The aim of this study was to investigate the predictive value of copeptin levels in the development of contrast-induced nephropathy (CIN).
MethodsA total of 274 patients diagnosed with ST-elevation myocardial infarction (STEMI) and who had undergone primary percutaneous coronary intervention were included in the study. The patients were divided into two groups according to the presence (CIN+) or absence (CIN-) of CIN. These groups were compared in terms of demographic characteristics, laboratory findings and risk factors.
ResultsCopeptin levels (10.68±6.43 vs. 7.07±05.53 pmol/l; p<0.001) and peak creatinine (1.46±1.20 vs. 1.03±0.20 mg/dl; p=0.005) were significantly higher in the CIN+ group than in the CIN- group. Female gender was significantly more prevalent in the CIN- group compared to the CIN+ group (19% vs. 8.6%; p<0.05). Copeptin level at hospital admission (OR: 2.36, p=0.005) was found to be an independent predictor for CIN development.
ConclusionCopeptin level is an independent predictor of CIN development in patients with acute STEMI that can be detected rapidly and easily. This result indicates that physicians should be aware of the possibility of CIN development in patients with high copeptin levels and preventive measures should start early.
Análise do valor preditivo dos níveis de copeptina no desenvolvimento da nefropatia induzida por contraste (NIC).
MétodosForam incluídos no estudo 274 doentes, diagnosticados com enfarte do miocárdio com elevação do segmento ST (STEMI) e submetidos a angioplastia coronária primária. Os doentes foram divididos em dois grupos de acordo com a confirmação de NIC. Estes grupos foram comparados quanto às características demográficas, achados laboratoriais e fatores de risco.
ResultadosO nível de copeptina (10,68±6,43 versus 7,07±5,53; p<0,001) e o pico dos níveis de creatinina (1,46±1,20 versus 1,03±0,20; p=0,005) foram significativamente superiores nos grupos de NIC (+) quando comparados com os grupos de NIC (-). A percentagem do género feminino foi significativamente superior no grupo NIC (-) quando comparada com o grupo NIC (+) (19% versus 8,6%; p<0,05). O nível da copeptina no momento do internamento (OR: 2,36, p=0,005) foi considerado como um valor preditor independente no desenvolvimento da NIC.
ConclusãoO nível da copeptina é um fator preditor independente do desenvolvimento da NIC em doentes com STEMI agudo. Pode ser detetado rápida e facilmente e este resultado indica que os médicos devem ser mais cuidadosos em relação ao desenvolvimento da NIC em doentes com níveis de copeptina levados. Medidas preventivas devem ser tomadas precocemente.
Contrast-induced nephropathy (CIN) is an iatrogenic disease occurring after the intravascular injection of iodinated radiographic contrast media and is a significant cause of acute renal failure.1 The mechanism of CIN is as yet not clearly understood. It is associated with increased in-hospital mortality and subsequently contributes to morbidity and prolonged hospitalization, and increases the costs of health care.2,3 The incidence of CIN varies depending on the study population, although it has decreased in recent years due to use of less nephrotoxic contrast agents and better prevention strategies.4 However, CIN developing after coronary angiography is still a major cause of mortality and morbidity.5 It is therefore clinically important to predict the risk of developing CIN and there is a need to identify new biomarkers for its rapid and accurate diagnosis.
Pituitary vasopressin plays important roles in the regulation of osmotic pressure and homeostasis. Copeptin is the C-terminal part of pro-arginine vasopressin and may serve as a potent biomarker of cardiovascular disease.6 Vasopressin plays an important role in water homeostasis, but has a very short half-life and is unstable in vitro, which makes it difficult to quantify. The function of copeptin remains unknown, but it is secreted in equimolar quantities to vasopressin and has the advantage of high stability in blood samples. Owing to its involvement in the adrenocorticotropic hormone cycle, copeptin has been proposed to be a marker of severe stress reactions as well as of hemodynamic triggers.7,8 In recent years, studies have also found that copeptin may increase albuminuria9 and predict the outcomes of adverse cardiac and renal events.10 We therefore hypothesized that copeptin may be a sensitive biomarker to predict changes in renal function, with clinical importance in the early diagnosis and assessment of progression of CIN in STEMI patients. The association of copeptin and CIN in STEMI patients was also investigated in the present study.
