Infective endocarditis (IE) is a serious disease with significant in-hospital mortality (15–30%) despite advances in medical and surgical therapy.
AimsTo perform a clinical characterization of patients undergoing cardiac surgery for IE and to identify factors that predict in-hospital mortality.
MethodsWe retrospectively analyzed 145 patients with IE admitted between January 2006 and October 2017.
ResultsThe median age was 72 years. IE was acquired mainly in the community (69%), and involved the native aortic valve in 54% of patients, biological prosthetic valves in 22.1% and mechanical valves in 10.3%. Staphylococcus spp. (31.0%) were the most frequent etiological agents. Cardiac surgery was emergent in 29 patients, urgent in 108, and elective in eight. The main indications were heart failure (57.9%), large vegetations (20%), systemic embolism (17.2%) and valve dysfunction (15.2%). Overall, biological valves were implanted in 62.1% of patients and mechanical valves in 37.2%. A total of 19 patients (13.1%) died. Predictors of mortality were preoperative atrial fibrillation and lower left ventricular ejection fraction, postoperative severe valve regurgitation associated with cardiogenic shock, sepsis, septic shock associated with cardiogenic shock, cardiac tamponade, need for renal replacement therapy and, although without statistical significance, emergent surgery.
ConclusionsThere is a need for better indicators to enable early identification of surgical candidates for IE, implementation of a heart team, and better surgical strategies, including more rapid intervention, more specific postoperative care, and optimal antibiotic therapy.
A endocardite infeciosa (EI) tem uma mortalidade intra-hospitalar significativa (15–30%), apesar dos avanços no tratamento médico e cirúrgico.
ObjetivosCaracterização clínica de doentes submetidos a cirurgia cardíaca por EI e identificação de fatores preditores de mortalidade intra-hospitalar.
Material e MétodosAnálise retrospetiva de 145 doentes com EI admitidos entre janeiro de 2006 a outubro de 2017.
ResultadosA mediana das idades foi de 72 anos. A EI foi maioritariamenre adquirida na comunidade (69%), atingiu a válvula aórtica nativa em 54% dos casos, em 22,1% as próteses biológicas e em 10,3% as mecânicas. Os Staphylococcus spp (31%) foram causa mais frequente. A cirurgia cardíaca foi emergente em 29 casos, urgente em 108 e eletiva em 8. As principais indicações foram insuficiência cardíaca (57,9%), vegetações de grandes dimensões (20%), embolização sistémica (17,2%) e disfunção protésica (15,2%). Foram implantadas próteses biológicas em 62,1% dos doentes e próteses mecânicas em 37,2%. Faleceram 19 doentes (13,1%) e os preditores de mortalidade foram, no período pré-operatório, a fibrilhação auricular e a fração de ejeção do ventrículo esquerdo, e no pós-operatório, a regurgitação valvular grave associada ao choque cardiogénico, a sepsis, o choque séptico associado ao choque cardiogénico, o tamponamento cardíaco, a necessidade de realização de terapêutica de substituição renal, e, ainda que tendencialmente, a cirurgia de caráter emergente.
ConclusõesA identificação precoce de melhores indicadores de candidatos a cirurgia, implementação de umHeart Team e uma melhor estratégia cirúrgica (maior precocidade, cuidados pós-operatórios mais específicos, antibioticoterapia otimizada) são uma necessidade real na EI.
Common indications for cardiac surgery in patients with infective endocarditis (IE) include refractory heart failure (HF), disseminating infection with periannular extension, multi-resistant microorganisms, recurrent embolic events and the presence of prosthetic material.1 Among the aims of surgery are excision of infected tissue, restoring cardiac integrity and valve function, and removal of potential sources of emboli.2 Irrespective of the surgical approach and the procedures used, such as mitral valve repair or homograft aortic root replacement, long-term results of emergent and urgent surgery are worse than those from elective surgery: 10-year survival ranges from 40% to 60%.3
This study had two aims: to characterize patients undergoing cardiac surgery for IE in sociodemographic and clinical terms, including the type of valve and microbiological agents involved, type of surgery and surgical indications, complications and mortality; and to identify predictors of in-hospital mortality.
MethodsStudy type and patient populationThis retrospective single-center study enrolled 145 patients who underwent cardiac surgery for IE and were admitted between January 2006 and October 2017, including those initially diagnosed and admitted for cardiac surgery at the reference hospital as well as patients transferred from other hospitals in Portugal.
IE was diagnosed using the modified Duke criteria.4
The following information was collected anonymously from patients’ electronic and/or paper medical records: sociodemographic data, pre-existing comorbidities, initial valve status, mode of acquisition of endocarditis, microbiological data and transthoracic and transesophageal echocardiographic data – assessing, among other parameters, valve regurgitation, length and location of the vegetation, ventricular function, presence of abscesses or fistulas – in the pre- and postoperative periods, as well as data on extracardiac involvement. The following were also recorded: antimicrobial treatment, pre- and postoperative complications (including HF, perivalvular extension defined by abscesses, pseudoaneurysms or fistulas, and cerebral or peripheral embolism), surgical indications, and mortality. Antimicrobial therapy was considered appropriate if it included antibiotics recommended in the current guidelines.
