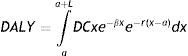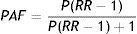Atrial fibrillation is the most prevalent sustained arrhythmia. This paper estimates the burden and cost of illness attributable to atrial fibrillation in Portugal based on demographic and health statistics.
MethodsMortality data by cause of death came from the European Detailed Mortality Database of the World Health Organization (WHO). Hospital data were taken from the Portuguese diagnosis-related groups database. The burden of disease was measured using DALYs (disability-adjusted life years), a metric adopted by the WHO. Costs studied included resource use and lost productivity. The burden and cost of illness are those attributable to atrial fibrillation and its main complication, ischemic stroke.
ResultsIn Portugal, 4070 deaths were attributable to atrial fibrillation in 2010, corresponding to 3.8% of all deaths. In total, the burden of disease attributable to atrial fibrillation was estimated at 23084 DALYs: 10521 resulting from premature deaths (1.7% of the total DALYs due to death in 2010 in Portugal), and 12563 resulting from disability. The total estimated direct costs attributable to atrial fibrillation at 2013 prices were €115 million: €34 million for inpatient care and €81 million for outpatient care. Indirect costs resulting from lost production due to disability were estimated at €25 million.
ConclusionsAtrial fibrillation has an important social impact in Portugal due to its associated mortality and morbidity, and was responsible in 2013 for a total cost of €140 million, about 0.08% of gross domestic product.
A fibrilhação auricular é a disritmia persistente mais prevalente. Pretendemos estimar a carga e custos da doença atribuíveis à fibrilhação auricular em Portugal com base nas estatísticas demográficas e de saúde.
MétodosUtilizou-se informação sobre mortalidade por causa da OMS-Europa. Dados hospitalares foram provenientes da base de dados dos GDH. A carga da doença foi medida pelos DALY (disability-adjusted life years) ou anos de vida perdidos ajustados por incapacidade, uma métrica adotada pela Organização Mundial de Saúde. Os custos incluíram os consumos de recursos e as perdas de produtividade. A carga e os custos da doença estimados são os atribuíveis à fibrilhação auricular e à sua principal complicação, o acidente vascular cerebral isquémico.
ResultadosEm Portugal, no ano 2010, podem atribuir-se à fibrilhação auricular 4070 mortes correspondendo a 3,8% do total das mortes ocorridas. A carga da doença atribuível à fibrilhação auricular foi estimada em 23.084 DALY: 10.521 decorrentes das mortes prematuras (1,7% dos DALY por morte em Portugal em 2010) e 12563 devidos à incapacidade gerada pela morbilidade. O total estimado de custos diretos para o sistema de saúde a preços de 2013 atribuíveis à fibrilhação auricular foi de 115 M€ (milhões de euros): 34 M€ em internamento e 81 M€ em ambulatório. Os custos indiretos gerados pela produção perdida devidos à incapacidade causada pela doença foram estimados em 25 M€.
ConclusõesA fibrilhação auricular tem um importante impacto social em Portugal devido à mortalidade e morbilidade geradas, podendo-se-lhe atribuir em 2013 um custo total de 140 M€, cerca de 0,08% do produto interno bruto.
atrial fibrillation
disability-adjusted life year
diagnosis-related group
International Classification of Diseases, Ninth Edition, Clinical Modification
myocardial infarction
national health service
population attributable fraction
relative risk
World Health Organization
Atrial fibrillation (AF) is the most common sustained arrhythmia. It was estimated in 2010 that 33.5 million individuals worldwide,1 and nearly nine million individuals in the European Union (EU),2 had AF; the number in the EU is predicted to double to nearly 18 million by 2060. AF is associated with advanced age, male gender and various comorbidities including hypertension, heart failure, valve disease and coronary artery disease.3–7
AF can be silent, only being diagnosed when a complication develops.8,9 The main complication is systemic thromboembolism leading to stroke; AF patients are at 3–5 times higher risk of ischemic stroke, and more severe stroke.7,10–14 It is estimated that 14% of AF patients in Portugal have suffered stroke. AF is thus an important cause of mortality and morbidity in itself and due to the associated risk of ischemic stroke.15
Against this background, it is important to assess the economic impact and burden and cost of illness of AF in Portugal, which is the aim of the present study.
