We investigated viscoelastic properties of the arterial wall in women with previous preeclampsia (PE) compared to those with normal pregnancy (NP).
MethodsIn a cross-sectional study 45 women with previous PE and 55 with NP were included, matched for age (PE: 38±6 vs. NP: 38±5 years, NS) and body mass index: PE: 25±4 vs. NP: 26±4 g/m2, NS) studied respectively, 76±34 and 86±48 months after delivery. We assessed arterial distensibility – pulse wave velocity (PWV, Complior) and reflected waves (augmentation pressure [AP], mmHg) and augmentation index (AIx) – in the central pressure wave and blood pressure (BP) on 24-h ambulatory BP monitoring (ABPM).
ResultsPE showed higher (p<0.01) peripheral systolic blood pressure (SBP): PE 131±18 vs. NP 121±19, and central SBP: PE 122±18 vs. NP 110±19 mmHg, with less amplification of central-peripheral pressure: PE 10±4 vs. NP 12±5, p=0.041, and higher (p<0.05) AP: PE 10±3 vs. NP 8±2, and AIx: PE 26±5 vs. NP 20±5 mmHg, but PE and NP did not differ in pulse wave velocity. On ABPM, PE (n=39) vs. NP (n=33) had higher nighttime SBP: PE 121±10 vs. NP 108±10 mmHg and lower percentage nocturnal SBP fall: PE 11±6 vs. NP 18±11%, both p<0.02. During follow-up, the need for antihypertensive medication was seven times higher in PE than in NP.
ConclusionWomen with previous PE have a greater risk of hypertension, higher nighttime BP values, blunted nocturnal BP fall and changes in central pressure suggestive of increased reflected waves and peripheral vascular resistance. These factors may contribute to their higher cardiovascular risk after pregnancy.
Investigar alterações hemodinâmicas e das propriedades viscoelásticas da parede arterial em mulheres com prévia pré-eclâmpsia (PE) versus mulheres com antecedentes de gravidezes normais (NT).
MétodosEstudo transversal em mulheres, 45 com prévia PE e 55 NT, emparelhadas para idade: PE: 38±6 versus NT: 38±5 anos, n.s., índice massa PE: 25±4 versus NT: 26±4 kg/m2, n.s, e 76±34 (NT) e 86±48 (PE) meses após o parto. Avaliamos rigidez arterial – velocidade onda pulso (PWV, Complior) e ondas refletidas (pressão de aumentação AP) e índice de aumentação (AIx,%) na onda de pressão central e a pressão arterial de 24 horas (MAPA).
ResultadosPE apresentaram pressão sistólica (PAS) periférica PE: 131±18 versus NT: 121±19 e PAS central PE: 122±18 versus NT: 110±19 mais elevadas (p<0,01), menor amplificação central-periférica da pressão diferencial, PE: 10±4 versus NT: 12±5 mmHg, p=0,041, e valores mais elevados (p<0,05) da AP: PE: 10±3 versus NT: 8±2 mmHg e do AIx%: PE: 26±5 versus NT: 20±5%. A PWV foi semelhante nas PE e NT. Na MAPA PE versus NT a PAS noturna foi mais elevada PE: 121±10 versus NT: 108±10 mmHg com menor descida noturna da PAS: PE: 11±6 versus NT: 18±11%, ambos p<0,02. Durante o follow-up na PE a prescrição de anti-hipertensores foi 6-7 vezes mais frequente do que na NT.
ConclusãoMulheres com PE prévia apresentam um risco maior de hipertensão, pressão noturna mais elevada, menor descida tensional noturna e alterações da pressão central sugestivas de aumento das ondas refletidas e das resistências vasculares periféricas. Estas alterações poderão contribuir para um risco cardiovascular aumentado em mulheres com antecedentes de PE.