In-hospital mortality was defined as all in-hospital deaths, including those of patients transferred to other intensive care units. Indications for surgery (HF, prevention of embolic events and uncontrolled infection), time to surgery (emergent: within 24 hours; urgent: within a few days; elective: after at least 1-2 weeks of antimicrobial therapy)5 and postoperative complications were classified according to the current recommendations of the European Society of Cardiology (ESC).5
Statistical analysisContinuous variables are expressed as means ± standard deviation or medians (interquartile range [IQR]) as appropriate and were compared using the Student’s t test. Categorical variables were compared using the chi-square test or Fisher’s exact test. Statistical significance was set at <0.05 for all tests.
ResultsBaseline characteristics and medical historyTable 1 shows the baseline characteristics, cardiovascular risk factors, history of cardiovascular disease and other medical history and comorbidities in patients who underwent cardiac surgery for IE (total, survivors and non-survivors) during hospital stay.
Epidemiological data, cardiovascular risk factors, medical history and comorbidities, in all IE patients who underwent surgery (n=145) and in survivors (n=126) and non-survivors (n=19).
| Total (n=145) | Survivors (n=126) | Non-survivors (n=19) | p (survivors vs. non-survivors) | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Gender, n (%) | 0.129 | |||
| Male | 105 (72.4) | 94 (74,6) | 11 (579) | |
| Female | 40 (27.6) | 32 (25) | 8 (42,1) | |
| Age, years, median (IQR) | 72 (7) | 69 (14) | 72 (12) | 0.107 |
| Hospital stay, days, median (IQR) | 25 (31) | 16 (31) | 25 (22) | 0.457 |
| Cardiovascular risk factors | ||||
| Smoking, n (%) | 0.638 | |||
| Non-smoker | 97 (66.9) | 82 (65.1) | 15 (78.9) | |
| Smoker | 33 (22.8) | 30 (23.8) | 3 (15.8) | |
| Former smoker | 15 (10.3) | 14 (11.1) | 1 (5.3) | |
| Hypertension, n (%) | 121 (83.4) | 104 (82.5) | 17 (89.5) | 0.740 |
| Diabetes, n (%) | 48 (33.1) | 40 (31.7) | 8 (42.1) | 0.371 |
| Dyslipidemia, n (%) | 97 (66.9) | 82 (65.1) | 15 (78.9) | 0.231 |
| Obesity, n (%) | 24 (16.6) | 20 (15.9) | 4 (21.1) | 0.522 |
| Cardiovascular history | ||||
| MI, n (%) | 16 (11.0) | 12 (9.5) | 4 (21.1) | 0.228 |
| Stroke, n (%) | 8 (5.5) | 8 (6.3) | 0 (0) | 0.597 |
| HF, n (%) | 50 (34.4) | 42 (33.3) | 8 (42.1) | 0.453 |
| NYHA I | 18 (12.4) | 17 (13.5) | 1 (5.3) | 0.468 |
| NYHA II | 24 (16.6) | 19 (15.1) | 5 (26.3) | 0.316 |
| NYHA III | 7 (4.8) | 5 (4.0) | 2 (10.5) | 0.229 |
| LVEF, %, median (IQR) | 54 (12) | 56 (11) | 48 (17) | 0.027 |
| Previous endocarditis, n (%) | 5 (3.4) | 4 (3.2) | 1 (5.3) | 0.510 |
| Arrhythmias, n (%) | 29 (20.0) | |||
| AF | 20 (13.8) | 14 (11.1) | 6 (31.6) | 0.027 |
| Complete AV block | 6 (4.1) | 6 (4.8) | 0 (0) | |
| Atrial flutter | 3 (2.1) | 3 (2.4) | 0 (0) | |
| Prior PCI, n (%) | 16 (11.0) | 12 (9.5) | 4 (21.1) | 0.228 |
| Prior CABG, n (%) | 3 (2.1) | 2 (1.6) | 1 (5.3) | 0.346 |
| Devices, n (%) | 6 (4.1) | 6 (4.8) | 0 (0) | |
| Other history | ||||
| CKD stage, n (%) | ||||
| 1 | 23 (15.9) | 20 (15.9) | 3 (15.8) | 1.000 |
| 2 | 102 (70.3) | 90 (71.4) | 12 (63.2) | 0.462 |
| 3 | 16 (11.0) | 13 (10.3) | 3 (15.8) | 0.442 |
| 4 | 4 (2.8) | 3 (2.4) | 1 (5.3) | 0.433 |
| IV drug use, n (%) | 1 (0.7) | 1 (0.8) | 0 (0) | 1.000 |
| Hepatitis C infection, n (%) | 23 (15.9) | 17 (13.5) | 6 (31.6) | 0.083 |
AF: atrial fibrillation; AV: atrioventricular; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; HF: heart failure; IQR: interquartile range; IV: intravenous; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association functional class; PCI: percutaneous coronary intervention.