The purpose of studies on cost of illness is to measure the impact of a disease or risk factor in terms of use of economic resources and reduction in economic activity due to associated disability. Studies on burden and cost of illness are not strictly speaking economic evaluations, since they do not address specific interventions or compare alternative interventions; rather, they seek to paint an accurate picture of a particular health problem and its magnitude.
Despite the importance of such studies, few have been carried out in Portugal.16,17 In particular, there has been no study of the burden and cost of AF, but the limited information at the international level18,19 suggests that the costs involved are high.
Epidemiology of atrial fibrillation: incidence and prevalenceEstimates of the incidence and prevalence of AF vary considerably between different sources, depending on population characteristics and diagnostic criteria.20 Furthermore, since AF can be asymptomatic, the reported incidence and prevalence may be underestimated.20,21
IncidenceThe incidence of AF in the main European and American studies ranges between 0.1/1000 person/years in women aged 20–54 years and 40/1000 person/years in men aged ≥80 years and 69/1000 person/years for individuals aged ≥90 years.
Incidence increases progressively with age5; from age 50 onward it doubles for each decade.20 It is also higher in males in all age-groups (men are 1.5 times more likely to develop AF),22 although some authors report that this difference is smaller in older age-groups.20 A population-based cohort in the USA shows that the incidence is increasing.23
In the absence of studies estimating AF incidence in Portugal, the best source is the Rotterdam study,6,24 a prospective European study that analyzes the relevant age-groups; it has accordingly been used to calculate the burden of disease in the present study.
PrevalenceThe main source of information on the prevalence of AF in Portugal is the FAMA study,5 a cross-sectional study of a representative sample of the Portuguese population aged 40 and over that included 10477 individuals, 55% female, with a median age of 58 years. The estimated prevalence was 2.5% (95% confidence interval: 2.2%–2.8%), increasing with age (significantly higher in individuals aged 70 and over) but with no significant differences between the sexes or geographical regions.
Only 1.6% of the FAMA study population said they had been diagnosed with AF. Regarding previous clinical events, there were significant differences between the general population and individuals with AF in terms of history of stroke and myocardial infarction (MI) (stroke: 5% vs. 14%, p<0.001; MI: 3% vs. 10%, respectively; p<0.001).
MethodsBurden of diseaseBurden of disease is estimated by means of disability-adjusted life years (DALYs), a measure of the years of health lost due to disease or premature death. It includes two time-based indicators: years of life lost, the difference between age at death and standard life expectancy for that age; and years lost due to disability.25 Disability is assigned a severity weight between 0 (no disability; perfect health) and 1 (total disability or death). These weights were originally defined by expert panels at the World Health Organization (WHO), and were re-estimated for the Global Burden of Disease Study 2010 through a large-scale empirical investigation.27 The formula used is:
where:a – age of onset,
L – duration of disability or time lost due to premature mortality,
D – disability weight (between 0 and 1),
C – age-weighting correction constant (0.04),
e – expectation of life,
x – age (ranging between a and a+L),
¿ – parameter from the age-weighting function (0.1658), and
r – discount rate (3%).
The discount rate used in this calculation is 3%, and the calculation includes different weighting for different age-groups, with the middle age-group (20–50 years), the years when people tend to be raising children, being assigned greater weight.25
As there is no direct evidence available on the duration of diseases, information which is needed to calculate DALYs, we used the DisMod II model developed by Barendregt et al. for the WHO.26 This exploits the causal relations between the variables that describe a disease process by age-group and gender: incidence, prevalence, remission, case fatality, mortality, relative risk (RR) for mortality, and duration.
The model was calibrated using data from the Portuguese Institute of Statistics on the resident population and mortality in Portugal for 2010, as well as the findings of the FAMA study (on AF prevalence) and the Rotterdam Study (on AF incidence). The remission rate of AF was assumed to be zero.
In order to calculate DALYs due to disability, the degree of disability attributable to a given health problem must be specified. The weighting factors used to characterize the relevant conditions in this study were those published in the Global Burden of Disease Study 2010.27
Ideally, studies of burden of disease should refer to a specific year, but the heterogeneity of the information sources and the changes that the Portuguese national health service (NHS) has undergone in recent years mean that in practice it is more informative to use the most recent data available for each of the areas under study. Accordingly, the calculations were based on population and mortality statistics from the WHO for 2010, hospital data from 2011 and official NHS prices for 2013. The resulting figures are thus as up-to-date as possible.
