Takotsubo cardiomyopathy (TC) is characterized by the sudden onset of reversible left ventricular dysfunction, with a presentation similar to that of an acute coronary syndrome. Although cardiogenic shock is a rare occurrence in TC, if it does occur it may require the use of a left ventricular assist device.
We report the use of extracorporeal life support (ECLS) in a patient with TC and refractory cardiogenic shock. With ECLS it was possible to reduce inotropic support, and a normal left ventricular ejection fraction was documented by echocardiography on day 2.
This is, to our knowledge, the first reported case of TC with refractory cardiogenic shock treated with ECLS in Portugal.
A miocardiopatia de Takotsubo (MT) é caracterizada por uma disfunção ventricular esquerda súbita, tendo uma apresentação semelhante à de uma síndrome coronária aguda. Apesar do choque cardiogénico ser uma ocorrência rara na MT, caso ocorra, pode levar ao uso de dispositivos de assistência ventricular esquerda.
Reportamos o caso de uma doente com MT e choque cardiogénico onde foi utilizado suporte de vida extracorporal (SVEC). Com o SVEC foi possível reduzir o suporte aminérgico e documentada em ecocardiografia uma fração de ejeção normal ao 2.° dia.
No conhecimento dos autores este é o primeiro caso, em Portugal, de choque cardiogénico por MT em que é usado o SVEC como ponte para a melhoria.
Takotsubo cardiomyopathy (TC) (as it was first named in 1990 by Sato et al. in Japan), also known as stress-induced cardiomyopathy or broken heart syndrome, is a transient left ventricular apical ballooning syndrome characterized by left ventricular dysfunction, that usually occurs after a sudden emotional or physical stress. The Japanese word ‘takotsubo’ means ‘octopus pot’; pots used to catch octopuses have a round bottom and a narrow neck, thus trapping the creatures until they are pulled out by the fisherman.1–3
The presentation of TC is similar to that of an acute coronary syndrome (ACS), patients presenting with cardiac chest pain, raised levels of cardiac enzymes and acute changes on the electrocardiogram (ECG), although with absence of obstructive coronary artery disease.2,3 Series suggest that TC affects approximately 1–2% of patients initially diagnosed with an ACS.4
Different pathophysiological mechanisms have been proposed to explain this syndrome, such as occult atherosclerotic disease, multivessel spasm and microvascular dysfunction. The most widely accepted hypothesis nowadays is that of catecholamine excess causing calcium overload in cardiac myocytes, leading to disruption of contraction and ventricular function.5
The long-term prognosis is excellent, with only 1–2% of reported patients dying during hospitalization. Serious complications including cardiogenic shock and arrhythmias may, however, occur acutely. Supportive treatment is the mainstay of therapy.6,7
In cardiogenic shock, mechanical circulatory support can be lifesaving. Intra-aortic balloon pump (IABP) and extracorporeal life support (ECLS) are alternatives.8
We report the successful use of ECLS to treat a patient with TC presenting with cardiogenic shock.
Case reportA 72-year-old female patient presented to the emergency department with chest pain and dyspnea of one hour's duration. Her medical history consisted of hypertension, dyslipidemia and paroxysmal atrial fibrillation. She was under losartan 100 mg plus hydrochlorothiazide 12.5 mg, pitavastatin 4 mg and propafenone 300 mg.
At admission her blood pressure was 170/130 mmHg, heart rate 100 beats/min and oxygen saturation 70% on room air. On physical examination the patient had diminished cardiac sounds and bilateral crackles up to the lung apices. Furosemide, intravenous nitrates and oxygen were started. An ECG demonstrated ST-segment elevation in the precordial leads V2-V4, and cardiac biomarkers were mildly elevated. A transthoracic echocardiographic examination showed mechanical alterations in the myocardium supplied by the left anterior descending artery.
Despite therapy the patient progressed to cardiogenic shock; invasive ventilation and inotropic support were initiated, and she was transferred to our hospital for primary percutaneous coronary intervention.
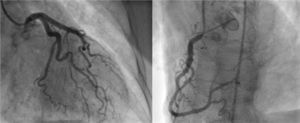
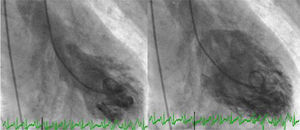
Angiography revealed absence of obstructive coronary disease or acute plaque rupture (Figure 1). Ventriculography showed apical and midventricular dysfunction with hyperkinesis of the basal myocardial segments and severely depressed ejection fraction (Figure 2). A second detailed transthoracic echocardiographic examination confirmed apical akinesis, basal hyperkinesis and severe systolic left ventricular (LV) dysfunction; apical ballooning syndrome was confirmed.
The patient was admitted to the coronary intensive care unit under midazolam, noradrenaline (60 μg/min) and dobutamine (1.8 μg/kg/min), and some minutes later developed pulseless monomorphic ventricular tachycardia, rapidly reverted to sinus rhythm. Despite aggressive therapy with fluids and increasing doses of noradrenaline and dobutamine she remained hemodynamically unstable. Since TC is a reversible condition, usually in a short period, therapy with ECLS was considered as a lifesaving alternative.
