In women with hypertrophic cardiomyopathy (HCM), pregnancy prompts major changes in hemodynamic and cardiac autonomic function that may precipitate heart failure (HF) or increase the risk of cardiac arrhythmia.
We report the clinical follow-up of two patients with non-obstructive HCM implanted with a cardioverter defibrillator (ICD) allowing for continuous analysis of heart rate (HR), heart rate variability (HRV) and cardiac arrhythmia throughout the entire course of pregnancy.
Both patients experienced increased HR and decreased HRV from the early stages of pregnancy, which persisted until delivery. Premature ventricular contractions (PVCs) and runs of non-sustained ventricular tachycardia (NSVT) reached a peak in the second and third trimesters, concurrent with sympathetic hyperactivity. In one patient with baseline NYHA class II HF symptoms, increased PVCs and NSVT were consistent with the deterioration of HF, supporting the decision to bring the delivery forward. While both patients experienced a persistent increase in sympathetic tone and ventricular ectopic activity, no life-threatening arrhythmias were documented.
During pregnancy, patients with hypertrophic cardiomyopathy develop progressive neuroautonomic imbalance, prompting an increase in non-sustained ventricular arrhythmia. This enhanced arrhythmia burden warrants close follow-up and rhythm assessment during the third trimester, especially in women who have heart failure symptoms before pregnancy. Implantable cardioverter defibrillators provide a continuous analysis of heart rate variability and arrhythmia burden that supports therapeutic decision-making during follow-up.
Em mulheres com miocardiopatia hipertrófica, a gravidez aumenta as variações hemodinâmicas e as alterações da função autonómica cardíaca que podem provocar insuficiência cardíaca ou aumentar o risco de arritmia. Reportamos o acompanhamento clínico de duas pacientes com miocardiopatia hipertrófica não obstrutiva, ambas implantadas com cardioversor-desfibrilhador (CID). A monitoração com CID permite a análise contínua da frequência cardíaca, da variabilidade da frequência cardíaca (VFC) e da arritmia durante toda a gravidez. As duas pacientes manifestaram aumentos da FC e diminuições da VFC desde o início da gravidez até ao parto. Observou-se um pico de frequência de extrassístoles ventriculares (EV) e de taquicardias ventriculares não sustentadas (TVNS) no segundo e terceiro trimestres da gestação, em correspondência da hiperatividade simpática. Numa das pacientes com classe funcional NYHA II, antes da gravidez, o aumento de EV e de TVNS contemporaneamente ao agravamento da insuficiência cardíaca levou à decisão de antecipar o parto. As duas pacientes demonstraram um aumento persistente da atividade simpática e da atividade ectópica ventricular, não existiram casos de arritmias ventriculares malignas. Durante a gravidez as pacientes com miocardiopatia hipertrófica desenvolvem um progressivo desequilíbrio autonómico que causa um aumento das arritmias ventriculares não sustentadas. O aumento do risco arrítmico necessita de um constante e frequente controle clínico e do ritmo cardíaco durante o terceiro trimestre, especialmente em mulheres com sintomas de insuficiência cardíaca antes da gravidez. O cardioversor-desfibrilhador implantável fornece uma análise continua da variabilidade da frequência cardíaca e das arritmias que podem apoiar as decisões terapêuticas durante a gravidez.
Maternal death is rare in pregnant women with hypertrophic cardiomyopathy (HCM). However, the deterioration of clinical conditions may occur in a small but significant proportion of patients1. Pregnancy prompts major changes in hemodynamic and cardiac autonomic function that may precipitate heart failure or increase the risk of cardiac arrhythmia. This paper reports the clinical and device follow-up of two patients with non-obstructive HCM implanted with a cardioverter defibrillator (ICD), thereby providing continuous analysis of heart rate (HR), heart rate variability (HRV) and cardiac arrhythmia throughout the entire course of pregnancy.
Case reportPatient A had been diagnosed with HCM at the age of 14 and implanted with a single-chamber ICD (Maximo VR, Medtronic) at the age of 22 based on massive left ventricular (LV) hypertrophy (>30mm maximal wall thickness), non-sustained ventricular tachycardia (NSVT) and a family history of unexplained sudden death in a first-degree relative <50 years of age2,3. She underwent a life-saving ICD intervention for ventricular fibrillation (VF) at the age of 26.
At week 1 of pregnancy she was 30 years old, had NYHA class II symptoms and was on treatment with atenolol 50mg daily. During pregnancy, she underwent routine clinical and ICD follow-up visits every two to three months. At each ICD interrogation, HR trends, HRV (expressed as SDNN, standard deviation of normal-to-normal RR intervals), single premature ventricular contractions (PVCs), series of PVCs (2–4 beats), runs of NSVT (>4 beats) and atrial fibrillation (AF) episodes were recorded.
