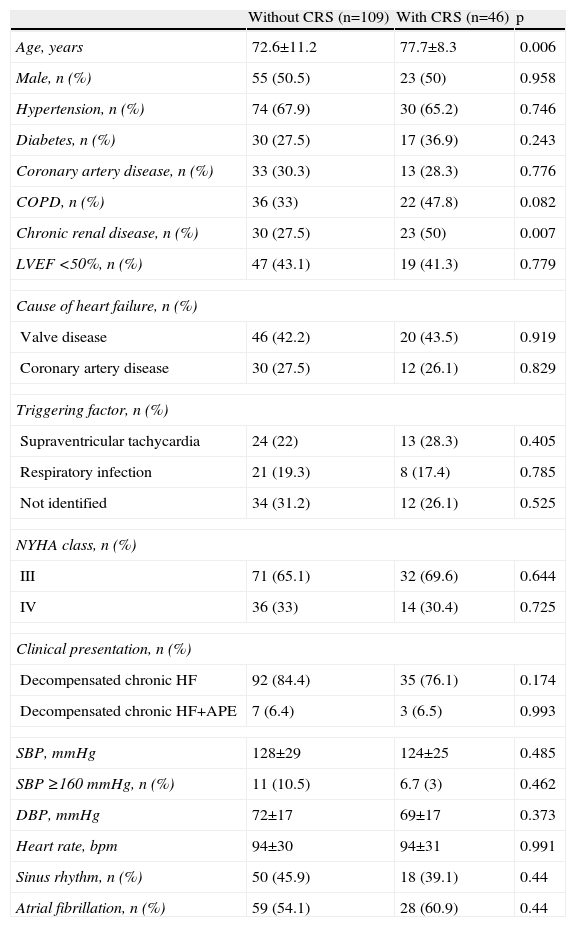
Characteristics of patients with cardiorenal syndromeTable 1 compares the baseline characteristics of the patients according to whether or not they developed CRS.
Baseline characteristics of patients without and with cardiorenal syndrome.
| Without CRS (n=109) | With CRS (n=46) | p | |
| Age, years | 72.6±11.2 | 77.7±8.3 | 0.006 |
| Male, n (%) | 55 (50.5) | 23 (50) | 0.958 |
| Hypertension, n (%) | 74 (67.9) | 30 (65.2) | 0.746 |
| Diabetes, n (%) | 30 (27.5) | 17 (36.9) | 0.243 |
| Coronary artery disease, n (%) | 33 (30.3) | 13 (28.3) | 0.776 |
| COPD, n (%) | 36 (33) | 22 (47.8) | 0.082 |
| Chronic renal disease, n (%) | 30 (27.5) | 23 (50) | 0.007 |
| LVEF <50%, n (%) | 47 (43.1) | 19 (41.3) | 0.779 |
| Cause of heart failure, n (%) | |||
| Valve disease | 46 (42.2) | 20 (43.5) | 0.919 |
| Coronary artery disease | 30 (27.5) | 12 (26.1) | 0.829 |
| Triggering factor, n (%) | |||
| Supraventricular tachycardia | 24 (22) | 13 (28.3) | 0.405 |
| Respiratory infection | 21 (19.3) | 8 (17.4) | 0.785 |
| Not identified | 34 (31.2) | 12 (26.1) | 0.525 |
| NYHA class, n (%) | |||
| III | 71 (65.1) | 32 (69.6) | 0.644 |
| IV | 36 (33) | 14 (30.4) | 0.725 |
| Clinical presentation, n (%) | |||
| Decompensated chronic HF | 92 (84.4) | 35 (76.1) | 0.174 |
| Decompensated chronic HF+APE | 7 (6.4) | 3 (6.5) | 0.993 |
| SBP, mmHg | 128±29 | 124±25 | 0.485 |
| SBP ≥160 mmHg, n (%) | 11 (10.5) | 6.7 (3) | 0.462 |
| DBP, mmHg | 72±17 | 69±17 | 0.373 |
| Heart rate, bpm | 94±30 | 94±31 | 0.991 |
| Sinus rhythm, n (%) | 50 (45.9) | 18 (39.1) | 0.44 |
| Atrial fibrillation, n (%) | 59 (54.1) | 28 (60.9) | 0.44 |
APE: acute pulmonary edema; COPD: chronic obstructive pulmonary disease; CRS: cardiorenal syndrome; DBP: diastolic blood pressure; HF: heart failure; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SBP: systolic blood pressure.