Venous-arterial (VA)-ECLS was performed with 19 French (F) venous femoral cannulation and 17F arterial femoral cannulation. Anticoagulation with unfractionated heparin was initiated, for a target activated clotting time of 150–200 s. Bypass flow was fixed initially at 2.8 l/min. The vital parameters stabilized rapidly after ECLS and inotropic support was reduced.
At the end of the second day pulsatility on the arterial pressure waveform was evident and another transthoracic echocardiogram was performed. This echocardiogram showed no regional wall motion abnormalities, and ejection fraction was nearly normal. ECLS flow was progressively reduced, and on day 3 the system was explanted.
During ECLS four units of red blood cells and one unit of platelets were transfused, to maintain hemoglobin concentration above 10 g/dl and a platelet count >70000 U/l.
Ventilator-associated pneumonia was diagnosed one day after ECLS withdrawal and the patient was started on antibiotics. Three days after ECLS the patient was in spontaneous ventilation and was transferred to her primary hospital.
DiscussionTC, also known as stress-induced cardiomyopathy, is an increasingly recognized clinical syndrome characterized by transient left ventricular dysfunction and wall motion abnormalities involving the apical and mid portions of the left ventricle, although atypical forms have also been described.9,10 It usually occurs in postmenopausal women after severe emotional or physical stress, although in some cases the trigger is unknown.4,5,11
Clinical presentation can mimic an acute coronary syndrome, with patients complaining of chest pain and dyspnea and with ST-segment elevation on the ECG and elevated cardiac enzymes. Angiography is crucial to the differential diagnosis, since in TC there are no significant obstructive coronary lesions.2–4
The diagnostic criteria proposed by the Mayo Clinic are: transient hypokinesis, akinesis, or dyskinesis in the left ventricular mid segments with or without apical involvement; regional wall motion abnormalities that extend beyond a single epicardial vascular distribution; frequently, but not always, a stressful trigger; the absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; new ECG abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and the absence of pheochromocytoma and myocarditis.4
The pathophysiology of TC is not well understood. Given that the onset of this syndrome is usually preceded by emotional or physical stress, direct catecholamine-mediated myocyte injury has been advocated as the most probable mechanism.11
Patients with TC generally have a good prognosis, with rapid improvement in LV systolic function within a few days or weeks.12 A variety of complications may occur in the acute phase of the disease, such as acute heart failure with pulmonary edema or cardiogenic shock, development of intraventricular pressure gradients, acute functional mitral regurgitation, right ventricular dysfunction, intraventricular thrombi resulting in stroke or arterial embolism, atrial fibrillation or malignant ventricular arrhythmias.13 In patients who develop such complications, treatment represents a real challenge. When the initial presentation of TC is cardiogenic shock, vasoactive drugs to maintain blood pressure and improve tissue perfusion seem a reasonable option. However, given the presumptive pathophysiology of this syndrome, vasopressors may cause more harm than benefit.5,8,11 In the patient presented, vasoactive drugs were started, given that ACS was the initial presumptive diagnosis. Worsening hemodynamics might have been partly due to this treatment. Mechanical support in hemodynamically unstable TC patients is the most logical alternative. Just after establishing TC as the correct diagnosis, the patient collapsed with low oxygen saturation, and so it was decided to initiate ECLS therapy as a bridge to recovery.
The first successful use of ECLS was reported in 1972, and since this report an increasing number of individuals have been saved thanks to this temporary form of life support.14
ECLS uses a modified heart-lung mechanical interface generally consisting of a centrifugal pump, a heat exchanger, and a membrane oxygenator. In VA-ECLS deoxygenated blood is withdrawn through a drainage cannula (from the venous system) by an external pump, passing through the oxygenator and returning to the patient through a reinfusion cannula inserted in an artery. Unlike veno-venous ECLS, VA-ECLS offers both respiratory and circulatory support.15
Bleeding remains the main complication in ECLS patients. Other less common complications include blood clots, pump or oxygenator failure, neurologic and musculoskeletal complications, limb ischemia, infection, renal failure and problems during cannulation.16 In a recent study Loforte et al. identified blood lactate level, creatine kinase-MB isoenzyme relative index at 72 h after ECLS initiation, and number of packed red blood cells transfused while on ECLS as significant predictors of mortality.17 Further studies are necessary to improve our knowledge of which patients will gain most from ECLS support.
In our case ECLS minimized cardiac work and improved tissue perfusion, enabling a significant reduction in doses of vasoactive drugs. Three days after implantation the patient was successfully weaned from support and cardiac function had completely recovered. There are some cases in the literature of TC successfully bridged to recovery with ECLS,18–24 but this is, to our knowledge, the first reported case of TC with refractory cardiogenic shock treated with ECLS in Portugal.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.