Cost of illnessThe first step in the analysis was to identify the conditions that are related to AF as well as the disease itself. The main complications of AF are heart failure and ischemic stroke, the latter being the most serious.28,29 The relation between AF and stroke is unequivocal: patients with AF have an age-adjusted risk that is five-fold higher than the general population, and stroke in AF patients is more likely to lead to severe disability.30 With regard to intracranial hemorrhage, the risk is similar to that of the general population, but it is higher in AF patients under anticoagulant therapy.30,31
The conditions considered in the analysis were AF and ischemic stroke, identified by the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD 9-CM) codes 427.31 (Atrial fibrillation), 427.32 (Atrial flutter), 433 (Occlusion and stenosis of precerebral arteries [with cerebral infarction]) and 434 (Occlusion of cerebral arteries [with cerebral infarction]).
The second step was to establish the quantitative relation between AF and stroke by estimating the fraction of the cost and burden of stroke that statistically is due to AF, using the epidemiological concepts of RR and population attributable fraction (PAF). RR in this case is the ratio between the risk of suffering stroke in a population with AF and the risk in a population without AF. The values for RR used in this study, taken from Kannel et al.,32 based on data from the Framingham Study and recently updated by Ball et al.,7 were 4.0 (50–59 years), 2.6 (60–69 years), 3.3 (70–79 years) and 4.5 (80–89 years).
According to Lin et al.,11 also based on the Framingham Study, mortality following stroke was significantly higher if the stroke was AF-related (25% vs. 14%; RR: 1.79). On the basis of this information and the RRs presented above, the RR for death due to stroke was estimated for a population with AF in comparison with a population without AF: 7.2 (50–59 years), 4.7 (60–69 years), 5.9 (70–79 years) and 8.1 (80–89 years).
The PAF is the proportion of cases that would not occur in a population if the risk factor were eliminated and can be calculated by the equation33:
where:PAF – population attributable fraction,
P – prevalence of AF, and
RR – relative risk of patients with AF suffering stroke.
On the basis of the estimates of AF prevalence in Portugal and the RRs presented above, the fractions of stroke and death from stroke attributable to AF were calculated (Table 1). For example, in men aged over 80, the prevalence of AF is 7.4% and the RR of stroke is 4.5, and so the proportion of the burden of disability and costs of stroke (the PAF) attributable to AF is 20.57%.
Fraction of stroke and death from stroke attributable to atrial fibrillation.
| Age-groups (years) | RR of stroke | RR of death from stroke | Prevalence of AF | Fraction of stroke attributable to AF (%) | Fraction of death from stroke attributable to AF (%) | |||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
| 40–49 | 4.0a | 7.2a | 0.1 | 0.2 | 0.30 | 0.60 | 0.62 | 1.22 |
| 50–59 | 4.0 | 7.2 | 1.7 | 0.4 | 4.85 | 1.19 | 9.54 | 2.42 |
| 60–69 | 2.6 | 4.7 | 1.6 | 1.6 | 2.50 | 2.50 | 5.56 | 5.56 |
| 70–79 | 3.3 | 5.9 | 8.2 | 5.5 | 15.87 | 11.23 | 28.83 | 21.37 |
| 80+ | 4.5 | 8.1 | 7.4 | 11.9 | 20.57 | 29.40 | 34.44 | 45.80 |
AF: atrial fibrillation; RR: relative risk.
As well as the burden of disease, AF is also responsible for costs to society and to the NHS, considered as direct costs attributable to atrial fibrillation, which in this study are divided between inpatient and outpatient care.
Costs of inpatient careThe centrally managed database of diagnosis-related groups (DRGs) of the Portuguese NHS for 2011 was used to estimate resource use for inpatient care, including other interventions covered by the DRGs such as outpatient surgery and day hospital sessions arising from AF and associated conditions.
Hospitalizations were identified on the basis of a primary diagnosis of AF or of ischemic stroke in accordance with the ICD 9-CM.