The median age of the overall study population was 72 years, with no significant difference between in-hospital survivors (n=126; median 69 years) and non-survivors (n=19; median 72 years) (Table 1). Overall, 27.6% of the total population were female and there was no significant difference in mortality according to gender (p=0.129).
The median hospital stay was 25 days and did not differ significantly between survivors and non-survivors (median 16 vs. 25 days; IQR 31 vs. 22; p=0.46).
HF had been diagnosed in 34.4% of patients prior to admission and surgery. There were no differences between survivors and non-survivors in either the proportion with HF (33.3% vs. 42.1%; p=0.45) or New York Heart Association (NYHA) functional class (Table 1).
Five patients (3.4%) had a previous history of IE. Overall, 83.4% of the patients had a history of hypertension, 33.1% of diabetes, and 66.9% of dyslipidemia; 33.1% were smokers and 16.6% ex-smokers. Medical history and comorbidities are listed in Table 1.
Preoperatively, atrial fibrillation (AF) was present in 20 patients (13.8%), 16 (11%) had undergone percutaneous coronary intervention (PCI), and three (2.1%) had undergone coronary artery bypass grafting.
All 145 patients had some degree of kidney dysfunction and 70% (n=102) had creatinine clearance between 60 and 90 ml/min/1.73 m2 (stage 2). A comparison between the survivors and non-survivors in terms of baseline characteristics showed statistically significant differences in only two of the parameters analyzed: left ventricular ejection fraction (LVEF) was lower in non-survivors (48% vs. 56%; p=0.027) and AF was more prevalent in the non-survivor group (31.6% vs. 11.1%; p=0.027).
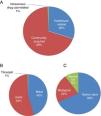
Mode of acquisition, valve involved and type of valve affectedFigure 1 shows the mode of acquisition of IE, the valve involved and the type of valve affected (native vs. biological or mechanical). Figure 2 compares these data between postoperative survivors and non-survivors.
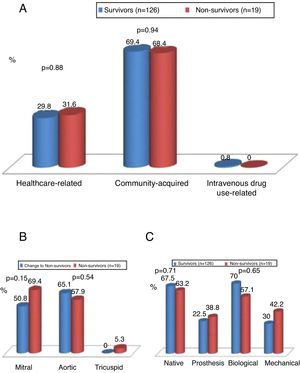
IE was community-acquired in 69% of cases and healthcare-associated in 30%. There were no significant differences between survivors and non-survivors with regard to community-acquired (69.4% vs. 68.4%; p=0.94) or healthcare-associated IE (29.8% vs. 31.6%; p=0.88) (Figure 2).
The aortic valve was most commonly involved (54%), followed by the mitral valve (45%). Mortality did not differ significantly in individuals according to the valve involved (Figure 2).
Both the aortic and mitral valves were involved in 25 patients. Native valves were more commonly affected (69.7%), followed by biological valves (22%) and then mechanical valves (10.3%). Mortality did not differ significantly according to the type of valve affected (native, biological or mechanical) (Figure 2).
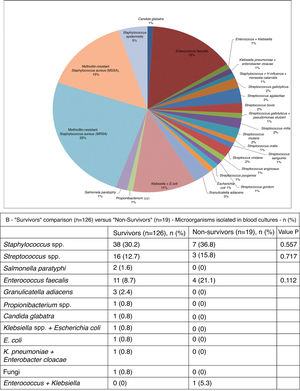
As can be seen in Figure 3A, in blood cultures from the 101 patients in whom the etiological agent could be isolated, the most frequent was methicillin-resistant Staphylococcus aureus (MRSA) (25%). Other microbes isolated included methicillin-sensitive S. aureus (MSSA) (15%), Streptococcus spp. (15%), Enterococcus faecalis (15%) and Staphylococcus epidermidis (5%) (Figure 3B). The microbial etiology of IE did not differ significantly between the two groups.
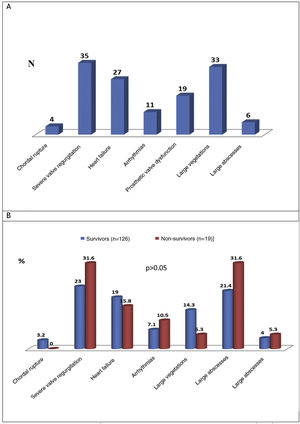
Cardiac and extracardiac complicationsThe preoperative cardiac and extracardiac complications of IE are shown in Figure 4A and these complications in the survivor and non-survivor groups are compared in Figure 4B.