MethodsPatient populationAfter approval from the Ethics Committee, a total of 274 consecutive patients (229 male, 45 female) admitted to our hospital due to acute STEMI who had undergone primary percutaneous coronary intervention (PCI) (angioplasty and/or stent implantation) between December 2016 and April 2017 were prospectively included in the study. The clinical and demographic characteristics of the patients were recorded. The following diagnostic criteria for STEMI were used: ST-segment elevation in ≥2 contiguous leads (≥2 mm in precordial leads, ≥1 mm in limb leads) or new-onset left bundle branch block; ischemic type chest pain lasting more than 30 min; and a two-fold or greater elevation in serum creatine kinase-myocardial band (CK-MB) and troponin levels. Patients with severe infection, those allergic to contrast media and those undergoing chronic dialysis were excluded from the study, as were those under treatment with any thrombolytic agents or who died within 48-72 hours in the coronary intensive care unit. A 12-lead ECG recording was obtained for all patients just after admission and the type of myocardial infarction (MI) was determined. Blood samples were obtained at the time of admission and during follow-up and analyzed on a Coulter LH 780 analyzer (Beckman Coulter Ireland Inc., Mervue, Galway, Ireland). Echocardiography (Vingmed, GE, Horten, Norway) was performed by an experienced cardiologist at the coronary intensive care unit just after the PCI procedure, and left ventricular ejection fraction was calculated using the modified Simpson method. Daily blood samples were taken and changes in serum creatinine were determined. Patients were divided into two groups according to the presence (CIN+) or absence (CIN-) of CIN, which was defined as impairment of renal function measured as either a 25% increase in serum creatinine from baseline or a 0.5 mg/dl (44 μmol/l) increase in absolute serum creatinine within 48-72 hours of intravenous contrast administration.11 A diagnosis of anemia was made if hemoglobin was <11.5 g/dl.
Coronary angiography and in-hospital follow-upAll PCI procedures were performed via the femoral route by an experienced cardiologist using an Axiom Artis Zee system (Siemens, Germany). Non-ionic low osmolality contrast medium (Omnipaque 350 mg/ml; GE Healthcare, Cork, Ireland) was used for the procedures. All patients were given 300 mg aspirin, 600 mg clopidogrel or 180 mg ticagrelor loading dose prior to the procedure, and 100 U/kg heparin was administered after visualization of the arterial anatomy. Use of glycoprotein IIb/IIIa inhibitors was at the discretion of the physician. All patients were transferred to the intensive care unit after the procedure and treatment continued with 100 mg aspirin, 75 mg clopidogrel or 90 mg ticagrelor twice daily. The decision for concurrent use of statins, angiotensin-converting enzyme inhibitors and beta-blockers was made according to the guidelines of the American College of Cardiology/American Heart Association. Use of nephrotoxic agents and non-steroidal anti-inflammatory drugs was avoided. Patients who did not have congestive heart failure were administered 1 ml/kg/h of 0.9% isotonic saline solution for 24 hours. Oral fluid intake was begun 90 min after the procedure for patients with good general status. Blood pressure and electrocardiographic monitoring were performed at the intensive care unit and control blood samples were obtained. Plasma creatinine values were monitored for 72 hours after the procedure.
Statistical analysisThe statistical analysis was performed using SPSS 15.0 for Windows Evaluation Version. Normality of distribution was assessed using the Kolmogorov-Smirnov test. Continuous variables were presented as mean ± standard deviation or median (interquartile range) and categorical variables were summarized as frequencies. Differences between the two groups in continuous variables were determined by the independent samples t test. Categorical variables were compared by the chi-square test or Fisher's exact test. Logistic regression analysis was used to determine the effect of potential prognostic factors on the occurrence of CIN, and independent predictors were determined by inclusion of significant risk factors in the logistic regression model. Receiver operating characteristic (ROC) curve analysis was used to determine the optimum cut-off level of sensitivity and specificity. A p-value of <0.05 was accepted as statistically significant with 95% confidence interval and 5% margin of error.
