Sudden cardiac death is the leading cause of death in athletes during sport. It is a tragic event that generates significant media attention and discussion throughout society as to whether everything possible had been done to prevent it. Regular physical exercise causes cardiac remodeling at both the mechanical and electrical level, known as athlete's heart, resulting in an electrocardiogram (ECG) considered abnormal compared with the ECGs of the general population. Some of these electrocardiographic changes are considered normal or physiological in athletes, while others suggest underlying cardiac disease with the potential to cause sudden cardiac death. There is thus an urgent need to define the electrocardiographic patterns that allow or prohibit participation in sports, and to differentiate them in terms of gender, ethnicity and age. The purpose of this review is to present the latest data on the electrocardiographic changes considered benign or pathological that are typically found in athletes and to critically analyze the most recent criteria for classifying ECGs in this population (the Seattle criteria), comparing them with previous guidelines and with the latest studies on the subject. This article also examines the question of including ECGs in preparticipation screening programs, the US and European approaches to the subject, and the most up-to-date data on the sensitivity, specificity and cost-effectiveness of the ECG in athletes.
A morte súbita cardíaca é a principal causa de morte em atletas durante a prática desportiva. É um evento trágico, com grande impacto nos média, gerando discussão dentro da comunidade no intuito de perceber se tudo foi feito para o evitar. A prática regular de exercício físico causa uma remodelagem cardíaca, tanto a nível mecânico como elétrico, conhecida como «coração de atleta», que se repercute num eletrocardiograma considerado «anormal» quando comparado com o da população geral. Algumas destas alterações do eletrocardiograma são consideradas normais/fisiológicas em atletas, enquanto outras traduzem, efetivamente, doença cardíaca de base, com potencial de causar morte súbita cardíaca. Assim, urge definir quais os padrões eletrocardiográficos que «permitem» ou, por outro lado, «proíbem» a prática desportiva, diferenciando-os em função do género, etnia e idade. Esta revisão pretende reunir a informação mais atual sobre as alterações eletrocardiográficas consideradas benignas ou patológicas encontradas tipicamente em atletas e analisar, de forma crítica, os critérios mais recentes para a classificação do eletrocardiograma nesta população (os Critérios de Seattle), comparando-os com as guidelines anteriores e com os estudos mais recentes sobre o tema. É também objetivo desta revisão dar a conhecer a problemática da inclusão do eletrocardiograma no programa de rastreio pré-desportivo, as perspetivas americana e europeia, e os dados mais recentes sobre a sensibilidade, especificidade e custo-efetividade do uso do eletrocardiograma em atletas.
American Heart Association
accessory pathway
arrhythmogenic right ventricular dysplasia
atrioventricular
Brugada syndrome
dilated cardiomyopathy
electrocardiogram, electrocardiographic
European Society of Cardiology
hypertrophic cardiomyopathy
implantable cardioverter-defibrillator
long QT syndrome
left ventricular
left ventricular hypertrophy
sudden cardiac death
short QT syndrome
sudden unexplained death
ventricular fibrillation
ventricular tachycardia
Wolff-Parkinson-White
Sudden cardiac death (SCD) in an athlete is a tragic event that generates significant media attention and discussion throughout society as to whether everything possible had been done to prevent it.
An athlete has been defined as an individual engaged in regular physical training and participating in official sports competition with an emphasis on excellence and achievement.1 Physical exercise is a very important component of primary and secondary cardiovascular prevention, and the 2012 European Society of Cardiology (ESC) guidelines on cardiovascular disease prevention recommend 2.5–5 hours/week of moderate-intensity physical activity or 1–1.5 hours/week of vigorous-intensity exercise, the benefits being greater with more hours of exercise.2
SCD is the leading cause of death (75–85%)3,4 in athletes during sports.5 The risk of cardiovascular death or isolated coronary artery disease is significantly lower in physically active and fit individuals, but the risk of sudden death increases 2–4.5 fold during high-intensity exercise.1,6–8
The purpose of this review is to present the latest data on the electrocardiographic changes found in athletes related to cardiac remodeling that is considered physiological or related to underlying heart disease, the latter being associated with SCD.
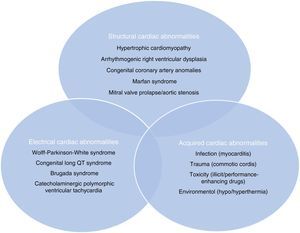
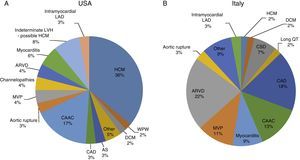
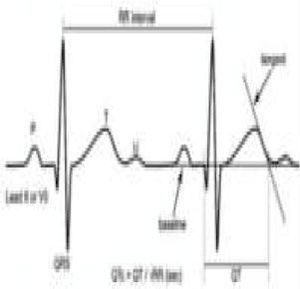
Sudden cardiac death and electrocardiographic screeningSCD is defined as unexpected, non-traumatic cardiac death within one hour of symptom onset in an individual with no known potentially lethal cardiovascular condition. It may be due to various causes, which are conventionally divided into congenital abnormalities (structural or electrical), which are more common in individuals aged under 35, and acquired abnormalities, which are more frequent after the age of 351,3,6,9,10 (Figure 1). The main causes of SCD vary depending on the athlete's age and geographical region, due to genetic and environmental factors; for example, in the USA the most common cause of SCD in individuals aged under 35 is hypertrophic cardiomyopathy (HCM), followed by congenital coronary artery anomalies,1,11,12 while in Italy arrhythmogenic right ventricular dysplasia (ARVD) is the most prevalent cause (Figure 2).13 In athletes aged over 35, the leading cause of SCD is atherosclerotic coronary artery disease.14
Structural, electrical and acquired abnormalities associated with sudden cardiac death in athletes. Adapted from Chandra et al.1
Causes of sudden cardiac death in athletes in the USA and Italy. (A) Distribution of cardiovascular causes of sudden death in 1435 young competitive athletes. From the Minneapolis Heart Institute Foundation Registry, 1980–2005 (adapted from Maron et al.12,20). (B) Causes of sudden death in athletes in the Veneto region, Italy, 1979–1999 (adapted from Corrado et al.13). ARVD: arrhythmogenic right ventricular dysplasia; AS: aortic stenosis; CAAC: congenital coronary artery anomalies; CAD: coronary artery disease; CSD: conduction system disease; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; LAD: left anterior descending artery; LVH: left ventricular hypertrophy; MVP: mitral valve prolapse; WPW: Wolff-Parkinson-White syndrome.