The most common preoperative cardiac complications (n=135) were severe valve regurgitation (24.1%), vegetations measuring more than 10 mm (22.8%), and manifestations of HF (18.6%). There was no significant difference in the proportion of these complications in survivors and survivors (Figure 4B). The most commonly found preoperative extracardiac complications in IE patients were systemic embolism (17.2%), acute kidney injury (defined as an increase in plasma creatinine of ≥0.3 mg/dl within 48 h or of ≥50% above the known or presumed baseline value from the previous seven days)6 or worsening kidney function (14.5%), sepsis (10.3%), and respiratory failure (9%) (Table 2). These extracardiac complications did not differ significantly between the survivor and non-survivor groups (Table 3).
Preoperative extracardiac complications in the overall study population.
| Systemic embolism | 25 (17.2) |
| Respiratory failure | 13 (9.0) |
| Sepsis | 15 (10.3) |
| AKI | 21 (14.5) |
| Gastrointestinal bleeding | 1 (0.7) |
| Seizures | 1 (0.7) |
| Liver dysfunction and worsening renal function | 1 (0.7) |
Data are n (%).
AKI: acute kidney injury.
Comparison of preoperative extracardiac complications between survivors and non-survivors.
| Survivors (n=126) | Non-survivors (n=19) | p | |
|---|---|---|---|
| Systemic embolism | 22 (17.5) | 3 (15.8) | 1.00 |
| Respiratory failure | 12 (9.5) | 1 (5.3) | |
| Sepsis | 7 (5.6) | 8 (42.1) | <0.001 |
| AKI | 17 (13.5) | 4 (21.1) | 0.48 |
| Gastrointestinal bleeding | 1 (0.8) | 0 | |
| Seizures | 1 (0.8) | 0 | |
| Liver dysfunction and worsening renal function | 1 (0.8) | 0 |
Data are n (%).
AKI: acute kidney injury.
Systemic embolism, the most common extracardiac complication, presented as ischemic stroke in 12 cases, hemorrhagic stroke in two cases, splenic infarction in six, renal infarction in three, embolization to the left arm in one case and embolization to the right leg in another.
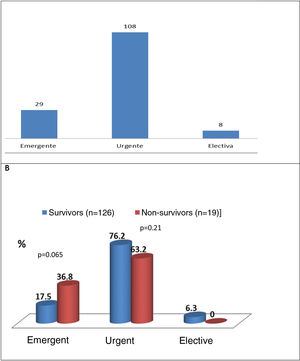
Type of cardiac surgerySurgery performed within a few days (urgent)5 was the most common approach (74.5%), followed by emergent (within 24 h)5 (20%) and elective (5.6%) surgery (Figure 5A). Figure 5B shows that there was a trend for higher mortality in the emergent surgery group (36.8% vs. 17.5%; p=0.065) compared to patients operated on urgently (63.2% vs. 76.2%; p=0.21) or electively (no deaths).
Reasons for cardiac surgery in infective endocarditisThe most common reason for cardiac surgery (Table 4) was refractory HF (57.9%) due to severe valve regurgitation, cardiogenic shock or valve dysfunction, followed by uncontrolled infection (31%) (large vegetations and abscesses) and to prevent cardioembolism (11% of cases). Mortality was significantly higher in patients with HF in cardiogenic shock caused by severe regurgitation (35.7% vs. 5.7%; p=0.005).
Reasons for surgery in the overall population and in survivors and non-survivors.
| Total (n=145) | Survivors, n=126 | Non-survivors, n=19 | p (survivors vs. non-survivors) | |
|---|---|---|---|---|
| HF | 84 (57.9) | 71 (56.3) | 13 (68.4) | 0.320 |
| Severe valve regurgitation + cardiogenic shock | 9 (6.2) | 4 (5.7) | 5 (35.7) | 0.005 |
| Valve dysfunction and persistent HF | 22 (15.2) | 20 (28.6) | 2 (14.3) | 0.337 |
| Severe valve regurgitation and persistent HF | 50 (34.5) | 44 (62.9) | 6 (42.9) | 0.164 |
| Severe valve regurgitation + refractory PE | 3 (2.1) | 2 (2.9) | 1 (7.1) | |
| Uncontrolled infection | 45 (31.0) | 39 (31.0) | 6 (31.6) | 0.956 |
| Large vegetations (>10 mm) | 23 (15.9) | 21 (52.5) | 2 (40.0) | 0.665 |
| Large abscesses | 14 (9.7) | 11 (27.5) | 3 (60.0) | 0.166 |
| Large vegetations + chordal rupture | 6 (4.1) | 6 (15.0) | 0 (0) | |
| Pseudoaneurysm + fistula | 2 (1.4) | 2 (5.0) | 0 (0) | |
| Prevention of embolism | 16 (11.0) | 16 (12.7) | 0 (0) |
Data are n (%).
HF: heart failure; PE: pulmonary edema.
Overall, 90 biological valves were implanted, 75 in the survivor group and 15 in the non-survivor group, and 54 mechanical valves, 49 in the survivor group and five in the non-survivor group, and one mitral valve repair was performed with excision of vegetation (Table 5). Native valves were replaced by biological valves in most cases (n=18 in mitral position and n=21 in aortic position). Mechanical valves were placed in mitral position in nine patients and in aortic position in 18 patients.