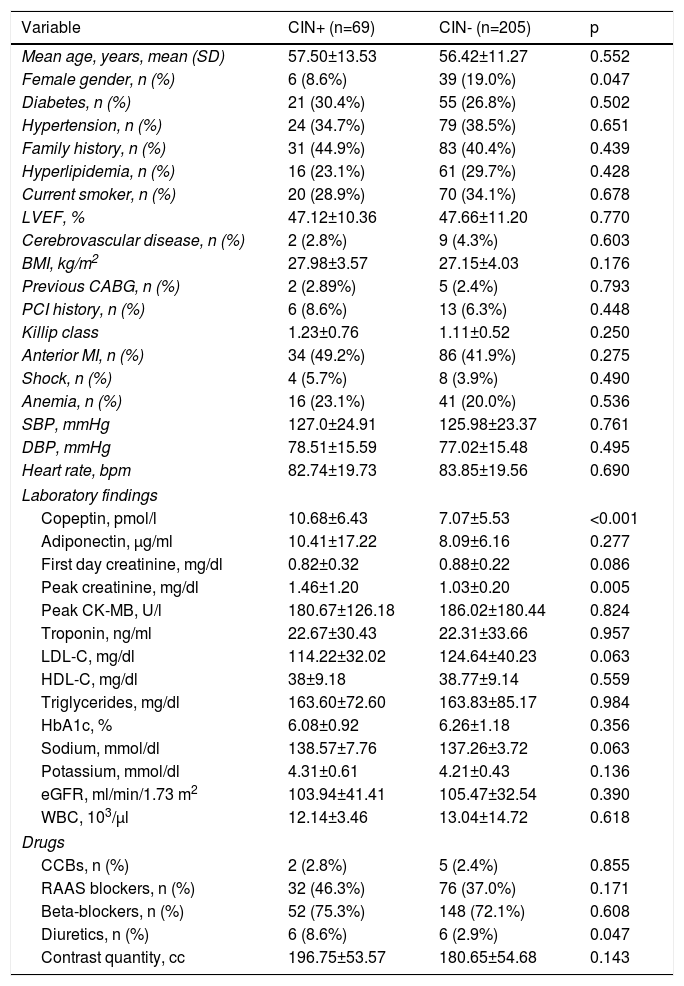
ResultsThe study population (n=274, mean age 56.69±11.85, 16.4% female) was divided into two groups as follows: 69 (25.1%) CIN+ and 205 (74.8%) CIN-. There was no significant difference in mean age between the groups, but the prevalence of females was significantly higher in the CIN- group than in the CIN+ group (19% vs. 8.6%; p<0.05). No differences were observed between the groups with regard to other demographic characteristics. Copeptin levels (10.68±6.43 vs. 7.07±05.53 pmol/l; p<0.001) and peak creatinine (1.46±1.20 vs. 1.03±0.20 mg/dl; p=0.005) were significantly higher in the CIN+ group compared to the CIN- group. No differences were observed between the groups with regard to other laboratory findings. Previous prescription of diuretics was significantly higher in the CIN+ group (8.6% vs. 2.9%; p=0.047). No significant difference was observed between the groups in quantity of contrast media used (196.75±53.57 vs. 180.65±54.68 cc; p=0.143) (Table 1). Four patients (5.8%) who developed CIN required dialysis in hospital follow-up.
Baseline characteristics of patients with and without contrast-induced nephropathy.