There is no consensus on how to combat SCD, mainly because of a lack of evidence of its true incidence either in the general population or in athletes or of the frequency of the different causes; there are also few data on the sensitivity and specificity of the electrocardiogram (ECG) for detecting pathological cases. This lack of data, together with the rarity of the event, makes use of the ECG in preparticipation screening difficult to implement, since the process must be cost-effective.
Studies on the incidence of SCD report varying figures (Table 1), mainly due to different methods for collecting information on deaths; in some cases this is limited to media reports or insurance claims, while in others the source is official government records.3,15 A prospective observational study in Italy, where preparticipation ECG screening and reporting of SCD are obligatory, revealed an incidence of 3.6/100000 athletes, a similar figure to that reported in more recent studies in the USA.3,16–18
Incidence of sudden death stratified by athletic or general population and the years of the study.
| Country | Lead author | Population | Years | Incidence per 100000 person-years |
|---|---|---|---|---|
| Italy | Corrado | Athletes | 1980–1981 | 3.6 |
| Italy | Corrado | Athletes | 2007–2008 | 0.4 |
| Israel | Steinvil | Athletes | 1985–1997 | 2.54 |
| Israel | Steinvil | Athletes | 1998–2009 | 2.66 |
| USA | VanCamp | Athletes | 1983–1993 | 0.33 |
| USA | Maron | Athletes | 1985–2006 | 0.44 |
| Denmark | Holst | Athletes | 2000–2006 | 1.21 |
| Denmark | Holst | Children | 2000–2006 | 3.76 |
| Japan | Tanaka | Children | 1989–1997 | 1.32 |
| USA | Eckart | Military recruits | 1997–2001 | 13 |
| USA and Canada | Atkins | All children ages 1–11 | 2005–2007 | 3.73 |
| USA and Canada | Atkins | All children ages 12–19 | 2005–2007 | 6.37 |
Adapted from Link and Estes.71
On the basis of such studies, Italy began to include an ECG in preparticipation screening as well as physical examination and clinical history, resulting in a decrease of 89% in SCD in athletes between 1979 and 2004.9,18,19 In the USA, the recommendations of the American Heart Association (AHA) for preparticipation cardiovascular screening of competitive athletes include only medical history and physical examination, citing the high number of false positives and low cost-effectiveness of large-scale ECG screening programs. The ESC, on the other hand, while not stipulating that ECG assessment is mandatory, recommends it in all athletes (Table 2).
American Heart Association recommendations for preparticipation cardiovascular screening of competitive athletes.
| Personal history |
| (1) Exertional chest pain/discomfort |
| (2) Unexplained syncope/near-syncope |
| (3) Excessive exertional and unexplained dyspnea/fatigue, associated with exercise |
| (4) Prior recognition of a heart murmur |
| (5) Elevated systemic blood pressure |
| Family history |
| (6) Premature death (sudden and unexpected, or otherwise) before age 50 years due to heart disease, in at least one relative |
| (7) Disability from heart disease in a close relative <50 years of age |
| (8) Specific knowledge of certain cardiac conditions in family members: HCM or dilated cardiomyopathy (DCM), long QT syndrome (LQTS) or other channelopathies, Marfan syndrome, or clinically important arrhythmias |
| Physical examination |
| (9) Heart murmur |
| (10) Femoral pulses to exclude aortic coarctation |
| (11) Physical stigmata of Marfan syndrome |
| (12) Brachial artery blood pressure (sitting position) |
HCM: hypertrophic cardiomyopathy.
Adapted from Maron et al.20
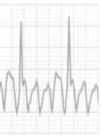
In 60%–80% of athletes the ECG shows changes from what is considered normal,5,21 due to cardiac remodeling, both mechanical and electrical, known as athlete's heart, which gives rise to the large number of false positives in some studies. This phenomenon results from physiological adaptation of the autonomic nervous system, with increased vagal tone and decreased sympathetic tone, and changes in cardiac dimensions, including increased left ventricular (LV) wall thickness and LV cavity size, which may be reflected on ECG and echocardiography. This remodeling permits enhanced filling of the left ventricle in diastole and augmentation of stroke volume, allowing generation of a large and sustained cardiac output even at rapid heart rates.1,21Table 3 summarizes the ECG alterations considered normal in athletes.10 Various studies have also shown that there is considerable variability in ECG patterns between genders, ages, ethnicities and types of sport.4,21–30
Normal electrocardiographic findings in athletes.