Preeclampsia (PE) is defined as the development of hypertension and proteinuria from week 20 of gestation onwards and is characterized by generalized maternal endothelial dysfunction.1–3 PE affects 3–5% of first pregnancies, and is the leading cause of maternal-fetal morbidity and responsible for 12% of maternal mortality during pregnancy and in the postpartum period.1–3 In severe cases, PE may be associated with seizures (eclampsia), coagulation disorders (HELLP syndrome) and intrauterine growth restriction. The causes of PE are not fully understood. Recent theories focus on changes in placental development in early pregnancy that cause inflammation and oxidative stress in the placenta, leading to the release of placental hormonal factors into the maternal circulation.4 These factors cause the generalized endothelial dysfunction that is characteristic of the pathophysiology of PE. It has also been suggested that PE is associated with the classic risk factors of cardiovascular disease (CVD), leading to the term ‘metabolic syndrome of pregnancy’.5 Various changes that occur during a normal pregnancy, including insulin resistance,6 dyslipidemia,7 hypercoagulability8 and hyperdynamic circulation,9 are more marked in PE. Although most changes in PE regress following delivery, some functional and structural changes may persist in women who suffer PE; their risk of future cardiovascular (CV) complications may be greater,10 due not only to the coexistence of CV risk factors but also to the subclinical vascular changes that occur in PE. However, according to recent reviews,11 it is not certain whether women with previous PE have increased CV risk. The risk of these women developing hypertension also remains to be clarified and if it occurs, whether it is accompanied by changes in circadian blood pressure rhythm and in aortic distensibility. The augmentation index (AIx) and aortic pulse wave velocity (PWV) are recognized markers of the hemodynamic properties of the arterial wall, aortic distensibility and peripheral resistance, and the changes assessed by these parameters are strong predictors of risk and of CV and renal events.12–16 A few studies have shown increased AIx during PE17–19 but there are no data on what happens to this marker years after childbirth.
Various studies have suggested that women with previous PE have a higher risk of developing CVD in later life,20–23 and some authors have identified genetic factors that increase the risk of CVD and PE.21 Of the studies investigating CV changes in women with previous PE, some assessed simple hemodynamic parameters such as blood pressure (BP), cardiac output and heart rate (HR), while others assessed biochemical parameters, including lipid profile, glucose metabolism and oxidative stress, and peripheral vascular resistance.24 The aim of the present study was to assess the hemodynamic properties of the arterial wall (aortic stiffness), 24-h BP profile on ambulatory BP monitoring (ABPM) and prevalence of hypertension in women with a history of PE in the previous 2–10 years, compared to women with normal pregnancies and a similar time since childbirth, matched for age and body mass index (BMI).
MethodsThis cross-sectional study was approved by the hospital's ethics committee and participants gave their informed consent. Over a period of four months, women aged 25–50 years with a history of PE in their only previous pregnancy according to hospital records were contacted. PE was defined as hypertension (systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg) from week 20 of gestation, associated with proteinuria ≥300 mg/24 h.2,25,26 Women with a history of CV events, renal or liver failure or diabetes, as well as those who had smoked at any stage in their lives, were excluded. At the same time, a similarly-sized sample of women with previous pregnancies considered normal (BP consistently <140/90 mmHg) over a similar period were selected as the control group and were matched with the PE group for each five-year age-group and for BMI values between 20 and 35 kg/m2.