| Variable | CIN+ (n=69) | CIN- (n=205) | p |
|---|---|---|---|
| Mean age, years, mean (SD) | 57.50±13.53 | 56.42±11.27 | 0.552 |
| Female gender, n (%) | 6 (8.6%) | 39 (19.0%) | 0.047 |
| Diabetes, n (%) | 21 (30.4%) | 55 (26.8%) | 0.502 |
| Hypertension, n (%) | 24 (34.7%) | 79 (38.5%) | 0.651 |
| Family history, n (%) | 31 (44.9%) | 83 (40.4%) | 0.439 |
| Hyperlipidemia, n (%) | 16 (23.1%) | 61 (29.7%) | 0.428 |
| Current smoker, n (%) | 20 (28.9%) | 70 (34.1%) | 0.678 |
| LVEF, % | 47.12±10.36 | 47.66±11.20 | 0.770 |
| Cerebrovascular disease, n (%) | 2 (2.8%) | 9 (4.3%) | 0.603 |
| BMI, kg/m2 | 27.98±3.57 | 27.15±4.03 | 0.176 |
| Previous CABG, n (%) | 2 (2.89%) | 5 (2.4%) | 0.793 |
| PCI history, n (%) | 6 (8.6%) | 13 (6.3%) | 0.448 |
| Killip class | 1.23±0.76 | 1.11±0.52 | 0.250 |
| Anterior MI, n (%) | 34 (49.2%) | 86 (41.9%) | 0.275 |
| Shock, n (%) | 4 (5.7%) | 8 (3.9%) | 0.490 |
| Anemia, n (%) | 16 (23.1%) | 41 (20.0%) | 0.536 |
| SBP, mmHg | 127.0±24.91 | 125.98±23.37 | 0.761 |
| DBP, mmHg | 78.51±15.59 | 77.02±15.48 | 0.495 |
| Heart rate, bpm | 82.74±19.73 | 83.85±19.56 | 0.690 |
| Laboratory findings | |||
| Copeptin, pmol/l | 10.68±6.43 | 7.07±5.53 | <0.001 |
| Adiponectin, μg/ml | 10.41±17.22 | 8.09±6.16 | 0.277 |
| First day creatinine, mg/dl | 0.82±0.32 | 0.88±0.22 | 0.086 |
| Peak creatinine, mg/dl | 1.46±1.20 | 1.03±0.20 | 0.005 |
| Peak CK-MB, U/l | 180.67±126.18 | 186.02±180.44 | 0.824 |
| Troponin, ng/ml | 22.67±30.43 | 22.31±33.66 | 0.957 |
| LDL-C, mg/dl | 114.22±32.02 | 124.64±40.23 | 0.063 |
| HDL-C, mg/dl | 38±9.18 | 38.77±9.14 | 0.559 |
| Triglycerides, mg/dl | 163.60±72.60 | 163.83±85.17 | 0.984 |
| HbA1c, % | 6.08±0.92 | 6.26±1.18 | 0.356 |
| Sodium, mmol/dl | 138.57±7.76 | 137.26±3.72 | 0.063 |
| Potassium, mmol/dl | 4.31±0.61 | 4.21±0.43 | 0.136 |
| eGFR, ml/min/1.73 m2 | 103.94±41.41 | 105.47±32.54 | 0.390 |
| WBC, 103/μl | 12.14±3.46 | 13.04±14.72 | 0.618 |
| Drugs | |||
| CCBs, n (%) | 2 (2.8%) | 5 (2.4%) | 0.855 |
| RAAS blockers, n (%) | 32 (46.3%) | 76 (37.0%) | 0.171 |
| Beta-blockers, n (%) | 52 (75.3%) | 148 (72.1%) | 0.608 |
| Diuretics, n (%) | 6 (8.6%) | 6 (2.9%) | 0.047 |
| Contrast quantity, cc | 196.75±53.57 | 180.65±54.68 | 0.143 |
BMI: body mass index; CABG: coronary artery bypass grafting; CCBs: calcium channel blockers; CIN+: patients with contrast-induced nephropathy; CIN-: patients without contrast-induced nephropathy; CK-MB: creatine kinase-myocardial band; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; WBC: white blood cell count.
Continuous variables are reported as mean ± standard deviation or median (interquartile range). Categorical variables are reported as n (%).