| 1. Sinus bradycardia (≥30 bpm) |
| 2. Sinus arrhythmia |
| 3. Ectopic atrial rhythm |
| 4. Junctional escape rhythm |
| 5. 1st-degree atrioventricular (AV) block (PR interval >200 ms) |
| 6. Mobitz type I (Wenckebach) 2nd-degree AV block |
| 7. Incomplete RBBB |
| 8. Isolated QRS voltage criteria for LVH |
| Except: QRS voltage criteria for LVH occurring with any non-voltage criteria for LVH such as left atrial enlargement, left axis deviation, ST-segment depression, T-wave inversion or pathological Q waves |
| 9. Early repolarization (ST elevation, J-point elevation, J-waves or terminal QRS slurring) |
| 10. Convex (‘domed’) ST-segment elevation combined with T-wave inversion in leads V1–V4 in black/African athletes. |
| These training-related ECG alterations are physiological adaptations to regular exercise, considered normal variants in athletes and do not require further evaluation in asymptomatic athletes. |
Adapted from Drezner et al.10
The guidelines take into consideration the bias due to athlete's heart in order to improve the accuracy of screening. Implementation of the 2010 European guidelines on ECGs in athletes, rather than those on athletes with cardiovascular disease of 2005, resulted in a reduction of 43%–59% in the number of ECGs classified as abnormal,32,33 with a parallel reduction in the number of false positives (from 16%–17% to around 10%).34,35 The report on the ‘Seattle Summit’,10 published at the end of 2012, as well as updating the criteria for classifying abnormal ECGs in athletes, highlights the concerns of some that physicians may lack experience in interpreting ECGs in athletes and that this hampers its use for preparticipation screening. Investment in medical training and the use of aids to interpretation, with standardized criteria for athletes, have been proposed as a way to improve this situation. Drezner et al. compared the accuracy of ECG interpretation among different physician specialties: 16 primary care specialists, 22 primary care residents, 12 sports medicine physicians and 10 cardiologists, first without and then with an ECG criteria tool based on the ESC consensus statement for interpretation of the 12-lead ECG in athletes, and observed an improvement in sensitivity from 70% to 91% and in specificity from 89% to 94% across all the different medical specialties.10,36
Electrocardiographic patternsThe ECG changes in those who undertake regular and intense exercise may be the result of physiological cardiac remodeling, and thus do not require further investigation, or may indicate an increased likelihood of SCD and thus require a thorough diagnostic workup that in most cases will result in disqualification from participation in competitive sports and treatment to prevent a fatal event. In the ESC guidelines these alterations are divided into two groups depending on whether they are training-related.31
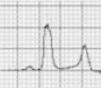
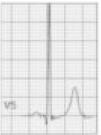
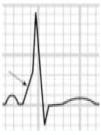
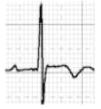
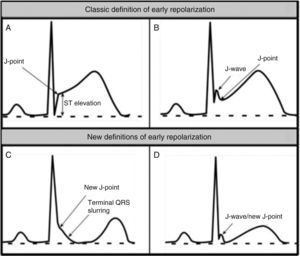
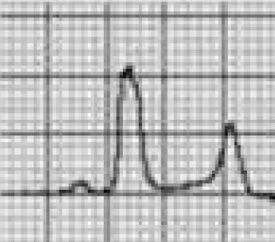
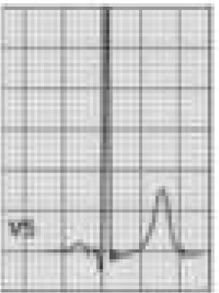
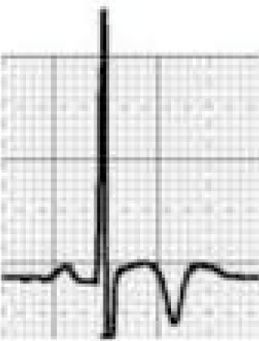
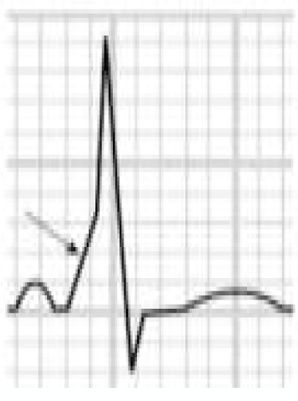
Benign training-related electrocardiographic changesFigures 3 and 4 and Table 3 show the ECG patterns associated with athlete's heart. Sinus bradycardia, defined as heart rate <60 bpm, is found in up to 80% of trained athletes, and in highly trained athletes, marked bradycardia (down to 30 bpm) is considered normal; sinus arrhythmia is also common (>50% of athletes, particularly young ones).21 Traditional examples of early repolarization referred to ST-segment elevation of >1 mV only, but newer definitions also include J waves or terminal QRS slurring, most commonly seen in the precordial leads (Figure 4) and are present in up to 45% of Caucasian athletes and 63%–91% of Afro-Caribbean athletes; 13%–25% of black athletes present J-point elevation with a domed ST segment in leads V1–V4, followed by T-wave inversion in the same leads and do not require further assessment in the absence of symptoms, positive family history or abnormal physical examination.5,21
Electrocardiographic patterns associated with athlete's heart. AV: atrioventricular; HR: heart rate; LV: left ventricular; RBBB: right bundle branch block. Adapted from Drezner et al.5
(A and B) Classic definition of early repolarization based on ST elevation at QRS end (J-point); examples without (A) and with (B) a J-wave; (C and D) new definitions of early repolarization showing slurred QRS downstroke and new J-point (C) and J-wave (D) without ST elevation. Adapted from Drezner et al.5
The most commonly used criterion for left ventricular hypertrophy (LVH), that of Sokolow-Lyon (SV1 + [RV5 or V6] >3.5 mV), is found in 45% of male and 10% of female athletes. The isolated presence of this criterion is no longer regarded as abnormal in athletes, since it almost always reflects increases in cardiac chamber size and/or wall thickness that are a physiological response to regular exercise, and its measurement is unreliable.5 However, the physician should ensure that there are no additional non-voltage criteria for LVH such as left atrial enlargement, left axis deviation, ST-segment depression, T-wave inversion or pathological Q waves that could indicate HCM or another condition.37
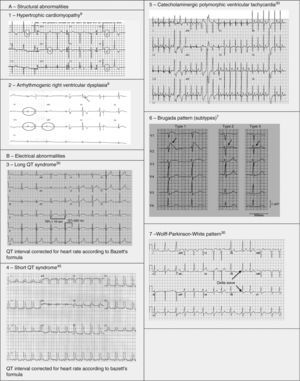
Pathological non-training-related electrocardiographic changesTable 4 presents the criteria for pathological ECG alterations for various cardiac conditions that may be detected in preparticipation screening, while Figure 5 shows some ECG traces illustrating cardiac abnormalities that have been identified as associated with SCD. Since most of these conditions, such as channelopathies and HCM, are diagnosed by ECG, the physician must be familiar with the typical ECG patterns for each disease in order to improve the diagnostic accuracy of preparticipation screening, particularly in the age-groups most likely to be affected.
