Congenitally corrected transposition of the great arteries is a rare heart defect that can be associated with systemic ventricular dysfunction and conduction disturbances.
The use of cardiac resynchronization therapy in patients with congenital heart disease is not fully established.
The authors report a case of cardiac resynchronization therapy in a 31-year-old woman with a history of congenitally corrected transposition of the great arteries and heart failure, who had undergone two heart surgeries and had a DDDR pacemaker implanted.
To our knowledge this is the first case reported in Portugal of cardiac resynchronization therapy by transvenous access in a patient with congenitally corrected transposition of the great arteries.
A transposição congenitamente corrigida das grandes artérias é uma cardiopatia rara associada a disfunção do ventrículo sistémico e perturbações da condução.
A utilização de terapia de ressincronização cardíaca em doentes com cardiopatia congénita ainda não está totalmente estabelecida.
Os autores reportam um caso de ressincronização cardíaca numa paciente de 31 anos, com história de transposição congenitamente corrigida das grandes artérias e insuficiência cardíaca, já submetida a duas cirurgias cardíacas e portadora de pacemaker DDDR.
Do nosso conhecimento, trata-se do primeiro caso descrito em Portugal de terapêutica de ressincronização cardíaca, por acesso transvenoso, numa paciente com transposição congenitamente corrigida das grandes artérias.
Congenitally corrected transposition of the great arteries (ccTGA) is a rare heart defect that accounts for less than 1% of all congenital heart disease. In this anomaly, the morphologically right atrium (RA) enters the morphologically left ventricle (LV), which gives rise to the pulmonary artery (PA), and the morphologically left atrium (LA) enters the morphologically right ventricle (RV), which gives rise to the aorta. There is thus double discordance, i.e. atrioventricular (AV) and ventriculoarterial.1
Ventricular dysfunction is common in patients with adult congenital heart disease (CHD) with a systemic RV and is related to electromechanical dyssynchrony.2 Over 25% of such individuals progress to symptomatic heart failure (HF), which is occasionally refractory to drug therapy and is associated with significant morbidity and mortality.3 It is therefore important to develop new therapeutic strategies for this patient group.
In patients with dilated cardiomyopathy and severe LV systolic dysfunction, electromechanical dyssynchrony and HF, and under optimal drug therapy, cardiac resynchronization therapy (CRT) has been shown in randomized trials to reduce overall mortality and hospitalization for major cardiac events4,5 and to improve quality of life, functional class and echocardiographic parameters.6,7 However, the use of CRT in patients with CHD is not fully established.
Case reportThe authors report the case of a 31-year-old Caucasian woman with ccTGA, pulmonary stenosis and ventricular septal defect (VSD). She had undergone two heart surgeries, the first a palliative Blalock-Taussig shunt procedure at 15 months of age, and the second at age 15 for VSD closure, placement of a conduit between the morphologically LV and the PA, and ligation of the Blalock-Taussig shunt. Due to complete atrioventricular block (AVB) following surgery, a DDDR pacemaker was implanted, with both leads through the left subclavian vein and the generator in a left pre-pectoral pocket. A new active fixation lead was subsequently implanted via the left subclavian vein due to ventricular lead dysfunction. Her history also included transient ischemic attack at age 16.
The patient remained in NYHA class II during follow-up, including while pregnant with her daughter. In early 2012, despite optimal medical therapy with ramipril, carvedilol, furosemide and digoxin, her clinical condition worsened to NYHA class III.