The unit costs used in the analysis were taken from Order in Council 163/2013, which defines the prices associated with DRGs and other health interventions. The costs of AF- and stroke-related admissions were calculated by summing the product of the number of patients in each DRG and the price of the corresponding DRG.
Costs of outpatient careOutpatient costs include direct medical costs (consultations, emergencies, diagnostic and therapeutic interventions, drugs, physiotherapy sessions, etc.), and direct non-medical costs (urgent and non-urgent patient transportation and institutionalization).
It is considerably more difficult to estimate outpatient costs than inpatient costs with any precision, since there is no equivalent database on which to calculate the relevant resource use. Resource use was estimated on the basis of the literature and on the findings of a panel of experts from various medical specialties convened to define resource use in patients with AF and ischemic stroke.
Most of the resources identified were for AF patients both with and without ischemic stroke, and so simply adding them together would result in double counting. To avoid this error, the numbers of patients in each of these two groups were calculated by applying the PAF to the population with ischemic stroke (columns 6 and 7 in Table 1). The patterns of resource use for the two groups were thus determined separately and then multiplied by the corresponding number of patients.
In calculating outpatient resource use, it is important to consider only patients with diagnosed AF. According to the FAMA study,5 36% of cases of AF in patients aged 40 and over are undiagnosed, and so calculation of the outpatient resource use was based on the prevalence of diagnosed AF. However, to appreciate the wider picture, it should be borne in mind that the prevalence of diagnosed AF in Portugal is increasing, as in other European countries, probably due to aging populations and better diagnosis.30
Indirect costs of atrial fibrillationIn calculating the indirect costs of AF, only costs associated with lost production due to the disease were included (excluding losses due to premature death).
The first step in the calculation was to estimate how AF affects employment in different age-groups and by gender. The employment rates for the general population were based on data from the Portuguese Institute of Statistics for the second half of 2013. The daily monetary value corresponding to loss of production was calculated using a human capital approach, on the basis of average daily labor costs, including employers’ contributions to social insurance.34 To calculate the total costs at 2012 prices of lost productivity, the mean daily labor costs for 2012 were estimated on the basis of data for 2009 (the last year for which official figures are available from the Ministry of Labor and Social Security) and the mean annual increases in earnings between 2009 and 2012 from the Office for Strategy and Planning of the same Ministry. Since the aim was to calculate the cost of productivity lost per day due to AF, the average annual labor costs were divided by 230 working days, resulting in an estimate of €96.53 in 2012 for the age-groups under consideration, and the total indirect costs attributable to AF were estimated on this basis. Since earnings remained stable between 2012 and 2013, this figure was considered appropriate to represent indirect costs in 2013.
Total indirect costsThe absenteeism due to ischemic stroke attributable to AF must be taken into account as well as that of AF itself. Calculation of the indirect costs of stroke directly attributable to AF requires use of the PAF (Table 1). Absenteeism related to AF and ischemic stroke may have different reasons but given the nature of AF, it is only ischemic stroke that leads to prolonged absence from work due to physiotherapy sessions and/or other reasons.
ResultsBurden of diseaseDisability-adjusted life years due to deathThe first step in quantifying the burden of disease arising from mortality attributable to AF is to calculate the number of deaths and DALYs due to diseases associated with AF. Data on mortality from AF and stroke in Portugal were taken from the WHO's European Detailed Mortality Database (http://data.euro.who.int/dmdb/). Most deaths from stroke in this database are not specified as ischemic or hemorrhagic, so on the basis of the DRGs and the opinions of the expert panel, we estimated that 30% in women and 40% in men are hemorrhagic, and that in 2010 there were 813 deaths from AF (303 men and 510 women) and 9316 deaths from ischemic stroke.