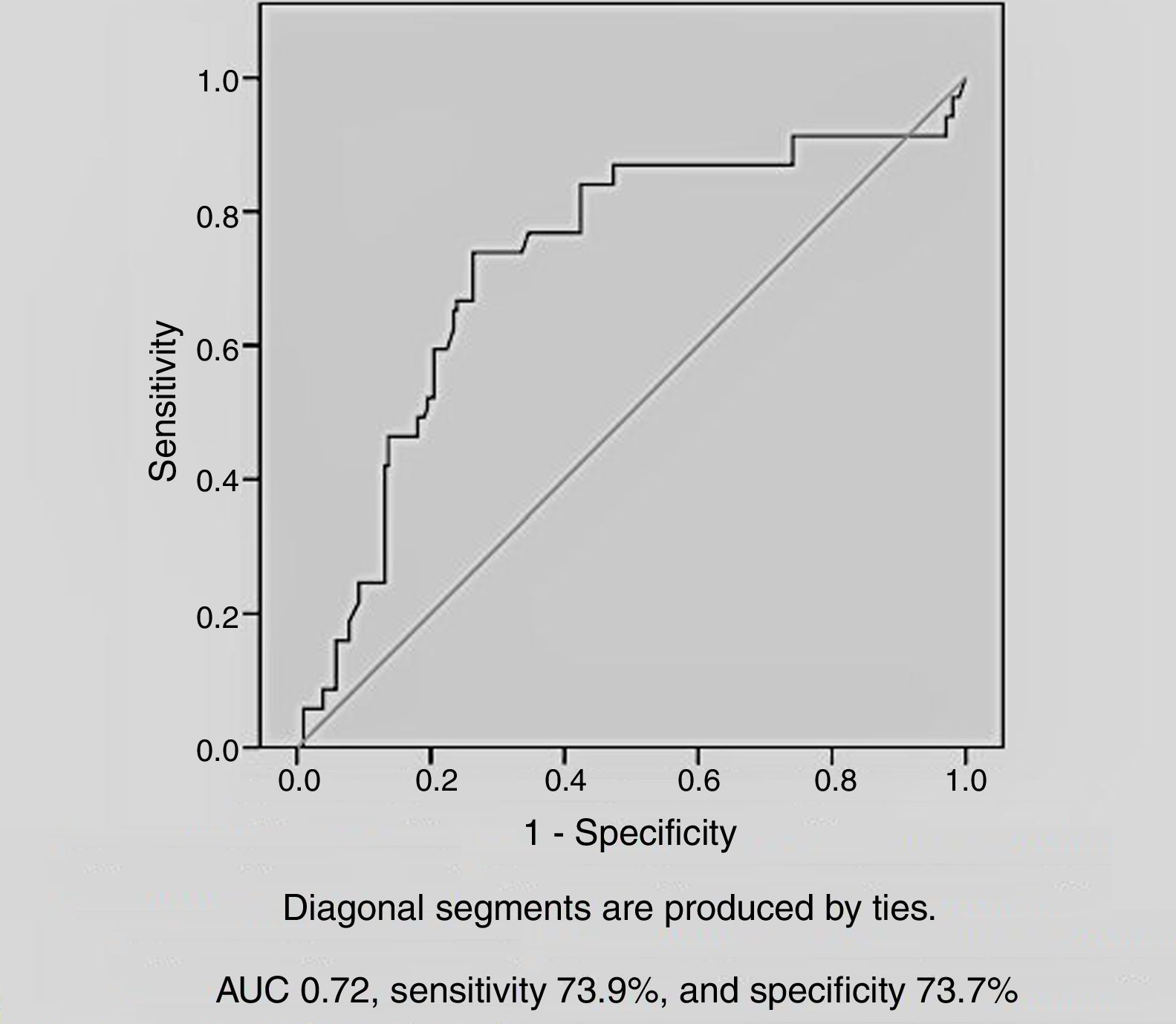
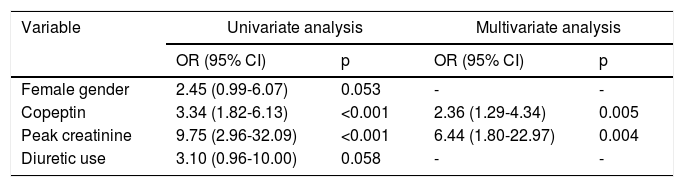
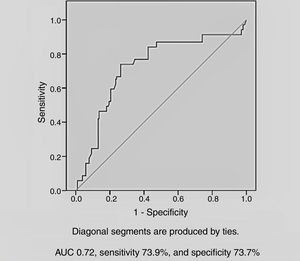
Among the demographic and laboratory findings (Table 1), variables associated with CIN were analyzed as potential risk factors and were assessed by logistic regression analysis. Copeptin level at admission (odds ratio [OR]: 2.36, p=0.005), and peak creatinine (OR: 6.44, p=0.004) were found to be independent predictors of development of CIN. Female gender and diuretic use were not found to be independent predictors of CIN development (Table 2). In ROC curve analysis, admission copeptin above a cut-off of 7.72 pmol/l predicted CIN with sensitivity of 73.9% and specificity of 73.7% (area under the curve: 0.72; 95% confidence interval: 0.65-0.79; p<0.001) (Figure 1).