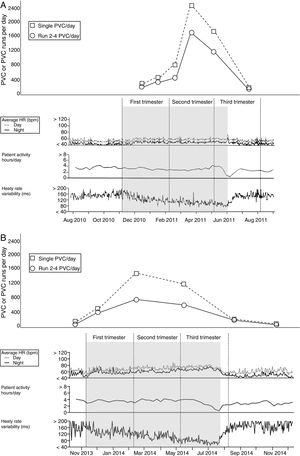
Data retrieved from the ICD showed increased HR and decreased SDNN from the early stages of pregnancy (week 4), with a peak and plateau in the second and third trimesters (Figure, panel A). She experienced eight runs of NSVT between week 12 and week 30, together with a substantial increase of single PVCs (from 280 to 2500 per day on average) and series of PVCs (from 190 to 1500 per day on average) from week 18 that persisted until delivery (Figure, panel A). The patient did not experience any episodes of AF. Compared to baseline, during the third trimester, patient A experienced progressive worsening of heart failure symptoms (transition from NYHA class II to III), increased pulmonary artery pressure (PAP) as assessed by Doppler echocardiography (from 30mmHg to 45mmHg) and increased ventricular ectopic activity, as recorded by the ICD (Figure, panel A). As a result, cesarean delivery was scheduled at week 30 and the pre-term infant was temporarily admitted to the neonatal intensive care unit. Drug therapy did not change throughout follow-up. At ICD follow-up (12 weeks after delivery), HR, SDNN and PVCs were found to be at pre-pregnancy levels.
PVC, PVC series, HR, daily activity (hours) and HRV (SDNN) throughout the entire course of pregnancy as retrieved from the ICD memory in patient A (panel A) and patient B (panel B).
The number of PVCs is shown at the midpoint between two close clinical evaluations and ICD follow-up visits.
PVC, premature ventricular contraction; HR, heart rate; HRV, heart rate variability; SDNN, standard deviation of normal-to-normal RR intervals.
Patient B had been diagnosed with HCM at the age of 12 and implanted with a primary prevention single-chamber ICD (Entrust VR, Medtronic) at the age of 30 based on massive LV hypertrophy and NSVT2. She never experienced ICD interventions.
At week 1 of pregnancy, Patient B was 37 years old, asymptomatic, and on atenolol, 25mg daily.
She underwent routine clinical and ICD follow-up visits throughout pregnancy (every two to three months). At each ICD interrogation, HR trends, HRV, single PVCs, series of PVCs, runs of NSVT and atrial fibrillation (AF) episodes were retrieved from the ICD memory.
During pregnancy, data retrieved from the ICD showed increased HR and decreased SDNN from week 4 with a peak and plateau in the third trimester (Figure, panel B). Patient B experienced an increase in single PVCs (from 34 to 1400 per day on average) and series of PVCs (from 70 to 500 per day on average) from week 16 until delivery (Figure, panel B). During the third trimester, she did not experience any deterioration of heart failure symptoms or AF episodes, and PAP remained within the normal range (max. 26mmHg). Atenolol was not up-titrated during follow-up. The patient underwent uncomplicated cesarean delivery at week 34 after oligohydramnios. The baby was born in good health. During routine ICD follow-up 10 weeks after delivery, HR, SDNN and ventricular ectopic activity were found to be at pre-pregnancy levels.
DiscussionIn healthy women, pregnancy is associated with hemodynamic and cardiac autonomic changes4. Indeed, HR rises as a compensatory response to reduced systemic vascular resistance and HRV decreases due to a shift in autonomic balance towards sympathetic hyperactivity5. The extent to which this occurs in pregnant women with HCM and whether it may affect the risk of cardiac arrhythmia and prognosis is currently unknown. For the first time, we used an ICD for continuous beat-to-beat analysis of HR, HRV and cardiac arrhythmia throughout the entire course of pregnancy in two high-risk HCM patients.
HRV analysis showed a persistent rise in sympathetic tone early in pregnancy. On the one hand, fluctuations in plasma estrogen and progesterone may explain the increased sympathetic outflow that, in turn, triggers PVCs and NSVT as a result of beta-adrenergic stimulation of the hypertrophic myocardium. On the other hand, the increase in ventricular ectopic activity may occur as a result of disease-related failure to compensate for expanded blood volume and tachycardia, particularly in the context of massive LV hypertrophy and diastolic dysfunction. Irrespective of whether enhanced ectopic activity is primary or secondary in origin, cardiac rhythm assessment is reasonable even in the early stages of pregnancy in HCM patients. Nonetheless, sympathetic hyperactivity, PVCs and NSVT showed a peak in the second and third trimesters, concurrent with maximum volume overload, HR, cardiac output and circulating catecholamines. This marked increase in ventricular arrhythmia was consistent with deterioration of heart failure symptoms and supported the decision to anticipate the delivery in patient A. Accordingly, close clinical follow-up including rhythm assessment is warranted in HCM patients during the third trimester, especially in women who are symptomatic before pregnancy. Heart failure symptoms and cardiac arrhythmia may indicate the need for cesarean and/or early delivery.
Finally, while both patients experienced a persistent increase in ventricular ectopic activity, no life-threatening arrhythmia was documented. This is consistent with previous studies that have shown that sustained ventricular arrhythmia is uncommon in pregnant women with HCM, and that PVCs and NSVT do not predict per se the risk of death1,6,7.
ConclusionsDuring pregnancy, patients with hypertrophic cardiomyopathy develop progressive neuroautonomic imbalance prompting an increase in non-sustained ventricular arrhythmia. This enhanced arrhythmia burden warrants close follow-up and rhythm assessment during the third trimester, especially in women who have heart failure symptoms before pregnancy. Implantable cardioverter defibrillators provide a continuous analysis of heart rate variability and arrhythmia burden that supports therapeutic decision-making during follow-up.
Conflict of interestDr. Pietro Francia served as consultant and received speaker's fees from Boston Scientific.