Electrocardiographic criteria for pathological alterations in athletes that require further diagnostic investigation.
| ECG alterations | Criteria for further assessment | Illustration | ECG alterations | Criteria for further assessment | Illustration |
|---|---|---|---|---|---|
| LBBB, RBBB, and IVCD | QRS >120 ms | ||||
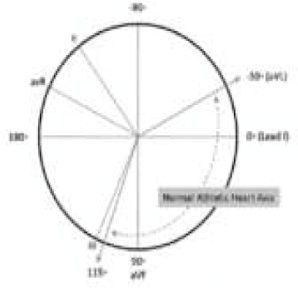
| Q waves | >3 mm in depth and/or 40 ms duration in any lead except III, aVR, aVL and V1 | QRS axis deviation | Further left than –30°, further right than +115° | ||
| ST-segment depression | >0.5 mm below the PR isoelectric line between the J-junction and beginning of the T wave in V4, V5, V6, I, aVL >1 mm in any lead | QTc abnormalities | >470 ms in men >480 ms in women <340 in any athlete | ||
| T-wave inversion | ≥1 mm in leads other than III, aVR, and V1/2 (except V2 and V3 in women aged <25 years) | BrS | BrS type 1: coved-type ST segment in V1 and V2 that gradually descends into an inverted T wave | ||
| Atrial abnormalities | Right: P-wave amplitude >2.5 mm Left: (i) a negative component of the P wave in V1 or V2 of 40 ms duration and 1 mm amplitude; (ii) total P-wave duration of >120 ms | Ventricular preexcitation | Delta wave and PR interval <120 ms | ||
| Right ventricular hypertrophy | Age >30 years: (i) R >7 mm in V1; or (ii) R/S >1 in V1; or (iii) sum of R in V1 and S in V5 or V6 >10.5 mm Age <30 years: the above plus right atrial abnormalities, T-wave inversion in V2/3, and/or right-axis deviation >115° | VES, cardiac block and supraventricular arrhythmia | Atrial fibrillation/flutter, supraventricular tachycardia, complete heart block, or ≥2 VES on a 12-lead ECG | ||
BrS: Brugada syndrome; ECG: electrocardiographic/electrocardiogram; IVCD: intraventricular conduction delay; LBBB: left bundle branch block; RBBB: right bundle branch block; VES: ventricular extrasystoles.
Adapted from Uberoi et al.39
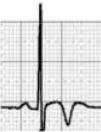
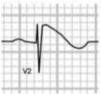
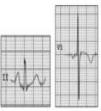
HCM is mainly a hereditary disease, typically of autosomal dominant inheritance, with a prevalence of 0.2% in the general population and 0.07%–0.08% in athletes.1 It is the leading cause of SCD in athletes, and is responsible for 11%–36% of cases.3,15,37,38 The disease affects the cardiac muscle, leading to thickening of a portion of the myocardium in the absence of a recognizable cause. A common pattern is asymmetrical septal hypertrophy, in which there is poor ventricular compliance along with microvascular dysfunction, which can result in ischemia during exertion. It is a clinically and genetically heterogeneous disease, but over 90% of patients present ECG alterations at rest,37 even though more than 80% are asymptomatic until a fatal event.16 SCD in HCM is a consequence of myocardial disarray and the presence of electrically unstable fibrotic areas that can lead to ventricular tachycardia (VT) and ventricular fibrillation (VF)37 (Figure 5A-1).