Regression analysis of potential factors predicting the development of contrast-induced nephropathy.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Female gender | 2.45 (0.99-6.07) | 0.053 | - | - |
| Copeptin | 3.34 (1.82-6.13) | <0.001 | 2.36 (1.29-4.34) | 0.005 |
| Peak creatinine | 9.75 (2.96-32.09) | <0.001 | 6.44 (1.80-22.97) | 0.004 |
| Diuretic use | 3.10 (0.96-10.00) | 0.058 | - | - |
CI: confidence interval; OR: odds ratio.
CIN is an important cause of concern for physicians and is a significant cause of iatrogenic acute renal failure.1 A biomarker that predicts CIN development could thus enable measures to be taken to prevent renal failure. In this study, we determined that copeptin is a independent predictor of CIN in patients with STEMI. To the best of our knowledge, this study is the first to determine such a relationship between copeptin and CIN. The results indicate that the CIN rate increases significantly in patients with high copeptin levels who have undergone primary PCI for STEMI.
The mechanism of CIN is not yet fully understood. Reactive oxygen species (ROS)-induced combined hypoxic and toxic injury is important in the development of CIN.12 Renal oxygenation is significantly decreased and medullary hypoxia leads to ROS production and thus increased oxidative stress.13 While ROS trigger vasoconstrictive signals such as angiotensin II and endothelin I,14 they also reduce the bioavailability of nitric oxide.15 There are also studies indicating that contrast administration leads to vasoconstriction in the renal arteries, reducing renal blood flow.16 As is evident, the underlying pathophysiology of CIN is complex and further studies are needed to understand this issue.
The incidence of CIN varies depending on the population. The main risk factors for its development include impaired renal function, heart failure, age (>65 years), diabetes, nephrotoxic drugs, decreased intravascular volume, long-standing hypotension, anemia, renal transplantation, female gender, high doses of contrast medium and multiple contrast injections within 72 hours.17 Contrast-medium osmolality is also important. Use of low-osmolality instead of high-osmolality contrast medium has been shown to be better for prevention of CIN.18 Therefore, in our study we opted to use low-osmolality contrast medium, aimed to maintain sufficient hydration and avoided the use of nephrotoxic drugs.
The incidence of CIN is higher in patients who undergo coronary angiography than in outpatients (10-15% vs. <5%),17 and previous studies have shown rates up to 25% in STEMI patients.18 The higher incidence in this group may be associated with the high-risk profile of these patients.19 The rate was found to be 25.1% in our study. We consider that this high rate may be related to the fact that our study population was composed of high-risk patients with many comorbidities. It also appears that the use of intra-arterial contrast administration is one of the causes of the high CIN rate in patients undergoing coronary intervention; intra-arterial administration is associated with more risk than intravenous administration.20 In addition, timing is important in determining the incidence of CIN, since creatinine elevation is relatively slow, 48-72 hours being required to identify many cases of CIN, and therefore studies assessing CIN at 24-48 hours after contrast exposure underestimate its rate.21 CIN incidence may be assumed to be low in patients undergoing elective PCI, due to early discharge of these patients and lack of blood tests in the early post-discharge period.22 Our study has shown that CIN is still common among hospitalized patients, but its development can be predicted and its incidence can be decreased if appropriate measures are taken. The current European guidelines on myocardial revascularization recommend risk assessment for acute renal damage for prevention of CIN.25 This indicates the importance of risk detection for CIN.