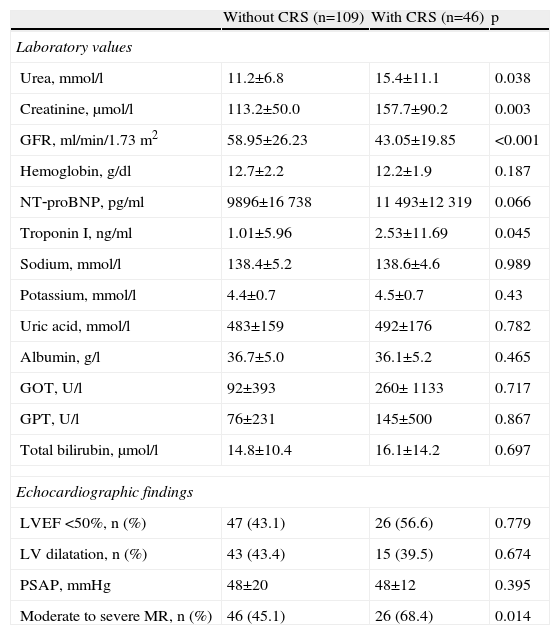
Table 2 shows laboratory values at admission and echocardiographic findings for the two patient groups.
Laboratory values at admission and echocardiographic findings of patients without and with cardiorenal syndrome.
| Without CRS (n=109) | With CRS (n=46) | p | |
| Laboratory values | |||
| Urea, mmol/l | 11.2±6.8 | 15.4±11.1 | 0.038 |
| Creatinine, μmol/l | 113.2±50.0 | 157.7±90.2 | 0.003 |
| GFR, ml/min/1.73 m2 | 58.95±26.23 | 43.05±19.85 | <0.001 |
| Hemoglobin, g/dl | 12.7±2.2 | 12.2±1.9 | 0.187 |
| NT‐proBNP, pg/ml | 9896±16 738 | 11 493±12 319 | 0.066 |
| Troponin I, ng/ml | 1.01±5.96 | 2.53±11.69 | 0.045 |
| Sodium, mmol/l | 138.4±5.2 | 138.6±4.6 | 0.989 |
| Potassium, mmol/l | 4.4±0.7 | 4.5±0.7 | 0.43 |
| Uric acid, mmol/l | 483±159 | 492±176 | 0.782 |
| Albumin, g/l | 36.7±5.0 | 36.1±5.2 | 0.465 |
| GOT, U/l | 92±393 | 260± 1133 | 0.717 |
| GPT, U/l | 76±231 | 145±500 | 0.867 |
| Total bilirubin, μmol/l | 14.8±10.4 | 16.1±14.2 | 0.697 |
| Echocardiographic findings | |||
| LVEF <50%, n (%) | 47 (43.1) | 26 (56.6) | 0.779 |
| LV dilatation, n (%) | 43 (43.4) | 15 (39.5) | 0.674 |
| PSAP, mmHg | 48±20 | 48±12 | 0.395 |
| Moderate to severe MR, n (%) | 46 (45.1) | 26 (68.4) | 0.014 |
CRS: cardiorenal syndrome; GFR: glomerular filtration rate; GOT: glutamic oxalacetic transaminase; GPT: glutamic pyruvic transaminase; LV: left ventricular; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; PASP: pulmonary artery systolic pressure.
During hospital stay, patients with CRS were medicated with higher daily doses of furosemide (maximum daily dose 163±114 vs. 104±70 mg, p<0.001), and more frequently required inotropic support (26.1% vs. 7.3%, p=0.002) and renal replacement therapy (15.2% vs. 0%, p<0.001). No differences were observed in the use of intravenous vasodilators (24.8% vs. 23.9%, p=0.392), levosimendan (3.7% vs. 6.5%, p=0.435) or noninvasive ventilation (7.3% vs. 15.2%, p=0.130).
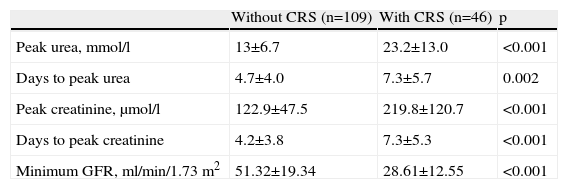
Table 3 shows peak values of renal function parameters in the two groups.
Peak values of renal function parameters of patients without and with cardiorenal syndrome.
| Without CRS (n=109) | With CRS (n=46) | p | |
| Peak urea, mmol/l | 13±6.7 | 23.2±13.0 | <0.001 |
| Days to peak urea | 4.7±4.0 | 7.3±5.7 | 0.002 |
| Peak creatinine, μmol/l | 122.9±47.5 | 219.8±120.7 | <0.001 |
| Days to peak creatinine | 4.2±3.8 | 7.3±5.3 | <0.001 |
| Minimum GFR, ml/min/1.73 m2 | 51.32±19.34 | 28.61±12.55 | <0.001 |
CRS: cardiorenal syndrome; GFR: glomerular filtration rate.
