Atrial flutter with 1:1 conduction is a relatively common entity in clinical practice as an effect of treatment with anticholinergic antiarrhythmic drugs (class IA) or those that slow the flutter rate (class IC) 1. Less common are cases of spontaneous occurrence 2. The presence of an atrioventricular (AV) node with an increased capacity for rapid conduction, especially under conditions of increased sympathetic activity, has been suggested as the underlying mechanism 3,4.
We present the case of a patient with atrial flutter being investigated for recurrent presyncope, who developed 1:1 AV conduction during exercise testing, with ventricular rate of 240bpm.
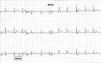
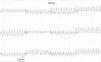
A 67-year-old man, with a history of hypercholesterolemia and persistent atrial flutter, was referred for cardiological consultation to investigate exercise-induced presyncopal episodes. He was under therapy with 2.5mg bisoprolol, 20mg atorvastatin and acenocoumarol. The baseline ECG showed typical atrial flutter, with a cycle length of 220ms and 4:1 AV conduction at 70bpm (Figure 1). Laboratory tests were normal, including thyroid hormones. During 24-hour Holter monitoring, the patient remained asymptomatic but there was evidence of atrial flutter throughout the recording, with a maximum heart rate of 140bpm and a minimum night-time rate of 38bpm. Echocardiography showed mild dilatation of both atria, but no other alterations. A standard symptom-limited exercise test was performed (Bruce treadmill protocol) under therapy with 2.5mg bisoprolol. Nine minutes into the test, he presented a sudden increase in heart rate to 240bpm (Figure 2), accompanied by a 40mmHg fall in blood pressure, triggering presyncopal symptoms.
In a recent study, Turitto et al. 4 analyzed the characteristics of 19 patients with spontaneous 1:1 conduction compared to patients with higher conduction ratios. They found that the former had less structural heart disease and longer cycle length (265 vs. 241ms) and more often presented syncope or presyncope.
We found only one study in the literature on the effect of exercise on cycle length in atrial flutter 5. Van den Berg et al. compared cycle length at rest and at peak exercise in 15 patients; 11 showed increased cycle length, of whom six developed 1:1 conduction. The authors suggest exercise-induced inhibition of vagal tone and increases in atrial filling and size as possible mechanisms to explain increased cycle length.
In the case presented, baseline cycle length was 220ms, increasing to 250ms at peak exercise. This, together with increased sympathetic activity induced by exercise despite beta-blocker therapy, could have promoted 1:1 conduction across the AV node, leading to an elevated heart rate with hemodynamic repercussions.
Although this case was unusual, the possibility of 1:1 conduction should be considered in patients with symptomatic atrial flutter, particularly when the baseline flutter wave cycle length is long. However, a short baseline cycle length does not exclude the possibility of prolongation during exercise, as occurred in the present case. Given the risk of these patients developing potentially fatal arrhythmias, radiofrequency ablation of the flutter circuit is the treatment of choice. Further studies will be required to identify the characteristics that predict the conduction behavior of flutter waves during exercise. In the meantime, exercise testing in patients with symptomatic atrial flutter is a simple way to identify those at risk of abnormally rapid conduction.