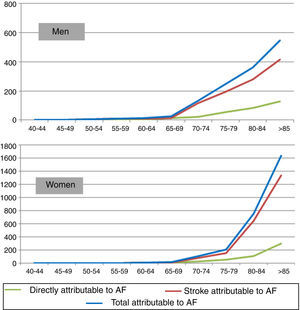
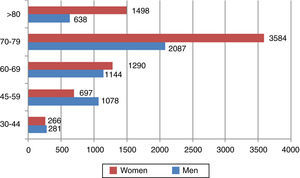
On the basis of these data and the standard life expectancy by gender and age-group, DALYs due to death were calculated. We estimate that in 2010 33753 DALYs were lost from deaths due to AF and stroke. Applying the PAF presented in Table 1 to the figures for mortality and DALYs from death due to stroke, the burden of stroke attributable to AF in Portugal in 2010 is estimated at 4070 deaths (3.8% of all deaths) and 10521 DALYs due to death (1.7% of all DALYs from premature death). Figure 1 shows the distribution of deaths for men and for women by age-group.
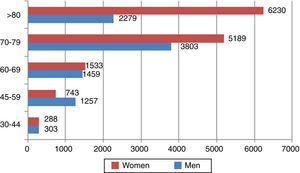
Figure 2 shows the distribution of DALYs due to death attributable to atrial fibrillation by age-group and gender. Stroke was responsible for the majority of life years lost due to premature death attributable to AF in both sexes.
It can also be seen in Figure 2 that the distribution of burden of disease at more advanced ages differs markedly between men and women, mainly due to differences in the number of men and women in older age-groups. Thus, there are more DALYs due to death attributable to AF per 100000 population in men up to the age of 79 (437 vs. 304 for women), but this pattern is reversed over the age of 80 (928 for men vs. 1446 for women).
Disability-adjusted life years due to disabilityThe severity weight for disability due to AF is 0.145. For stroke, two representative cases – moderately severe stroke with long-term sequelae (weight 0.076) and moderately severe stroke with long-term sequelae and cognitive deficit (weight 0.312) – were considered and the mean of the two (0.194) was adopted for this study.
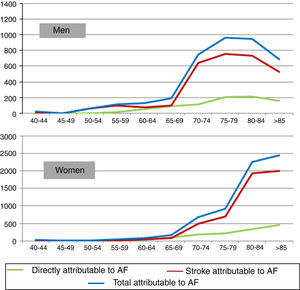
It is estimated that 14% of AF patients in Portugal have suffered stroke. Calculation of the severity weight in these patients entails adding to the mean weight for AF (0.145) a part of the corresponding disability weight for stroke. The mean RR of stroke for the population with AF is 3.71. The proportion of stroke attributable to AF is (RR-1)/RR, hence the fraction of strokes in AF patients attributable to AF itself is 73.05%. Considering that 14% of AF patients have suffered stroke, the mean disability weight of AF is (0.145+0.14*0.7305*(0.194−0.145))=0.15. On this basis, AF caused a loss of 12563 DALYs due to disability in 2010, over 5000 for men and over 7000 for women (Figure 3).
Adding DALYs due to premature death and due to disability gives a total burden of disease attributable to AF of 23084 DALYs (Figure 4), of which DALYs due to disability account for around 54%.
Cost of illnessDirect costsCosts of inpatient careThe DRG database was used to identify 6598 episodes with a primary diagnosis of AF and 20907 with a primary diagnosis of ischemic stroke, corresponding to 31 and 23 different DRGs, respectively. Table 2 summarizes the total costs of inpatient care attributable to AF. The PAF of ischemic stroke used in the cost analysis is a population-weighted mean of the PAFs in Table 2. The total cost of inpatient care (including outpatient DRGs) attributable to AF is €34503800. The data shown in Table 2 reveal that the cost of hospitalization for AF itself accounts for 55% of all costs attributable to AF, with ischemic stroke accounting for the remainder.
Costs of inpatient care attributable to atrial fibrillation.
| Disease | No. of episodes | Total cost (€) | PAF (%) | Attributable cost (€) |
|---|---|---|---|---|
| Inpatient DRGs | ||||
| AF | 6339 | 17569100 | 100 | 17569100 |
| Ischemic stroke | 20903 | 113310028 | 14 | 15523595 |
| Outpatient DRGs | ||||
| AF | 259 | 1410227 | 100 | 1410227 |
| Ischemic stroke | 4 | 6409 | 14 | 878 |
| Subtotal | ||||
| AF | 6598 | 18979327 | 100 | 18979327 |
| Ischemic stroke | 20907 | 113316437 | 14 | 15524473 |
| Total | 34503800 | |||
AF: atrial fibrillation; DRG: diagnosis-related group; PAF: population attributable fraction.