Types of surgical procedure.
| Survivors (n=126) | Non-survivors (n=19) | |
|---|---|---|
| On native mitral valve or valve in mitral position | ||
| Replacement of native mitral valve with biological valve | 14 | 4 |
| Replacement of native mitral valve with mechanical valve | 11 | 2 |
| Replacement of biological valve in mitral position with another biological valve | 6 | 1 |
| Replacement of biological valve in mitral position with mechanical valve | 1 | 0 |
| Replacement of mechanical valve in mitral position with biological valve | 3 | 1 |
| Replacement of mechanical valve in mitral position with another mechanical valve | 4 | 0 |
| Mitral valvuloplasty and vegetation removal | 2 | 0 |
| On native aortic valve or prosthesis in aortic position | ||
| Replacement of native aortic valve with biological valve | 19 | 2 |
| Replacement of native aortic valve with mechanical valve | 18 | 0 |
| Replacement of biological valve in aortic position with another biological valve | 17 | 2 |
| On more than one native or prosthetic valve and/or adjacent tissues | ||
| Replacement of native mitral and aortic valves with biological valves | 8 | 3 |
| Replacement of native mitral and aortic valves with mechanical valves | 9 | 1 |
| Replacement of native mitral valve with biological valve + replacement of biological aortic valve with another biological valve | 3 | 0 |
| Replacement of native mitral valve with mechanical valve + tricuspid annuloplasty | 2 | 0 |
| Replacement of biological valve in mitral position and biological valve in aortic position with other biological valves | 2 | 1 |
| Replacement of a mechanical valve in aortic position with another mechanical valve + clearance and closure of mitral/aortic abscess cavity | 3 | 2 |
| Replacement of mechanical prosthesis in aortic position with biological valve + replacement of native mitral valve with biological valve | 1 | 0 |
| Replacement of native aortic valve with mechanical valve + mitral valve repair + tricuspid valve repair | 1 | 0 |
| Replacement of biological valve in aortic position with another biological valve + replacement of native mitral valve with biological valve + tricuspid annuloplasty | 2 | 0 |
The most frequently observed postoperative complications (Table 6) were uremia requiring hemodialysis (24.8%) (non-specific signs and symptoms not all present simultaneously, including nausea, vomiting, encephalopathy, seizures, bleeding, hypervolemia, pleuritis, pericarditis, dyspnea, hyperkalemia and creatinine clearance <8−10 ml/min/1.73 m2 and referral by a nephrologist for hemodialysis),7 acute kidney injury,6 third-degree atrioventricular (AV) block (12.4%), AF (8.3%) and sepsis (6.9%). Of all the complications analyzed, three were significantly more common in the non-survivor than the survivor groups (Table 6B): sepsis (36.8% vs. 2.4%; p<0.001), cardiac tamponade (15.8% vs. 1.6%; p=0.016), and cardiogenic shock plus septic shock (15.8% vs. no death).
Postoperative complications in the overall population and comparison of some of these complications between survivors and non-survivors.
| Postoperative complications in the overall population | |
|---|---|
| AF | 12 (8.3) |
| 3rd-degree AV block | 18 (12.4) |
| Sepsis | 10 (6.9) |
| AKI | 24 (16.6) |
| Stroke | 3 (2.1) |
| Respiratory failure | 8 (5.6) |
| Respiratory infection | 7 (4.8) |
| Cardiac tamponade | 5 (3.4) |
| Suprapubic cystostomy | 1 (0.7) |
| Large pleural effusion requiring thoracentesis | 2 (1.4) |
| Cardiogenic shock and septic shock | 3 (2.1) |
| Toxic erythema | 2 (1.4) |
| Gynecological infection | 1 (0.7) |
| Major drainage requiring resternotomy | 2 (1.4) |
| Pulmonary thromboembolism | 1 (0.7) |
| Disuse atrophy | 2 (1.4) |
| Liver dysfunction + worsening kidney function | 1 (0.7) |
| Urinary tract infection | 2 (1.4) |
| Left femoral thrombosis requiring thrombectomy | 1 (0.7) |
| Postoperative dialysis | 36 (24.8) |
| Comparison of some of these complications between survivors and non-survivors | |||
|---|---|---|---|
| Survivors (n=126) | Non-survivors (n=19) | p | |
| AF | 11 (8.7) | 1 (5.3) | |
| 3rd-degree AV block | 15 (11.9) | 3 (15.8) | 0.707 |
| Sepsis | 3 (2.4) | 7 (36.8) | <0.01 |
| AKI | 23 (18.3) | 1 (5.3) | |
| Stroke | 3 (2.4) | 0 (0) | |
| Respiratory failure | 7 (5.6) | 1 (5.3) | |
| Respiratory infection | 7 (5.6) | 0 (0) | |
| Cardiac tamponade | 2 (1.6) | 3 (15.8) | 0.016 |
| Suprapubic cystostomy | 1 (0.8) | 0 (0) | |
| Large pleural effusion requiring thoracentesis | 2 (1.6) | 0 (0) | |
| Cardiogenic shock and septic shock | 0 (0) | 3 (15.8) | 0.002 |
Data are n (%).