Arrhythmogenic right ventricular dysplasiaARVD has a prevalence of 1:2000–1:5000 in the general population, but figures for its prevalence in athletes vary widely in published studies, ranging from 4% in the USA to 22% in Italy.3,15,16 It is a mainly inherited disease that affects the myocardium, due to mutations in genes coding for the desmosome proteins that are essential to intercalated discs and thus to the structure of cardiomyocytes. The resulting structural changes give rise to reentrant circuits which predispose to sustained monomorphic VT and SCD.39,40 Phenotypically ARVD is characterized by aneurysmal dilatation of the right ventricle, which usually results in right ventricular dysfunction and histological alterations such as fibrofatty replacement of the myocardium, necrosis, apoptosis, and inflammation. The definition of this entity has widened with the recent recognition of biventricular and predominantly left ventricular variants; the mainly right ventricular location is due to the greater shear force on myocytes due to the thinner ventricular wall on the right side, but genetic alterations, particularly in desmoplakins, can result in a left ventricular phenotype. The risk of SCD due to ARVD increases five-fold during competitive sports compared to sedentary activity, and 80% of patients present resting ECG changes (Figure 5A-2).1,37,41
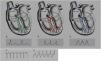
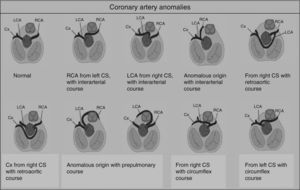
Congenital coronary artery anomaliesCongenital coronary artery anomalies are the other main cause of SCD in athletes aged under 35 years, accounting for 12%–33% of cases.1,15,16,42,43 These anomalies involve the site of implantation of the coronary arteries in the sinuses of Valsalva, the most frequent being the circumflex artery arising from the right sinus or from the right coronary artery.42 The functional repercussions depend mainly on the course of the artery (Figure 6): interarterial (between the aortic root and the pulmonary artery root), retroaortic, or prepulmonary. The course that represents the highest risk of ischemia is interarterial, due to the risk of compression, particularly during exertion.44 In such cases, SCD results from ventricular arrhythmia caused by myocardial ischemia occurring during exercise. Although an episode of syncope associated with chest pain may raise suspicions, death is usually the form of presentation of these anomalies, which are extremely difficult to diagnose using conventional methods such as ECG, echocardiography or exercise testing. The gold standard is cardiac magnetic resonance imaging or computed tomography coronary angiography.1,42,43
Other structural abnormalities with less impact include DCM, aortic dissection or rupture due to Marfan syndrome (which accounts for 3% of SCD1,16), mitral valve prolapse, and aortic stenosis due to congenital bicuspid aortic valve.1,38
Electrical cardiac abnormalitiesElectrical cardiac abnormalities are the most frequent cause of sudden unexplained death (SUD) on autopsy studies, particularly in children and young adults. Postmortem genetic testing indicates that channelopathies caused by mutations in ion channels are responsible for 25%–35% of SUD.16,45 Inherited cardiac arrhythmias and conduction disorders that can cause SCD include the following:
- (1)
LQTS and short QT syndrome (SQTS) are inherited or acquired channelopathies, often associated with the use of drugs including antihistamines, antibiotics, antifungals, and antipsychotics, in which ventricular repolarization alterations lead to life-threatening polymorphic ventricular arrhythmias such as torsade de pointes, caused by abnormalities in K+, Na+ and Ca2+ ion channels. While SQTS is rare, affecting 1:10000 individuals, the prevalence of LQTS is relatively high (1:2000) and may even be underestimated due to changes in the definition of a ‘normal’ QT interval. Diagnosis is based on a combination of clinical and family history, ECG findings and genetic tests. A total of 13 genes have been identified that are involved in the condition, affecting Na+ or K+ channels in 95% of cases.43,45 LQTS is known to be responsible for 15%–20% cases of autopsy-negative sudden death45,46 (Figure 5B-3 and B-4).
- (2)
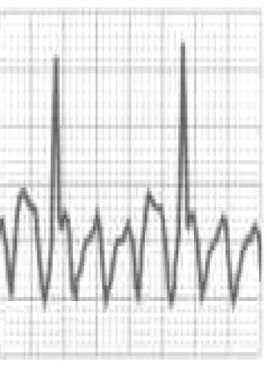
Catecholaminergic polymorphic ventricular tachycardia is a rhythm disorder originating from a ventricular ectopic focus that is triggered by exercise or emotional stress, and generally appears between the ages of 7 and 9. Patients usually have structurally normal hearts; the condition is caused by mutations in the genes coding for ryanodine receptors and calsequestrin, which regulate Ca+ channels, and predisposes to syncope, VF and SCD. The ECG is normal at rest but shows abnormalities during exercise testing, including adrenergic-mediated ventricular tachycardia as well as monomorphic extrasystoles that progress to bidirectional and polymorphic VT.1,45,46 Besides prohibiting sports, treatment consists of arrhythmia control with beta-blockers, verapamil or flecainide and an implantable cardioverter-defibrillator (ICD)47 (Figure 5B-5).
- (3)
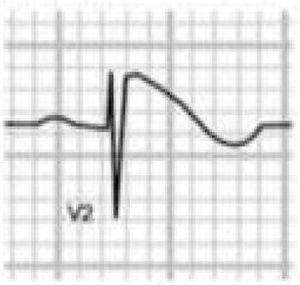
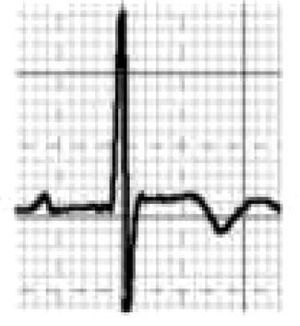
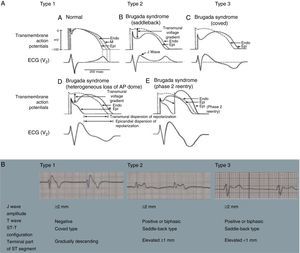
Brugada syndrome (BrS) is a genetic disease with autosomal dominant transmission and incomplete penetrance, of which 20%–25% of cases (and around 40% of cases with prolonged PR interval) are caused by mutations in the SCN5A gene affecting Na+ channels.45,46
BrS involves an imbalance between the positive currents (Na+ and K+) in phase 1 of the action potential (Figure 7A). This imbalance causes early repolarization, accentuating the notch caused by the transient K+ outflow,48 which leads to a paradoxical prolongation of the action potential dome in epicardial cells due to the delay in its formation.49 This causes the epicardium to repolarize after the endocardium, resulting in T-wave inversion and producing the characteristic coved ECG. However, if the epicardium still repolarizes before the endocardium, the T wave remains positive (saddleback ECG).50
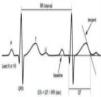
Figure 7.(A) Schematic representation of right ventricular epicardial action potential changes proposed to underlie the electrocardiographic manifestations of Brugada syndrome (adapted from Antzelevitch et al.49); (B) The three electrocardiographic patterns associated with Brugada syndrome: type 1 – coved ST-segment elevation of ≥2 mm followed by a negative T wave with little or no isoelectric separation in one or more right precordial leads (V1–V3); type 2 – ST-segment elevation followed by a positive or biphasic T wave, resulting in a saddleback configuration; and type 3 – ST-segment elevation in the right precordial leads of <1 mm, coved or saddleback configuration; diagnostic criteria for Brugada syndrome (adapted from Sheikh and Ranjan51).