Based on the resource use estimated by the expert panel and on unit costs, the outpatient costs attributable to AF and ischemic stroke were calculated for the year of the diagnosis or event and following years (Table 3). The total cost of outpatient care attributable to AF is around €22 million in the year of the diagnosis or event and €59 million for patients diagnosed in previous years. The outpatient cost attributable to AF accounts for 45.2% of the total costs attributable to AF.
Outpatient costs attributable to atrial fibrillation.
| No. of patients | Total cost (€) | ||||
|---|---|---|---|---|---|
| Incidence-mortality | Prevalence-(incidence-mortality) | Year of diagnosis | Patients diagnosed in previous years | Total | |
| AF | 10960 | 72160 | 7140340 | 37229346 | 44369687 |
| Stroke | 2171 | 16097 | 15185149 | 21431089 | 36616238 |
| Total | 22325489 | 58660435 | 80985925 | ||
AF: atrial fibrillation.
The total direct costs attributable to AF are €115.5 million, made up of €34.5 million for inpatient care (Table 2) and €81 million for outpatient care (Table 3). Ischemic stroke is responsible for 45% of all direct costs attributable to AF.
Indirect costsIndirect costs of atrial fibrillation without strokeWhen estimating the indirect costs of AF without stroke it was assumed that the disease's impact is only in terms of absenteeism, without early retirement.
Table 4 shows the estimates of number of working days lost per year for each disease due to consultations, exams, hospitalizations and convalescence. Length of hospital stay was estimated on the basis of DRGs. It was assumed that convalescence time for AF was equal to length of hospitalization. The employment rate of AF patients (low given the fact that many are past retirement age) was taken into account when calculating mean costs.
Indirect costs attributable to atrial fibrillation.
| Consultations | Exams | Hospitalization and convalescence | |||
|---|---|---|---|---|---|
| Year of diagnosis | Patients diagnosed in previous years | Year of diagnosis | Patients diagnosed in previous years | Year of diagnosis | |
| No. of working days lost | 2.17 | 1.66 | 1.68 | 1.3 | 6.5 |
| Cost per patient (€) | 52 | 40 | 40 | 31 | 60 |
| Total annual indirect costs (€) | 571335 | 2876961 | 436026 | 2227050 | 661444 |
| Total (€) | 6772816 | ||||
AF: atrial fibrillation.
The costs of consultations and exams for patients in the first year of diagnosis were calculated by multiplying the mean costs in the first year by the number of patients in the first year of diagnosis (incidence), while the costs for patients diagnosed in previous years were obtained by multiplying the mean costs in subsequent years by the prevalence (minus incidence). Finally, the total indirect costs arising from hospitalizations due to AF were calculated by multiplying the indirect costs of days spent in hospital and convalescing by the number of admissions. Adding these estimates together, we arrive at a total indirect cost of AF without stroke of €6.77 million.
Indirect costs of atrial fibrillation with strokeEstimating the indirect costs of AF with stroke is more complicated, since there are different subgroups of patients depending on the consequences of the stroke and the need for rehabilitation after the event. The findings of the expert panel were used to identify possible scenarios following stroke and the proportion of patients in each scenario, each of which involves different levels of absenteeism resulting from physiotherapy sessions and differences in ability to return to work. Table 5 summarizes the possible scenarios, the number of patients in each scenario, and the indirect costs per patient with AF and ischemic stroke for the population initially employed.
Indirect costs of ischemic stroke.