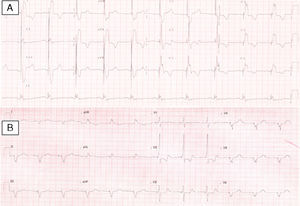
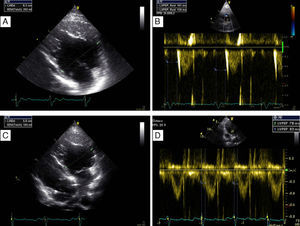
On physical examination, her blood pressure was 96/65 mmHg and a grade II-III/VI systolic murmur was audible at the fourth intercostal space on the left sternal border. The 12-lead electrocardiogram showed sinus rhythm, heart rate of 60 bpm and ventricular pacing with preserved AV synchrony, and QRS interval of 196 ms (Figure 1A). Transthoracic echocardiography (TTE) revealed a dilated (63 mm) systemic (morphologically right) ventricle (Figure 2A) with impaired global systolic function (≤35% by the Teichholz method – Simpson's method was not applicable due to the RV morphology – and dP/dt of 379 mmHg/s), moderate aortic regurgitation, moderate regurgitation of the systemic AV (morphologically tricuspid) valve, and estimated pulmonary artery pressure of 40 mmHg. There was no AV dyssynchrony (diastolic filling time >40%, with clear separation of the E and A waves and no A-wave truncation). The only parameter of interventricular dyssynchrony that could be quantified was a significantly prolonged systemic pre-ejection time (161 ms) (Figure 2B).
Transthoracic echocardiogram before (A and B) and after (C and D) implantation of a biventricular pacing system, showing decrease in diastolic systemic ventricular size (a possible indicator of reverse remodeling) from 63 mm (A) to 58 mm (C), and disappearance of systemic electromechanical delay as quantified by pre-ejection time falling from 161 ms (B) to 83 ms (D).
In a patient in NYHA class III despite optimal medical therapy, with a history of ccTGA, and pacemaker rhythm with mechanical dyssynchrony and systolic dysfunction of the systemic ventricle, it was decided to implant a CRT pacemaker (CRT-P).
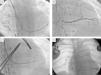
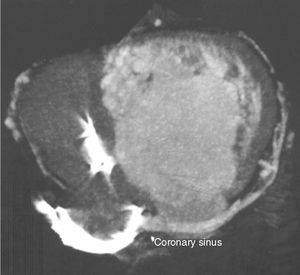
Previous computed tomography (CT) angiography (Figure 3) revealed the coronary sinus (CS) draining into the RA and several tributary veins, and absence of atherosclerotic lesions in the coronary arteries.
As the patient already had three old unipolar leads in the left heart that could not be used for the new system, it was decided to implant the system on the right.
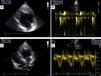
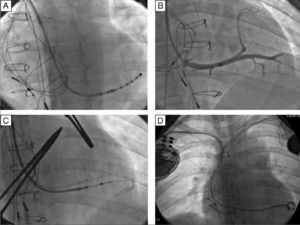
Vascular access was obtained via the right subclavian vein and a passive fixation lead (Fineline II Sterox 4457, Boston Scientific) was implanted in the pulmonary ventricle and an active fixation atrial lead (Fineline II EZ Sterox 4457, Boston Scientific) in the RA free wall. The CS was catheterized using a sheath and a diagnostic electrophysiological catheter (Figure 4A), and CS venography was performed to determine the most suitable tributary vein in which to place the systemic ventricular pacing lead. A bipolar lead (Boston Scientific) was introduced using a guidewire into the posterolateral vein (Figure 4C), with a good pacing threshold and no diaphragmatic stimulation, and the three leads were connected to the biventricular pacing system (Boston Scientific INVIVE) (Figure 4D). The procedure was uneventful and QRS interval decreased from 196 to 144 ms (Figure 1B).
Implantation of a cardiac resynchronization system via the right subclavian vein in a patient with a DDDR pacemaker in the left heart. (A) Catheterization of the coronary sinus with a diagnostic catheter followed by introduction of a guide catheter; (B) venography of the coronary sinus, showing two tributary veins suitable for delivery of the systemic ventricular pacing lead; (C) introduction of a guidewire through the catheter in the posterolateral vein followed by introduction of the systemic ventricular pacing lead; (D) final result following implantation of the resynchronization system, with leads in the right atrium, pulmonary vein and systemic ventricle.