BrS is estimated to cause 5%–20% of autopsy-negative SUD. Death usually occurs at rest due to vagal hyperactivity induced by prolonged training. Diagnosis is by ECG, which most often shows a type 1 pattern (Figure 7B), but can be difficult because the alterations are often intermittent and provocative testing with sodium channel blockers may be necessary to trigger the type 1 pattern. The only treatment to date is an ICD1,49,51–54 (Figure 5B-6).
- (4)
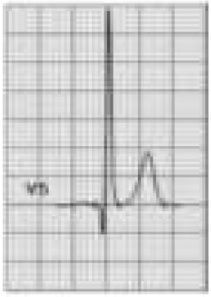
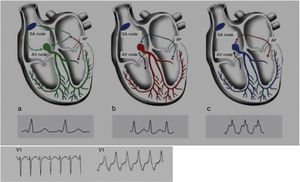
Wolff-Parkinson-White syndrome (WPW) has a prevalence of 0.1%–0.3% both in the general population and in athletes. It consists of ventricular pre-excitation through an anomalous AV accessory pathway (AP) that bypasses the separation of cardiac conduction between the atria and the ventricles. In sinus rhythm, the electrical impulse can pass through the AV node or through the AP, which is faster and thus gives rise to AV reentrant tachycardia. This is the most common tachyarrhythmia in WPW patients, accounting for 95% of all reentrant tachycardias, but around a third of these individuals have atrial fibrillation, which can degenerate into ventricular fibrillation, causing SCD. Figure 8 shows the pathophysiological mechanism of two variants of reentrant tachycardia that can occur in WPW: orthodromic and antidromic. WPW is diagnosed by ECG, which shows a delta wave, a short PR interval, prolonged QRS and, in some cases, repolarization abnormalities. Most individuals are asymptomatic (WPW pattern), while others (WPW syndrome) may experience palpitations, syncope and/or arrhythmias, with or without hemodynamic compromise, or even SCD. Athletes with WPW should undergo exercise testing to stratify SCD risk and to guide treatment. Intermittent pre-excitation during sinus rhythm, or complete and sudden disappearance of pre-excitation on exercise testing, are considered low risk, while high risk is defined as an RR interval <250 ms (heart rate 240 bpm) following induced fibrillation. The current guidelines state that only high-risk athletes should be treated, by catheter ablation of the AP, and can return to sports after three months1,45,55,56 (Figure 5B-7).
Figure 8.Pathophysiological mechanisms of Wolff-Parkinson-White syndrome (WPW). (a) Sinus rhythm with preexcitation of the WPW type: antegrade conduction via the atrioventricular (AV) node (green) and the accessory pathway (AP) (red). Rapid conduction over the AP leads to early repolarization, which correlates to the delta wave; (b) orthodromic reentrant tachycardia: antegrade conduction over the AP is blocked due to its longer refractoriness. Conduction to the ventricles occurs only via the AV node (black) and reaches the AP when it is able to conduct back to the atria (blue); (c) antidromic reentrant tachycardia: the AV node reaches refractoriness before the AP, and so antegrade conduction occurs only via the AP (red) and reaches the AV node when it is able to conduct back to the atria (black). Adapted from Estner et al.73
Despite some disagreement concerning its usefulness, the ECG is a well-established tool for assessment of athletes, providing valuable diagnostic and prognostic information on a wide range of cardiovascular conditions that are known to be associated with increased risk of SCD during sport. It is thus important to follow internationally standardized criteria that will enable ECG changes to be classified as normal or physiological or pathological. The Seattle criteria10 are the most recent, based on current knowledge, and provide clear guidelines on interpreting and classifying ECGs and relating them to the decision to allow or prohibit participation in sports. They are designed to provide a new tool for physicians to distinguish ECG patterns resulting from normal physiological adaptations in athletes from potentially fatal alterations.10 One of the main objectives is to reduce the number of false positives, the major limitation of previous guidelines, and comparative studies indicate that the number of ECG classified as abnormal is reduced by almost 80% when the Seattle criteria are used, falling from 26%–29.3% with the European criteria to 6%–11%.57,58 Asif et al. obtained a false positive rate of 1.7% on ECGs interpreted using the Seattle criteria,59 and this figure could have been improved still further by excluding certain ECG alterations that are still deemed abnormal even when found in isolation, such as left axis deviation and left atrial enlargement.60,61
The main limitation of the Seattle criteria is their lack of external validity, since there is considerable variability in ECG findings between genders, ethnicities and ages, whereas almost all studies in this area have been carried out in white males aged between 14 and 35 years. There is therefore a need for physicians to gain experience in interpreting ECGs in a wider range of contexts and for comparative studies in these different subgroups, so that the guidelines can include recommendations that are specific to particular subpopulations.