AF: atrial fibrillation; AKI: acute kidney injury; AV: atrioventricular.
There were 19 in-hospital deaths in the overall population (13.1%), of which septic shock (47.4%) and cardiogenic shock (36.8%) were the main causes (Table 7).
Non-survivors had significantly lower LVEF in the preoperative phase (median: 48% vs. 56%; p=0.027), and more prevalent AF (31.6% vs. 11.1%; p=0.027) (Table 1).
Other factors associated with significantly higher mortality were sepsis in the pre- and postoperative phase, need for emergent surgery in the first 24 hours (compared to urgent or elective surgery), severe valve regurgitation associated with a history of cardiogenic shock, and cardiac tamponade as a postoperative complication.
DiscussionThis study focuses on two aspects of this patient population which enable comparisons with results from similar studies: clinical characterization of IE patients undergoing surgery, and predictors of in-hospital mortality. With regard to clinical and demographic characteristics, male patients were more affected by IE (72.4%) than females, the most frequent microorganisms isolated were Staphylococcus aureus followed by Streptococcus spp., and the native aortic valve was most often involved. Surgery was most often urgent (within seven days)5 and the main indication was refractory HF, followed by large vegetations. The most common surgical complications were uremia requiring hemodialysis, acute kidney injury and third-degree AV block. Overall in-hospital mortality was 13.1% and septic and cardiogenic shock were the main causes of death. The main predictors of higher in-hospital mortality were the need for emergent surgery,5 lower preoperative LVEF, AF, pre- and postoperative sepsis or septic shock, cardiogenic shock, cardiac tamponade, and the need for renal replacement therapy in the postoperative period.
In this study, in-hospital mortality was 13.1%, which is similar to results from other retrospective studies such as Moreira et al.,8 with 15% mortality in 133 patients who underwent cardiac surgery for IE, and Farag et al.1 (18.3% in 360 operated patients), but higher than in Ferreira et al. (3.7% in 54 patients who underwent surgery for IE).9 Predictors of in-hospital mortality in this study were different from those described by Moreira et al.8 (chronic obstructive pulmonary disease, cerebral embolism, IE caused by Staphylococcus spp. and non-HACEK Gram-negative bacilli), by Ferreira et al.9 (operated and non-operated patients with IE: Charlson index ≥5, EuroSCORE >6, use of immunosuppressants, severe sepsis and/or septic shock, and inappropriate antibiotic therapy), and by Farag et al.1 (advanced age, preoperative diabetes, higher NYHA class, kidney failure and liver failure, longer operative, cardiopulmonary bypass (CBP) and aortic cross-clamp times, greater volume of red blood cells transfused, and fresh plasma and platelet transfusion). Many of the variables in these studies1,8,9 were not assessed in ours. Differences in baseline characteristics of the IE patients included in these studies may explain why they found predictors of mortality that were different from those in our study. For example, in Moreira et al.,8 diabetes, chronic kidney disease (CKD) and Staphylococcus spp. as the etiological agent were less frequent, while a history of endocarditis and the presence of vegetations >10 mm were more common. In the studies by Farag et al.,1 Moreira et al.,8 Ferreira et al.,9 mean patient ages were lower.
Certain comorbidities may have a significant impact on mortality. In our study, only eight patients had a history of stroke (5.5%) and all survived. CKD (creatinine clearance <60 ml/min/1.73 m2) was diagnosed in 13.8%; degree of renal dysfunction had no significant effect on survival, but mortality was significantly higher in patients requiring postoperative dialysis (78.9% vs. 16.7%; p<0.001). The proportion of patients with recent stroke and/or CKD was lower in our study than in Farag et al.1 Although fewer patients in our study had a history of stroke, and thus practical conclusions cannot be drawn, in Misfeld et al.10 patients with IE and cerebral embolism had a five-year survival of 46%, which was lower than in those without cerebral embolism. Among patients discharged from hospital, overall survival is over 60% at 10 years. Farag et al.1 found a mean survival of 69.4% at one year, 63.3% at five years and 63.3% at 10 years following cardiac surgery for IE.
The present study only considered patients who underwent cardiac surgery for IE and not patients admitted to the same center who were only treated medically. According to Cahill et al.,2 50–60% of patients with IE undergo cardiac surgery, while Moreira et al.8 observed that in-hospital mortality in patients with IE who underwent cardiac surgery was significantly lower than in those who did not (15.5% vs. 32.6%; p=0.028), as also observed by Ferreira et al.9 Our study did not make this comparison, but it would have been useful to do so in order to enable conclusions to be drawn on various parameters, including surgical indications and the most appropriate timing for surgery.