All women selected for the study underwent clinical re-evaluation during the study period, including clinical history, current medication, and assessment of weight, height, BMI, office BP and HR. Office brachial BP was measured using a validated automatic monitor (Omron 705IT), the mean of three readings, taken on the left arm at 5-min intervals with the patient seated, being included in the analysis. Aortic stiffness (as assessed by carotid-femoral PWV) was determined by the Complior method after a 10-min resting period in a supine position,12,16 using the method previously described by us.27 Aortic pulse wave analysis was used to determine the AIx and aortic-brachial pulse pressure amplification using the SphygmoCor system28,29 (SphygmoCor, AtCor Medical, Sydney, Australia), which employs arterial applanation tonometry, appropriate acquisition and software to analyze noninvasive recordings of arterial pulse pressure using the method described by us.27 The augmentation index of central (aortic) pressure is an index of peripheral wave reflection. Augmentation pressure (AP) is the pressure added to the descending wave by the ascending (reflected) wave. The AIx (defined as the ratio of AP to pulse pressure expressed as a percentage) is a measure of wave-reflection amplitude and arterial stiffness, and determines the point at which the reflected wave meets the descending wave. High AP and AIx indicate increased peripheral wave reflection or early arrival of the reflected wave, the result of increased PWV due to aortic stiffness. AIx is corrected for a HR of 75 bpm (adjusted AIx). Aortic Tr (time between the start of the systolic curve and the inflection point), systolic and diastolic pressures, central (aortic) and peripheral pressure difference, systolic pressure at end-diastole (in mmHg), and duration of ventricular ejection (in ms) were recorded. ABPM (Spacelabs 90207) was performed over a 24-h period on a weekday. BP readings were taken automatically every 20 min during the day (7 am–11 pm) and every 30 min at night (11 pm–7 am). Recordings were included when they contained >85% of valid data. Mean values±standard deviation (SD) were calculated for daytime and nighttime systolic, diastolic and overall BP and HR, together with nocturnal BP fall in absolute values and percentages.
Statistical analysisThe sample size required for an estimated difference of p<0.05 in the study variables was calculated as 40–50 women with previous PE and 50–60 controls. The statistical analysis was performed using SPSS version 13.0 (IBM Corporation, Somers, NY). The results are expressed as means±SD, for a level of significance of p<0.05. Differences in the parameters studied were analyzed using one-way ANOVA.
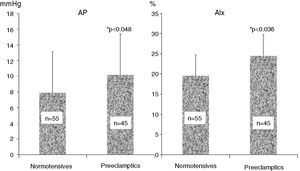
ResultsAnthropometric and clinical data for the two study groups are shown in Table 1. There were no significant differences between the groups in age, BMI, time since delivery (a mean of 6–7 years) or family history of hypertension. However, the percentage of patients under antihypertensive therapy was significantly higher (around 6.5 times) in the PE group. Table 1 also shows ABPM data for a subgroup of both populations who underwent this exam. Patients with previous PE presented higher office systolic and diastolic BP, nighttime systolic BP and HR and a lower percentage fall in nocturnal systolic BP. PWV did not differ between the groups. Table 2 and Figure 1 show the results of hemodynamic assessment by the SphygmoCor system. Central-peripheral amplification of systolic BP (Table 2) and differential pressure were lower in the PE group. On the other hand, radial and central systolic and diastolic BP were significantly higher in women with previous PE, as were those for AP (Figure 1) (7.9±2.4 mmHg in normotensives vs. 10.2±2.6 mmHg in preeclamptics) and AIx (19.8±4.9 mmHg in normotensives vs. 25.7±5.1 mmHg in preeclamptics), both p<0.05.
Anthropometric and clinical characteristics and 24-h ambulatory BP data in women with and without previous preeclampsia.