Female athletesEarly studies estimated the incidence of SCD in female athletes at 0–1:1300000. This figure was subsequently challenged by Harmon et al., who put it at 1:77000, although this was still 2.3 times less common than in male athletes.62 This difference has been explained by the fact that male gender is an independent risk factor for SCD, not only due to coronary artery disease.12 Most studies of female athletes focus on gender differences in repolarization alterations, which are considerably less common in women (5.9% vs. 21%), with statistically significant differences in the prevalence of J-point elevation (21.5% vs. 35%) and of ST elevation (0.5% vs. 3.3%).63 Rollin et al. demonstrated that although repolarization abnormalities are less prevalent in women, an early repolarization pattern is a risk factor for all-cause and cardiovascular mortality, with hazard ratios of 4.77 and 7.11, respectively.64
Afro-Caribbean athletesThe Seattle criteria were the first guidelines to mention Afro-Caribbean individuals specifically, due to the particularly high incidence of SCD in this group (5.6:10000018) and a series of typical ECG patterns that came to be seen as physiological adaptations to training. Black athletes are more likely to have voltage criteria for LVH (68% vs. 40%), repolarization alterations including J-point elevation with concave or convex ST segment (85% vs. 62%) and deep T-wave inversion (12% vs. 0%) than their white counterparts.1,22 Multivariate analysis showed that black ethnicity was a predictor of ECG abnormalities (relative risk of 2.3 compared to non-blacks).22 HCM, for example, is 10 times more prevalent in blacks than in whites, which makes the ECG particularly valuable in this group.1,65
Pediatric agesThe Seattle criteria have not been validated for athletes younger than 14. Characteristics associated with younger ages are the absence of gender-related differences on the ECG (probably due to the lack of influence of sex hormones),66 T-wave inversion in the precordial leads,67 and shorter PR, QRS and QT intervals (partly due to higher heart rates in children). These differences will tend to disappear as adulthood approaches, but there is no specific age at which this occurs, which can make it difficult to differentiate between persistent juvenile patterns from patterns associated with underlying heart disease.
Recommendations for athletesAs well as the lack of agreement on the use of the ECG in preparticipation screening, there are also differences between the US and European guidelines concerning recommendations for athletes with pathological ECG alterations. Table 5 summarizes the recommendations for different heart conditions according to the American College of Cardiology's Bethesda Conference guidelines and the ESC.1,43
Recommendations for sports participation among athletes with cardiac conditions according to the US and European guidelines.
| Clinical criteria and permitted sports | ||
|---|---|---|
| 36th Bethesda Conference72 | ESC72 | |
| HCM | ||
| Definitive diagnosis | Excluded from competitive sports | |
| Positive genotype/negative phenotype | May compete | Excluded from competitive sports |
| ARVD | Excluded from competitive sports | Excluded from competitive sports |
| CCAA | Excluded from competitive sports. Participation in all sports 3 months after corrective surgery | Not mentioned |
| Myocarditis | Excluded from competitive sports. Convalescent period of 6 months. Athletes may return to competition when test results normalize | Excluded from competitive sports. Convalescent period of 6 months. Athletes may return to competition when test results normalize |
| Marfan syndrome | If aortic root <40 mm, no mitral regurgitation, no familial sudden death, low-moderate intensity competitive sports permitted | Only recreational sports |
| LQTS | ||
| Definitive diagnosis | >0.47 s in males, >0.48 s in females: if symptomatic, excluded from competitive sports; if asymptomatic, restricted to competitive low-intensity sports | >0.44 s in males, >0.46 s in females: excluded from competitive sports |
| Positive genotype/negative phenotype | May compete | Excluded from competitive sports |
| SQTS | Only competitive sports and leisure-time sports with low static/dynamic demand. Sports with risk for patient or others due to (pre)syncope are relatively contraindicated | Not mentioned |
| BrS | Excluded from all competitive sports except those of low intensity | Excluded from competitive sports |
| CPVT | ||
| Definitive diagnosis | Excluded from competitive sports | Excluded from competitive sports |
| Positive genotype/negative phenotype | May still compete in low-intensity sports | Excluded from competitive sports |
| WPW | Asymptomatic: can participate in all competitive sports Symptomatic: EPS and ablation recommended; return to competitive sports is allowed after corrective ablation, provided that the ECG has normalized | Asymptomatic: can participate in all competitive sports EPS for both symptomatic and asymptomatic individuals; return to competitive sports is allowed after corrective ablation, provided that the ECG has normalized |
ARVD: arrhythmogenic right ventricular dysplasia; BrS: Brugada syndrome; CCAA: congenital coronary artery anomalies; CPVT: catecholaminergic polymorphic ventricular tachycardia; EPS: electrophysiological study; ESC: European Society of Cardiology; HCM: hypertrophic cardiomyopathy; LQTS: long QT syndrome; SQTS: short QT syndrome; WPW: Wolff-Parkinson-White syndrome.
The debate concerning the best way to identify athletes at risk for SCD continues, and there is still disagreement between the ESC and the AHA. The Seattle criteria aimed to resolve one of the major problems with preparticipation ECG screening, that of the high proportion of false positives observed using the criteria of previous guidelines.57,58,68 Achieving a false positive rate of 1.7%59 led to a change in focus from false positives to false negatives. There is currently no reason to doubt the safety of the Seattle criteria, but further prospective studies are needed to ascertain the outcome of all the athletes assessed.