At six-month follow-up, the patient had improved to NYHA class I-II, diastolic systemic ventricular size had decreased from 63 mm to 58 mm (Figure 2C), the systemic electromechanical delay had disappeared (pre-ejection time falling from 163 ms to less than 100 ms) (Figure 2D), ejection fraction had improved (from ≤35% to around 47% by the Teichholz method), and dP/dt had increased from 379 mmHg/s to 511 mmHg/s.
DiscussionAs more patients with ccTGA survive to adulthood, they are more likely to present systemic ventricular dysfunction and hence HF refractory to medical therapy.
ccTGA is associated with various conduction disturbances. The incidence of AVB is about 2%/year,1,8 and it affects around 50% of patients in 20-year follow-up, requiring pacemaker implantation and thus increasing the risk of electromechanical dyssynchrony.9 In the case presented, surgical closure of the VSD was complicated by complete AVB10 followed by the need for ventricular pacing. The patient's HF may be explained not only by exposure of the systemic (morphologically right) ventricle to high systemic pressures,11 but also by the negative hemodynamic effect of the electromechanical dyssynchrony produced by ventricular pacing.11
Considering that the patient did not meet the criteria for heart transplantation, and that another heart surgery would be associated with high risk, the less invasive strategy of CRT was decided upon. CRT has been shown to be useful in dysfunction of the systemic ventricle.2 Janousek et al. reported that 37% of patients were removed from the heart transplant waiting list after CRT-P implantation.12 However, these findings cannot be generalized, given the specific characteristics of patients with adult CHD and the lack of randomized trials.
In patients with ccTGA, the CS is usually located next to the systemic RV and drains into the RA, and is thus a viable site for placement of a pacing lead.2,3,11,13,14 In the case presented, however, it was decided to perform CT angiography before the procedure, in view of the heterogeneity of venous anatomy described in the literature2 and of the patient's previous surgeries.
The decision not to insert an implantable cardioverter-defibrillator (CDI) is also debatable, and the risks and benefits were carefully weighed in this patient. Neither the European15 nor the American16 guidelines recommend an ICD for primary prevention in patients with adult CHD. Oechslin et al.,17 assessing mode of death in around 200 adults with CHD, found that sudden death occurred in 26% of the population, a lower percentage than the 50–60% in patients with acquired heart disease.18,19 The defibrillation lead itself is associated with complications such as increased risk of major complications if the lead needs to be extracted,20 and of inappropriate shocks. In a study by Alexander et al.21 of patients with a mean age of 18 years, 42% with CHD, 25% had received inappropriate shocks at two years; in an analysis by Berul et al.22 of a population (46% with CHD) in whom almost half had an ICD for primary prevention, 21% received inappropriate shocks, while a study by Khairy et al.23 in 121 adult patients with tetralogy of Fallot, 56% of whom had received an ICD for primary prevention, 25% had inappropriate shocks. For this reason, it was decided not to implant an ICD.
The incidence of infections related to pacemakers and ICDs is around 1.9/1000 devices/year.24 Our patient's young age, and the total of six leads that had been implanted, put her at high risk for infection, and extracting the leads was accordingly considered an option. However, in view of her CHD with a systemic ventricle, the risk of the biventricular pacing leads being dislodged, and the 7% rate of major complications associated with extraction of three or more leads in female patients,20 it was decided that the risks outweighed the benefits.
In the case reported, the clinical, electrocardiographic and echocardiographic response to CRT was positive. However, the follow-up is still short; there are few reports of long-term follow-up of such cases in the literature.25 The best site and mode for pacing in these patients has also not been established.26,27
Cases have been reported of biventricular pacing systems implanted in patients with CHD, including ccTGA, and dysfunction of the systemic ventricle. However, this case is noteworthy because it is the first case described in Portugal of CRT by transvenous access in a patient with ccTGA and systemic ventricular dysfunction.
ConclusionsThis case highlights the feasibility of CRT in patients with ccTGA, dysfunction of the systemic ventricle and HF.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sousa PA, Cavaco D, Adragão P, Teixeira A, Ribeiras R, Martins M, et al. Ressincronização cardíaca em doente com transposição congenitamente corrigida das grandes artérias. Rev Port Cardiol. 2014;33:387.