We believe that copeptin, which has been associated with cardiovascular and renal disease in previous studies, can help in this regard. The close relationship between copeptin and renal disease has been revealed in many studies. Copeptin was found to be affected by mean blood pressure and blood volume and is elevated during pre-dialysis and dialysis periods,23 and is an early indicator of reduced glomerular filtration rate and renal function in diabetic patients.24,25 In addition, a community-based cohort study revealed an independent positive relationship between plasma copeptin level and progression to chronic kidney disease (CKD), demonstrating that its close relationship with renal function is valid not only in patients with CKD, but also in the general population,26 and copeptin is associated with increased risk of CKD even in the general population.27 Furthermore, elevated copeptin has proved a valuable prognostic factor for mid-term mortality in patients with both coronary artery disease and kidney disease.28 The relationship between copeptin and cardiac disorders such as heart failure, coronary artery disease and hypertension has been shown in many previous studies.29–31 Copeptin has been shown to be an independent marker for long-term prognosis in heart failure,32,33 it may be a marker for diagnosis of left ventricular dysfunction in hemodialysis patients,34 and is associated with coronary atherosclerosis in diabetic patients35,36 and with resistant hypertension.37 A rise in copeptin levels two days after STEMI has also been shown to be related to larger acute and chronic infarct size, and initially elevated copeptin level is related to myocardial function and remodeling four months after STEMI. These results highlight the role of copeptin as a biomarker of negative post-STEMI results.38 Copeptin has also been shown to be useful for diagnosing or excluding acute coronary syndrome (ACS) and for safe and early discharge or shortening duration of emergency room stay in low- or moderate-risk patients suspected of suffering ACS when the results of copeptin and troponin are used in combination, as copeptin is elevated in the early period of ACS.39,40 In addition to these findings, rapid copeptin measurement is suggested as a practical and useful tool for medium- and long-term risk assessment in the emergency room.41
In a study assessing acute renal damage in STEMI patients, Guerchicoff et al. investigated the relationship between acute renal damage and B-type natriuretic peptide (BNP), chemokine (C-C motif) ligand 23, D-dimer, endothelial cell-selective adhesion molecule, C-reactive protein, cystatin C, adiponectin and von Willebrand factor.22 Levels of these biomarkers on admission were not found to be significant predictors for CIN development, except for BNP. Although a significant relationship was seen between these biomarkers and CIN during hospital stay and after discharge, we consider that, except for BNP, they have limited benefit in predicting CIN development, and taking measurements within 48-72 hours of contrast administration is important for preventing CIN development. Our study differs from that of Guerchicoff et al., as it investigates the relationship between copeptin and CIN, and our study has revealed that copeptin levels on admission can predict CIN. This may help clinicians to assess the risk and to take appropriate measures. In addition, a statistically significant relationship was found between CIN and peak creatinine level in our study. However, since CIN has already developed by the time peak creatinine is detected, peak creatinine is not a predictive marker, but has only diagnostic value, and is not useful for taking measures to prevent CIN development. As in Guerchicoff et al., no relationship was found between adinopectin level on admission and CIN development in our study. We therefore consider that copeptin is a more valuable marker than adinopectin for prediction of CIN. Copeptin levels also have the advantage of being detected rapidly and easily.
Our findings indicate that physicians should be aware of the possibility of CIN development in patients with high copeptin levels and that preventive measures should start early.
Study limitationsThe present study has some limitations. It was based on a single center, included only STEMI patients and not those with other forms of MI, and did not fully analyze potential nephrotoxic agents. Another limitation is that this study did not include long-term results such as copeptin level after reversal of contrast nephropathy.
ConclusionCopeptin level is an independent predictor of CIN development in patients with acute STEMI. One of the advantages of measuring copeptin compared to other biomarkers is that copeptin at admission can predict CIN at an early stage. Assessment of copeptin level may be helpful for taking measures to prevent CIN development in patients who are to undergo PCI.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interestThe authors have no conflicts of interest to declare.