| Clinical data | Normotensives (n=55) | Preeclamptics (n=45) | p |
| Age (years) | 37.9±6.4 | 37.8±5.1 | NS (0.897) |
| BMI (kg/m2) | 25.0±4.1 | 26.2±4.2 | NS (0.174) |
| Months after delivery | 776±34 | 86±48 | NS (0.747) |
| Family history of hypertension, n (%) | 14/55 (25.4%) | 14/45 (31.1.%) | NS (0.211) |
| Antihypertensive therapy, n (%) | 4/55 (7.2%) | 21/45 (46.7%) | 0.0003 |
| Diuretics, n | 3 | 16 | |
| ACEIs, n | 3 | 8 | |
| ARBs, n | 1 | 10 | |
| Calcium antagonists, n | 1 | 2 | |
| Beta blockers, n | 0 | 1 | |
| Normotensives (n=33) | Preeclamptics (n=39) | p | |
| Office BP | |||
| 24-h SBP (mmHg) | 125.2±15.2 | 135.1±16.2 | 0.011 |
| 24-h DBP (mmHg) | 77.8±8.1 | 84.2±10.8 | 0.019 |
| 24-h HR (bpm) | 72.7±9.7 | 74.9±10.8 | NS (0.340) |
| 24-h ABPM | |||
| 24-h SBP (mmHg) | 126.2±10.2 | 130.1±13.2 | NS (0.245) |
| 24-h DBP (mmHg) | 78.8±8.1 | 81.5±10.8 | NS (0.106) |
| 24-h HR (mmHg) | 76.1±5.3 | 79.3±8.8 | NS (0.170) |
| Daytime SBP (mmHg) | 131.5±11.0 | 133.3±13.4 | NS (0.276) |
| Daytime DBP (mmHg) | 81.6±8.1 | 84.2±10.1 | NS (0.109) |
| Daytime HR (bpm) | 80.1±7.7 | 83.3±9.4 | NS (0.140) |
| Nighttime SBP (mmHg) | 108.3±10.1 | 121.4±10.3 | 0.007 |
| Nighttime DBP (mmHg) | 68.1±12.0 | 73.5±10.1 | NS (0.067) |
| Nighttime HR (bpm) | 65.6±4.7 | 72.6±9.4 | 0.014 |
| Nocturnal SBP fall (%) | 17.6±11.1 | 11.0±6.1 | 0.004 |
| Nocturnal DBP fall (%) | 19.4±13.1 | 14.1±8.6 | NS (0.056) |
| Pulse wave velocity (m/s) | 6.3±1.0 | 6.7±1.1 | NS (0.120) |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure.
Hemodynamic data from the SphygmoCor system in women with and without previous preeclampsia.
| Data | Normotensives (n=55) | Preeclamptics (n=45) | p |
| Radial SBP (mmHg) | 121.1±18.9 | 131.3±17.5 | 0.007 |
| Radial DBP (mmHg) | 74.9±12.6 | 86.4±11.9 | 0.001 |
| Radial PP (mmHg) | 46.3±11.5 | 45.0±9.3 | NS (0.542) |
| Central SBP (mmHg) | 110.0±18.2 | 122.2±17.7 | 0.001 |
| Central DBP (mmHg) | 75.9±12.1 | 87.4±12.2 | 0.001 |
| Central PP (mmHg) | 34.1±9.7 | 34.8±8.4 | NS (0.725) |
| SBP amplification | 11.1±5.6 | 9.2±3.8 | 0.041 |
| DBP amplification | −1.0±0.7 | −1.0±0.5 | NS (0.947) |
| PP amplification | 12.1±5.5 | 10.2±3.9 | 0.043 |
| HR (bpm) | 70.1±10.4 | 68.5±11.3 | NS (0.469) |
DBP: diastolic blood pressure; HR: heart rate; PP: pulse pressure; SBP: systolic blood pressure.
Various studies have suggested that women with previous PE present metabolic and CV changes compared to those with normal pregnancies, and have a higher risk of developing hypertension, CVD20–23 and endothelial dysfunction.24 The present study set out to compare the hemodynamic and vascular properties of women with and without previous PE of a similar age.
Firstly, we found that, around seven years after pregnancy, women with PE were 6–7 times more likely to be under antihypertensive therapy than women without, matched for age and BMI. This suggests that those with previous PE have a higher risk of developing sustained hypertension and confirms the idea that these women are far from making a full recovery.
Furthermore, 24-h ABPM in a subgroup of the study population showed that the circadian BP profile of the PE group differed from that of the control group. Although mean 24-h and daytime BP values were similar in the two groups, absolute nighttime systolic BP was significantly higher in the PE group, who also showed a lower percentage fall in nocturnal BP than the control group. It has been amply demonstrated that BP values on 24-h ABPM are significantly better predictors of risk for CV events than office BP, and that absolute nighttime BP and reduced physiological nocturnal fall are the most important indicators among ABPM parameters.30–34
Various previous studies have shown that women with established PE, and those at risk of developing it, have blunted nocturnal BP falls.35,36 Our results further demonstrate that changes in circadian profile persist long after PE, which highlights not only these patients’ increased risk of developing hypertension but also changes in 24-h BP regulation that are in themselves independent risk factors for CV events. The fact that women with previous PE were more often under antihypertensive therapy may have reduced the differences in ABPM values between the two groups. However, office BP was significantly higher in the PE group than in the control group, but the absence of any difference in ABPM daytime BP suggests this may have been due to the white-coat effect.