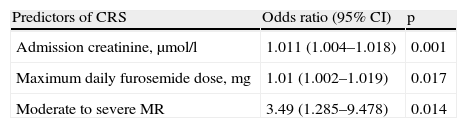
Logistic regression analysis was used to construct a predictive model of CRS which included admission creatinine, maximum daily furosemide dose and presence of mitral regurgitation, with a good model fit by the Hosmer‐Lemeshow test (p=0.340) that explained 31.8% of the variance observed in development of CRS (Nagelkerke R square) (Table 4).
Predictors of cardiorenal syndrome by binary logistic regression analysis.
| Predictors of CRS | Odds ratio (95% CI) | p |
| Admission creatinine, μmol/l | 1.011 (1.004–1.018) | 0.001 |
| Maximum daily furosemide dose, mg | 1.01 (1.002–1.019) | 0.017 |
| Moderate to severe MR | 3.49 (1.285–9.478) | 0.014 |
CRS: cardiorenal syndrome; CI: confidence interval; MR: mitral regurgitation.
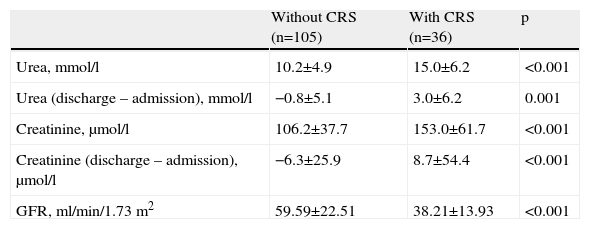
Patients who developed CRS had longer hospital stay (14.1±10.2 vs. 10.5±5.4 days, p=0.008). Of those who survived, 66.7% (n=24) did not recover baseline creatinine levels. Table 5 shows renal function at discharge and differences from admission values.
Renal function parameters at discharge compared to admission values.
| Without CRS (n=105) | With CRS (n=36) | p | |
| Urea, mmol/l | 10.2±4.9 | 15.0±6.2 | <0.001 |
| Urea (discharge – admission), mmol/l | −0.8±5.1 | 3.0±6.2 | 0.001 |
| Creatinine, μmol/l | 106.2±37.7 | 153.0±61.7 | <0.001 |
| Creatinine (discharge – admission), μmol/l | −6.3±25.9 | 8.7±54.4 | <0.001 |
| GFR, ml/min/1.73 m2 | 59.59±22.51 | 38.21±13.93 | <0.001 |
CRS: cardiorenal syndrome; GFR: glomerular filtration rate.
At discharge, both groups were medicated with similar doses of furosemide (70±26 vs. 66 mg±28 mg, p=0.370), and there were no significant differences in the proportion of patients prescribed angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) (81.7% vs. 82.9%, p=0.881), beta‐blockers (44.2% vs. 28.6%, p=0.102) or digoxin (30.8% vs. 17.1%, p=0.118). Patients with CRS tended to be less often medicated with aldosterone antagonists (56.7% vs. 40%; p=0.086).
Patients with CRS presented higher mortality in‐hospital (19.6% vs. 3.7%, p=0.001, odds ratio [OR] 6.4) and at 30 days (22.7% vs. 6.5%, p=0.004, OR 4.2); this trend was maintained at 90 days (27.3% vs. 15%, p=0.077). On multivariate analysis, development of CRS was an independent predictor of higher in‐hospital mortality.
DiscussionPatient characteristics and prevalence of cardiorenal syndromeIn contrast to the latest data for developed countries,1 the most common cause of HF in our study was valve disease rather than CAD. This is probably due to the age of our population and the fact that an ischemic cause was only considered in those with a history of acute coronary syndrome or documented ischemia, and so mitral regurgitation may have been the result of undiagnosed CAD in some cases.
The present study adds to the growing evidence that CRS is common among patients hospitalized for acute HF and that it is associated with worse prognosis, as reflected in longer hospital stay and higher mortality.3,5,6,9
The most important findings of this study were:
- (1)
around a third of patients developed CRS, defined as an increase in serum creatinine of ≥26.5 μmol/l;
- (2)
certain baseline characteristics are associated with development of CRS;
- (3)
CRS is a strong predictor of in‐hospital mortality.
The incidence of CRS was similar to that of previous studies,3,5,6 and it also developed relatively early during hospital stay (around the fifth day),5,6 which suggests that worsening renal function is due to mechanisms related to HF itself or to the effects of treatment during hospitalization, rather than progressive clinical worsening during prolonged hospitalization.6
The study also confirmed the association between certain baseline characteristics and the development of CRS, particularly older age and a history of chronic renal disease, reflected in worse renal function at admission.3,6,13 Interestingly, the group of patients who developed CRS presented significantly lower GFR at admission than in other studies.8,17 In contrast to previous studies, we found no association between development of CRS and a history of HF,6 diabetes,3,6 elevated SBP at admission,3,6 tachycardia3 or female gender,3 but this may be due to the small sample size.