Another major concern with the use of the ECG is its financial impact and its poor cost-effectiveness. According to Wheeler et al.,69 addition of electrocardiography to preparticipation screening saves 2.06 life years per 1000 athletes at an incremental cost of $89 per athlete, yielding a cost-effectiveness ratio of $42900 per life year saved, which is much less than other public health initiatives such as dialysis ($20000–$80000) or public access to defibrillators ($55000–$162000).
Asif et al.59 addressed another important question regarding opposition to the inclusion of electrocardiography in preparticipation screening by assessing what psychological impact this would have on athletes, including the effect of false positives. They concluded that there were no statistically significant differences in anxiety levels with and without the ECG, and that those who received an ECG were more satisfied with their screening, felt safer during competition, and stated the ECG positively impacted their training.
Another study demonstrated the benefit of early detection and treatment of LQTS in reducing the risk of SCD. Johnson et al.70 analyzed 353 individuals with LQTS, of whom 130 remained in competitive athletics. These received counseling and were treated with beta-blockers, left cardiac sympathetic denervation, and/or an ICD. In a mean follow-up of 5.1 years, none of the 130 athletes died and only one received appropriate ICD shocks. Although these results cannot be extrapolated to other conditions, they demonstrate the need for further studies to provide a solid basis of scientific evidence to guide treatment decisions and to determine eligibility for participation in sports.
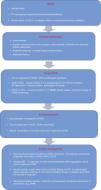
Evidence supporting the inclusion of the ECG in screening continues to mount. Nevertheless, in order to be effective, there is a need for improved medical training and for tools to assist with interpreting the ECG, since the cost-effectiveness of electrocardiography in preparticipation screening will depend on the competence of physicians as well as on their response to abnormal and borderline alterations. The algorithm for evaluating athletes (Figure 9) proposed by Chandra et al.1 to identify possible cardiac causes of sudden death highlights the role of the ECG. Acceptance of this algorithm, as well as improvements in training for primary health physicians, sports medicine specialists and cardiologists, are needed before including an ECG in preparticipation screening protocols, in order to avoid unacceptable levels of false positives.
Algorithm for evaluating athletes for conditions capable of causing sudden cardiac death. ARVC: arrhythmogenic right ventricular cardiomyopathy; CCAA: congenital coronary artery anomaly; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; LA: left atrial; LBBB: left bundle branch block; LQTS: long QT syndrome; LVH: left ventricular hypertrophy; MRI: magnetic resonance imaging; RBBB: right bundle branch block; SCD: sudden cardiac death; WPW: Wolff-Parkinson-White syndrome. Adapted from Chandra et al.1
Recent years have seen a considerable increase in the number of athletes, and participation in competitive sports begins at ever younger ages. It is thus increasingly urgent to develop a standardized method of assessing this population to reduce the risk of SCD. The latest studies indicate that the ECG, interpreted using appropriate criteria, is a valuable element in preparticipation screening. The Seattle criteria were an important step in improving the diagnostic accuracy of the ECG, but they are far from perfect, and there is a need to further study ECG patterns in different subpopulations and to understand their real pathological significance.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Machado M, Silva MV. Alterações eletrocardiográficas benignas e patológicas em atletas. Rev Port Cardiol. 2015;34:753–770.





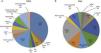











 ARVD: arrhythmogenic right ventricular dysplasia; AS: aortic stenosis; CAAC: congenital coronary artery anomalies; CAD: coronary artery disease; CSD: conduction system disease;
ARVD: arrhythmogenic right ventricular dysplasia; AS: aortic stenosis; CAAC: congenital coronary artery anomalies; CAD: coronary artery disease; CSD: conduction system disease; 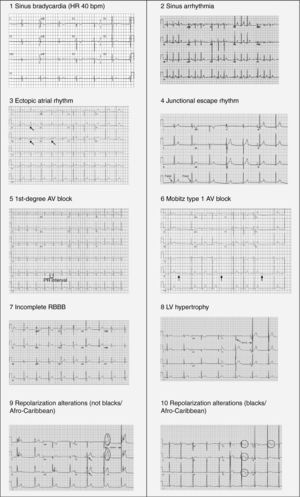
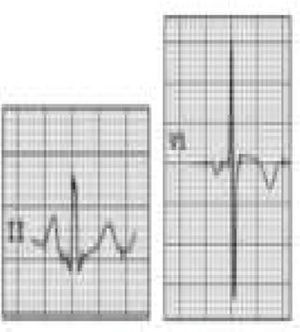
 WPW). (a) Sinus rhythm with preexcitation of the
WPW). (a) Sinus rhythm with preexcitation of the 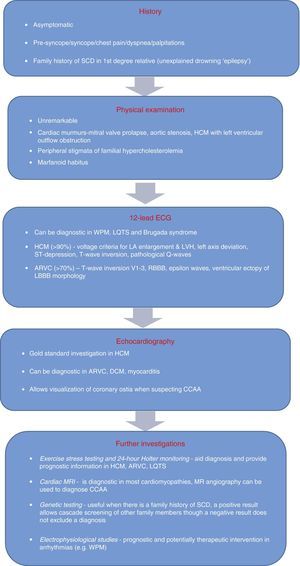 DCM: dilated cardiomyopathy;
DCM: dilated cardiomyopathy; 