The second objective of our study was to assess hemodynamic and vascular properties in women with previous PE by analysis of aortic pressure curves. Studies have demonstrated increased aortic stiffness and peripheral vascular resistance in pregnancies complicated by PE, 37 as well as increases in wave reflection from the periphery.19,38 Changes in aortic stiffness as assessed by carotid-femoral PWV and increased arterial wave reflection demonstrated by AIx as determined by applanation tonometry are known independent markers of target organ damage and increased CV risk.13–15,28,29,39 Given that women with previous PE are more likely to present multiple cardiometabolic risk factors and increased CV risk, changes in the viscoelastic properties of the arterial wall are to be expected in these women compared to those without such a history. In the present study, the PE group presented significantly increased aortic pulse wave AP and AIx, both measures of the amplitude of arterial wave reflection from the periphery, which in turn depends on the diameter and elasticity of small arteries and muscle arterioles at the main sites of pressure wave reflection. This suggests that women with previous PE have increased peripheral vascular resistance, which has also been demonstrated by a different method40 from that used in our study. In addition, we found that women with previous PE showed higher ventricular end-systolic pressure, reflecting increased hemodynamic overload. At the same time, the PE group presented lower central-peripheral amplification of systolic and differential pressures, consistent with greater aortic stiffness, compared to women with no history of PE. Curiously, no difference was found between the two groups in PWV, another measure of arterial stiffness. This apparent discrepancy between increased AIx but no significant change in PWV has also been reported during the clinical phase of PE.19 This may be explained by the fact that any changes in muscle tone, due for example to sympathetic activation, will mainly affect small arteries and hence wave reflection, leading to increased AIx, but without affecting the distensibility of elastic arteries such as the aorta, in which PWV is assessed. The value of AIx depends on aortic stiffness and is influenced by wave reflection from the peripheral arterial tree; it is an indicator of increased left ventricular work during systole and is a more direct and accurate measure of peripheral vasoconstriction than PWV. Furthermore, the effect of medication with inhibitors of the renin-angiotensin system in many women with previous PE cannot be discounted in improving aortic distensibility and lessening differences in PWV. Thus, in situations of vasoconstriction and increased peripheral arterial resistance AIx may change independently of PWV, as demonstrated by various authors.41
As mentioned above, few other studies24,42 have assessed arterial properties in women with previous PE and they used different methodologies from ours, but with similar sized populations. However, our study covered a significantly longer period since pregnancy.
The present study has certain limitations, particularly the size of the groups studied and the treatment prescribed, particularly antihypertensive therapy, which may have decreased the discriminative power of the comparative analysis of the two groups. In any event, the differences observed were pathophysiologically plausible.
In conclusion, the present study suggests that women with previous PE (assessed on average seven years after pregnancy) have a higher risk of sustained hypertension than women of similar age without PE, as well as changes in circadian BP profile, including higher nighttime values and blunted physiological nocturnal BP fall. They also present increased wave reflection and central pressure consistent with increased peripheral arterial resistance.
The study provides objective evidence that women with previous PE have changes in the hemodynamic properties of the arterial wall, central pressure and circadian BP profile compared to women with normal pregnancies, which may result from structural changes and contribute to their higher CV risk. The study thus highlights the need for early and continuous CV monitoring of all women with previous PE.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Polónia J, Olival C, Ribeiro S, et al. Avaliação das propriedades dinâmicas da pressão arterial em mulheres com antecedentes de pré-eclâmpsia. Rev Port Cardiol. 2014;33:345–351.