With regard to echocardiographic parameters, our study also found that CRS was equally prevalent in HF patients with normal and with reduced ejection fraction.6 Only one review in the literature,18 of patients with chronic HF, found an association between renal dysfunction and mitral regurgitation, which was identified as a predictor of renal failure, irrespective of age.
Although recent evidence13 has shown that GFR calculated by the MDRD formula is better at assessing renal function in HF patients than serum creatinine, which often overestimates renal function, particularly in elderly women, in our study serum creatinine at admission was a predictor of CRS.
Causes of cardiorenal syndromeThe mechanisms that cause CRS in acute HF patients are multiple and not fully understood.4,19 It is plausible that impaired renal perfusion is secondary to reduced cardiac output, which is a widely‐held view.20 However, CRS occurs most frequently at an early stage of treatment for acute HF, when patients are still hypervolemic,5 as was demonstrated in our study. Ljungman et al.21 also showed that renal blood flow is preserved until the cardiac index falls below 1.5 l/min/m2. Thus, the simplistic assumption that worsening renal function is a response to intravascular volume depletion is likely inaccurate and certainly does not identify a satisfactory therapeutic strategy (volume replacement) for persistently congested HF patients with CRS.13 Recent evidence14 has highlighted the importance of renal venous hypertension in the pathophysiology of CRS, but our study found no association between PASP and CRS. Drugs with proven effects on filling pressures, such as nitroglycerin, nesiritide and sodium nitroprusside, have not been shown to reduce the incidence of CRS during treatment for acute HF.22
Therapies used in acute HF patients are another potential cause of CRS, for example excessive diuresis leading to hypovolemia and early introduction of ACE inhibitors resulting in hypotension.
In agreement with our results, studies assessing the association between in‐hospital medication and development of CRS found that higher doses of loop diuretics were used in CRS patients.3,6,17 Higher doses of furosemide are associated with worse prognosis23 and CRS may be the mechanism responsible. On the other hand, it cannot be ruled out that administration of higher doses of diuretics is a consequence rather than a cause of advanced HF with coexisting renal failure, and would thus be merely a marker of worse prognosis rather than the mechanism.
Clinical significance and prognosis of cardiorenal syndromeAs in the ADHERE registry,24 our study found that patients who developed worsening renal function were treated with higher doses of diuretics and staged therapy with inotropic support and renal replacement, and had higher in‐hospital mortality. There are no prospective data on the clinical benefits of renal‐dose dopamine in acute HF, and retrospective data have shown an adverse effect of positive inotropic agents on morbidity and mortality.24
In agreement with previous studies,3,5,6 our study showed that CRS is associated with longer hospital stay, which has important implications in terms of both quality of life and health costs. This may be due to the need to postpone the introduction of ACE inhibitors, ARBs or beta‐blockers and to allow renal function to recover before discharge. Serum creatinine levels at discharge were consistently lower than peak values in patients who developed CRS, although most did not achieve baseline levels.
Our study also found that the development of CRS was a predictor of in‐hospital mortality,3,5,6 and confirmed that it was associated with worse prognosis after discharge.3,6,9
Nevertheless, it has yet to be fully clarified whether worsening renal function in itself contributes to increased mortality or whether it is merely a marker of more severe cardiac and/or renal dysfunction.6,13
Study limitationsOur study is limited by the relatively small number of patients. Nevertheless, as shown by calculation of its statistical power, the size of the population was sufficient to identify CRS as a predictor of prognosis, which was one of its main aims.
Another limitation was the fact that the choice of medications during hospitalization (ACE inhibitors, ARBs, beta‐blockers and aldosterone antagonists), or the time of their introduction or dosage, was not analyzed.
This study, like all retrospective studies, was limited by the information available in patients’ medical records.
ConclusionsThe development of CRS is common following admission for acute HF, even after excluding those with end‐stage chronic renal disease.
Serum creatinine levels at admission, high doses of furosemide and the presence of moderate to severe mitral regurgitation were identified as predictors of CRS.
Patients who developed CRS were treated with higher maximum daily furosemide doses and more often required inotropic support and renal replacement therapy. The development of CRS was associated with longer hospital stay and higher in‐hospital and medium‐term mortality.
Although there are various possible therapeutic approaches to CRS, no randomized study has shown any to have a positive impact on this complication.
Various studies, including clinical trials by the National Heart, Lung, and Blood Institute's Heart Failure Network,25 are underway to validate therapeutic strategies for these patients and to identify novel biomarkers of kidney damage that can be used for the early identification of patients at risk of developing CRS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Caetano F, Barra S, Faustino A, et al. Síndrome cardiorrenal na insuficiência cardíaca aguda: um círculo vicioso? Rev Port Cardiol. 2014;33:139–146.