A 68‐year‐old man with a small‐bowel carcinoid tumor, liver metastases, and chronic elevation of urinary 5‐hydroxyindolacetic acid levels was referred for cardiology consultation because of worsening fatigue, exertional dyspnea, hepatalgia and peripheral edema over the previous few months. The carcinoid syndrome had been known for four years, when the patient was considered unsuitable for cytoreductive surgery because of disease extent. Accordingly, he was medically treated with cytotoxic agents first, followed by octreotide LAR in conjunction with interferon alfa. On physical examination he presented in good general condition. A pansystolic murmur increasing with inspiration was heard in the xiphoid region. Systolic jugular vein expansion, a pulsatile and enlarged liver and leg edema were present. Pulse was regular and blood pressure was normal.
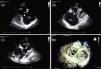
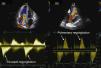
Two‐dimensional and Doppler echocardiography revealed thickened, retracted and immobile tricuspid (Figure 1a and b – stars) and pulmonary valves (Figure 1c – arrow), leading to severe bi‐valvular regurgitation (Figure 2). Three‐dimensional transthoracic echocardiography provided an en face view from the atrial side of the tricuspid valve fixed in a semi‐open position throughout systole, displaying the large regurgitant orifice (Figure 1d). Additional echocardiographic findings were right atrial and ventricular dilatation, a dilated and pulsatile inferior vena cava, and systolic bowing of the interatrial septum toward the left atrium. Right ventricular function was reasonably well preserved. The patient underwent tricuspid valve replacement with a mechanical prosthesis and his postoperative course was uneventful.
Transthoracic echocardiography: thickened, retracted and immobile tricuspid (a and b) and pulmonary valves (c); 3D en face view of the tricuspid valve open during systole (d). MV: mitral valve; PA: pulmonary artery; RA: right atrium; RV: right ventricle; RVOT: right ventricular outflow tract.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.