| Percentage of patients | No. of patients | Scenario | Months of absenteeism | Costs per patient (€) | |
|---|---|---|---|---|---|
| Consultations and exams | Physiotherapy sessions | ||||
| Year of diagnosis | |||||
| 13.08 | 270 | No further care required after stay in stroke unit | 0 | 94 | 0 |
| 11.79 | 244 | Unable to return to work following discharge | 12 | 0 | 5518 |
| 52.01 | 1075 | Six months of physiotherapy following discharge | 6 | 47 | 2759 |
| 22.29 | 461 | 12 months of physiotherapy following discharge | 9 | 23 | 4139 |
| 0.11 | 2 | Only admitted for rehabilitation | 1.5 | 82 | 690 |
| 0.36 | 7 | Stay in convalescent unit following stay in stroke unit (mean 44 days) | 1.47 | 82 | 674 |
| 0.36 | 7 | Stay in medium-term rehabilitation unit following discharge (mean 92 days) | 3.1 | 70 | 1410 |
| Diagnosed in previous years | |||||
| 11.76 | 1905 | No return to work | 12 | 0 | 5518 |
| 23.47 | 1268a | Chronic rehabilitation for three months | 1.57 | 49 | 720 |
| 64.77 | 13028 | Able to return to work | 0 | 56 | 0 |
According to the expert panel, 12% of patients who suffer stroke attributable to AF are unable to return to work, 52% need six months of physiotherapy after hospitalization and 22% need 12 months, leading to absenteeism of six and nine months, respectively, assuming that after six months of physiotherapy the patient loses half a working day for each physiotherapy session for the following six months.
The expert panel also calculated that only 0.83% of patients with stroke attributable to AF are unable to work for a period of less than three months. As for those with AF-related stroke AF in previous years, 12% have still not returned to work, while 23% are absent from work for the equivalent of one and a half months a year.
The estimates in Table 6 show that the indirect costs of ischemic stroke attributable to AF amount to almost €20 million. Thus, considering all aspects of the indirect costs of ischemic stroke, the total indirect costs attributable to AF are calculated to be €25 million.
Indirect costs of stroke attributable to atrial fibrillation.
| Consultations | Exams | Rehabilitation | Hospitalization and convalescence | ||||
|---|---|---|---|---|---|---|---|
| Year of diagnosis | Patients diagnosed in previous years | Year of diagnosis | Patients diagnosed in previous years | Year of diagnosis | Patients diagnosed in previous years | Year of diagnosis | |
| No. of working days lost | 2.74 | 1.56 | 1.17 | 0.8 | 22.6 | ||
| Cost per patient (€) | 66 | 37 | 28 | 19 | 542 | ||
| Total annual indirect costs (€) | 64656 | 171192 | 27593 | 86559 | 6549699 | 11352054 | 153999 |
| Total (€) | 18405752 | ||||||
AF: atrial fibrillation.
AF is the most common sustained arrhythmia in Portugal. According to the FAMA study, its prevalence in 2009 was 2.5% in the population aged 40 and over and more than 10% in those aged 80 and over; only 60% of cases were diagnosed. As in other countries, the prevalence of AF in Portugal is increasing, probably due to aging populations and better diagnosis. As an example of this trend, there were 4678 admissions with a primary diagnosis of AF in NHS hospitals in Portugal in 2008, while the corresponding figure for 2012 was 6765, an increase of 45%.
The main purpose of this study is to provide an estimate of the burden of disease in terms of DALYs and costs to society. The results are a clear statement of the seriousness of AF in Portugal. Stroke is highly disabling and frequently results in early retirement; as well as the costs involved, the burden of AF reflects the fact that stroke in AF patients is particularly lethal and, for those who survive, disabling.
For methodological reasons, the figures presented in this study underestimate the cost and burden of the disease, since they do not consider bleeding episodes, including intracranial hemorrhage, a major complication of the anticoagulant therapy used as prophylaxis against stroke in AF patients.
ConclusionsThis analysis shows that 4070 deaths can be attributed to AF in 2010 in Portugal, corresponding to 3.8% of all deaths, and that the total burden of disease attributable to AF is 23084 DALYs. The overall cost of illness is estimated at €140.7 million, around 0.08% of Portugal's gross domestic product. These figures confirm the importance of AF, but at the same time they make it clear that this is an area in which significant health gains can be made.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThis study received funding from Bristol-Myers Squibb Farmacêutica Portuguesa, SA and Laboratórios Pfizer Lda through an unrestricted grant to the Research & Development Association of Lisbon University School of Medicine (AIDFM). Bristol-Myers Squibb and Pfizer were not involved in any stage of the project.
Please cite this article as: Gouveia M, Costa J, Alarcão J, Augusto M, Caldeira D, Pinheiro L, et al. Carga e custo da fibrilhação auricular em Portugal. Rev Port Cardiol. 2014. http://dx.doi.org/10.1016/j.repc.2014.08.005