Thirty-day mortality following surgery for IE ranges from 15% to 20%,1,11–13 which is similar to the results from the present study.
HF was the main surgical indication for most of the patients included in the study (57.9%), as also observed by Farag et al.1 (around half their patients were in NYHA class IV). The main mechanisms underlying HF were severe valve regurgitation and valve dysfunction. Uncontrolled infection (large vegetations and abscesses) and the prevention of cardioembolism were other predictors of cardiac surgery. The ESC5 and Cahill et al.2 identify these three factors as the surgical indications with potential benefit for IE patients, which need to be identified early to improve surgical outcomes and thereby reduce early and long-term mortality. All the patients in our study cohort underwent cardiac surgery, but data on potential candidates who were not operated were not analyzed. According to Chu et al.,13 24% of candidates with guideline-recommended indications for surgery are not actually operated. Among the reasons given are poor prognosis irrespective of treatment (34%), hemodynamic instability (20%), death before surgery (23%), stroke (23%), sepsis (21%), and refusal for surgery (26%).2 In our study, surgery was most often urgent (within a few days)5 (74.5%) (76.2% in survivors and 63.2% in non-survivors (p=0.21), and emergent surgery was performed in 17.5% of patients in survivors and 36.8% in non-survivors (p=0.065) (Figure 5). Moreira et al.8 concluded that early cardiac surgery in IE (within seven days) was not associated with higher mortality compared to later surgery.
The profile of the microbial agents found in this study and their relative proportions were similar to those found in Farag et al.1 – predominantly Staphylococcus spp. (45%) (MRSA 25%, MSSA 15% and S. epidermidis 5%). Not all the tissues excised during surgery underwent histopathological or microbiological analysis (which is now the cornerstone of IE diagnosis),5,14 nor was the antibiotic therapy used in the postoperative phase characterized. These data are important to help evaluate some predictors of mortality.
As pointed out by Farag et al.,1 the type of microorganism isolated has changed, with Staphylococcus spp. now predominating as etiological agents, which due to their more aggressive and invasive nature may lead to the formation of large vegetations and abscesses. These alone are an indication for surgery (observed in 29.7% of cases in the present study). IE caused by Staphylococcus has repeatedly been associated with higher mortality,15,16 although this was not observed in our study.
Many other variables may be associated with higher mortality in cardiac surgery for IE patients. Several authors17,18 have demonstrated a relationship between the presence of vegetations >10 mm, large abscesses, false aneurysms and fistulas,11,19,20 and adverse events and prognosis, but in our study mortality was not significantly higher in the presence of these indicators of uncontrolled infection (although our small sample size should be taken into account).
The simultaneous presence of severe valve regurgitation and cardiogenic shock is associated with higher 30-day mortality. According to the ESC guidelines,5 surgery must be performed on an emergency basis,21 irrespective of the status of infection, when patients are in persistent pulmonary edema or cardiogenic shock despite medical therapy.22 As seen in the present study, mortality is higher in such patients. Surgery should be performed urgently when HF is less severe or there is severe aortic or mitral valve regurgitation with large vegetations, even in the absence of HF.23
As seen in other studies,1,8 native valves were most frequently affected, followed by biological and mechanical valves; however, no significant differences were recorded in in-hospital mortality according to the type of valve. This contrasts with the higher mortality found by other authors with prosthetic valves.1,24
The main cardiac complications before surgery for IE in this study were similar in type to those observed in other studies1,5,8 (in most cases urgent or emergent, and not uncommonly life-saving), but differ in their frequency, especially for severe valve regurgitation, refractory HF (15% in this study), large vegetations and valve dysfunction. These differences may be part of the reason for the different predictors of postoperative mortality in our study compared to the literature, as discussed above. Extracardiac complications, the most frequent of which were systemic embolism, respiratory failure, sepsis and renal dysfunction, in themselves present a range of preoperative risks and are observed in varying proportions in different studies.1,8 They influence the approach toward anesthesia, transfusion (and its effect on coagulation parameters), medical therapy and mechanical circulatory support, and affect decisions on what types of surgery are possible or desirable in terms of operative, cardioplegia, CBP and aortic cross-clamp times,1 type of valve or homograft to be implanted, extent of debridement or resection of intracardiac tissues, including paravalvular tissue, and possible involvement of conduction tissue and/or the valvular annulus, use of Dacron patches, and level of hypothermia.1 The severity of these complications varies so much, as do their impact on postoperative mortality, that it is difficult to compare predictors reported in studies published over the last 20 years. Some of these factors, according to Farag et al.,1 are the most effective predictors of mortality: operating, cardioplegia, CBP and aortic cross-clamp times, and massive transfusions (and their impact on coagulation parameters).
Biological valves were selected for most patients (62.1%); the reasons for this choice (such as age, need for oral anticoagulation, bleeding risk, and expected treatment adherence) were not stated on the medical records. In Lee et al.’s study,25 biological valves were used in the majority of patients (75.8%, n=71) requiring mitral valve replacement, and mechanical valves were used in 24.3%.
In our study, AF, sepsis and cardiac tamponade were the most common postoperative complications in patients who died during hospital stay. According to David et al., 26 the most common postoperative complications following surgery for IE are severe coagulopathy, repeat chest surgery for bleeding or cardiac tamponade (in our study associated with 15.8% mortality in the non-survivor group compared to 1.6% in the survivor group; p=0.01), aute kidney injury or uremia requiring hemodialysis (in our study, the most common postoperative complication; 24.8% of patients required dialysis with markedly higher associated in-hospital mortality: 78.9% vs. 16.7%; p<0.001), HF (again corroborated by our study, especially with regard to concomitant severe valve regurgitation and cardiogenic shock (35.7% vs. 5.7%; p=0.005), pneumonia, and third-degree AV block following radical resection of the aortic root due to an abscess and requiring pacemaker implantation.26
In our study, new-onset AF was less common (8.3%) in the postoperative period than reported in the literature (15–45%).27 Postoperative AF is associated with longer hospital stays and higher rates of complications and mortality28 (as found in our analysis).
This study only assessed in-hospital mortality (13.1%), for which septic shock and cardiogenic shock were the main causes. This figure is slightly lower than that in Farag et al.1 (18.3% at 30 days). In the study by Kang et al.,23 patients with IE who underwent early cardiac surgery were significantly less likely to have the composite endpoint of all-cause mortality, embolic events or recurrence of IE at six months. Farag et al.1 reported postoperative IE survival of 63.3% at five years, 63.3% at 10 years and 48.3% at 20 years.
This study and others show that, in the context of IE, surgery often plays a crucial and often life-saving role,14 such as in cases of rupture of the chordae tendineae, severe valve regurgitation, biological or mechanical valve dysfunction, refractory HF, sepsis unresponsive to antibiotics, large intracardiac vegetations and abscesses, and risk of cardioembolism.
In this study, several topics were not studied, such as the length and characteristics of patients’ hospital stay, including length of stay between the initial diagnosis and surgery and between surgery and discharge or in-hospital death, the duration of other treatments in the pre- and postoperative periods, and potential indicators of treatment quality,14 as well as complications which may have prolonged hospital stay and influenced in-hospital and medium- and long-term mortality.14
Due to the retrospective nature of our study, we were unable to investigate several items on clinical records which are essential to calculate the scores used to estimate mortality risk following cardiac surgery in patients with IE, such as the Association pour l’Etude et la Prévention de l’Endocardite Infectieuse (AEPEI), modified AEPEI and PALSUSE scores, and to analyze predictors of in-hospital mortality, particularly the parameters used to calculate EuroSCORE II, such as those related to whether the patient is considered to be in a critical state, which is also included in the AEPEI and PALSUSE scores. We were therefore unable to validate this excellent risk prediction tool in the IE patients included in our analysis. In the future, a prospective study could answer many of the questions raised by the results of this study.
LimitationsThis study has several limitations. It was a retrospective analysis (and therefore potentially important information was missing or incomplete in clinical records) on a relatively small sample over 11 years, a period during which changes took place in surgical approaches and criteria, techniques and prosthetic material, diagnostic methods, and intensive postoperative care that will undoubtedly have affected the quality of care and hence in-hospital mortality. Over this period patients also presented with increasingly frequent and severe comorbidities. Certain important data related to the surgical procedure itself are also missing, including operative, cardioplegia, CBP and aortic cross-clamp times; extent of debridement or resection of intracardiac tissues, including paravalvular tissue, and possible involvement of conduction tissue and/or the valvular annulus; use of Dacron patches; and level of hypothermia. In addition, we did not analyze the duration of pre- or postoperative antibiotic therapy, length of stay in other hospitals, or time between admission of a patient with indication for surgery to another hospital and the operation.
ConclusionIn our study, 13% of patients with IE died in the postoperative period. There is a need for better indicators to enable early identification of surgical candidates for IE; implementation of a heart team composed of a cardiologist (clinical or experienced in transthoracic and transesophageal echocardiography), a cardiothoracic surgeon, an internist/intensivist, an infectious disease specialist, a neurologist, a primary care physician and an imaging specialist; and better surgical strategies, including more rapid intervention, more specific postoperative care and optimal antibiotic therapy, to reduce in-hospital and medium- and long-term mortality.
In practical terms, early cardiac surgery (within seven days) should be considered by the heart team when Staphylococcus spp. are isolated in blood cultures, or in the presence of refractory HF or large vegetations (>10 mm), septic and/or cardiogenic shock, or LVEF<50%.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Guiomar N, Vaz-da-Silva M, Mbala D, Sousa-Pinto B, Monteiro JP, Ponce P, et al. Cirurgia cardíaca na endocardite infeciosa e preditores da mortalidade intra-hospitalar. Rev Port Cardiol. 2020;39:135–147.