Hemophilia A is an inherited coagulation disease characterized by factor VIII (FVIII) deficiency and is associated with high hemorrhagic risk, especially in its severe forms. As the average life expectancy of patients with hemophilia has increased, so has the prevalence of acute coronary events. There is however limited experience in dealing with them. The strategy of acting on acute coronary events in patients with hemophilia, as demonstrated in the present case, is a real challenge, not only due to the need for antiplatelet therapy (which is essential in the prevention of stent thrombosis, but increases hemorrhagic risk), but also due to the lack of specific recommendations related to the most adequate and safe replacement therapy in these situations. The authors describe the case of a 48-year-old man with unstable angina and a previous diagnosis of severe hemophilia A who underwent percutaneous coronary intervention under FVIII therapy without hemorrhagic complications.
A hemofilia A é uma doença hereditária da coagulação caracterizada por uma deficiência de fator VIII (FVIII) e que, principalmente nas suas formas graves, está associada a um risco hemorrágico elevado. Com o aumento da esperança média de vida destes doentes, a prevalência de eventos coronários agudos tem aumentado, mas a experiência na abordagem destes permanece escassa. A estratégia de atuação nos eventos coronários agudos em doentes hemofílicos, como demonstrado no presente caso, representa um verdadeiro desafio, não só pela necessidade de terapêutica anti‐agregante plaquetária (a qual, sendo indispensável para prevenção de trombose de stent, aumenta o risco hemorrágico), como também pela inexistência de recomendações específicas relacionadas com a terapêutica substitutiva mais adequada e segura nestas situações. Os autores descrevem o caso de um homem de 48 anos, com angina instável e diagnóstico prévio de hemofilia A grave, submetido a intervenção coronária percutânea sob terapêutica com FVIII sem complicações hemorrágicas.
Hemophilia A is a severe inherited coagulation disorder characterized by a deficiency of factor VIII (FVIII) coagulation activity and is associated with significant bleeding risk. This disease is characterized by alterations in the FVIII gene located on the long arm of the X chromosome and therefore affects almost exclusively males, with females usually only carrying the mutation.
The severity of the disease depends on the level of FVIII and is classified as mild (FVIII >5%), moderate (FVIII 2-5%) and severe (FVIII <1%).1
The clinical picture of hemophilia is characterized by spontaneous or trauma-related hemorrhagic episodes, in the most part, joint (hemarthroses) and muscle hemorrhages. Repeated joint hemorrhages lead to progressive functional disability (hemophilic arthropathy), while other types of hemorrhage, such as central nervous system hemorrhages, can be fatal if not correctly treated. Surgical procedures are a risk in people with this disease, so any invasive procedures require adequate therapy and monitoring by a multidisciplinary team.
In recent decades, there have been remarkable advances in hemophilia therapeutic options with increasing availability of factor concentrates, which are effective in the prevention and treatment of hemorrhagic episodes. These therapies have increased the average life expectancy of these patients from <30 years to about 70 years,2 similar to that of the general population. However, treatment with the missing factor is also responsible for the most serious iatrogenic complication in these patients: the development of antibodies (inhibitors) against the missing factor. These antibodies, which develop in 30-40% of patients and, in most cases, within the first 20 days of exposure to therapy,3 render therapy ineffective and severely compromise the therapeutic options and prognosis of these patients. The etiopathogenesis of inhibitor development seems to be multifactorial and depends on genetic and non-genetic factors.4 In the case of the latter, treatment intensity (defined as more than five days of treatment) is a well-established risk factor.5
In relation to the risk of thrombotic events, and contrary to what one might think, this remains despite the high hemorrhagic risk of these patients. Regardless of any protective effect that may be associated with the coagulation defect, the same factors that contribute to cardiovascular disease in the general population also have an impact on the hemophilia population. Individuals with hemophilia are particularly vulnerable to the development of metabolic syndrome, given the prevalence of obesity and inactivity resulting from hemophilic arthropathy.6 Hypertension also seems to be more frequent in hemophiliac patients than in the general population,7 and although the mechanism is not yet fully understood, it is thought to be associated with microangiopathy of the liver.8
Cardiovascular diseases are a major cause of morbidity and mortality worldwide. In recent years, due to the already mentioned increase in the life expectancy of hemophilia patients and the inherent presence of comorbidities associated with aging, there has been an increase in cardiovascular events in this population.6
The treatment of acute coronary syndrome (ACS) in hemophiliac patients is a real challenge because, if on the one hand anticoagulation and antiplatelet increase the hemorrhagic risk (already high in these patients), on the other hand, correction of anticoagulation with the factor in deficit increases the risk of thrombosis.
We describe the clinical case of ACS in a patient with severe hemophilia A, who underwent invasive procedures, without hemorrhagic or thrombotic complications, thus demonstrating the importance of a multidisciplinary approach in these situations.
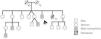
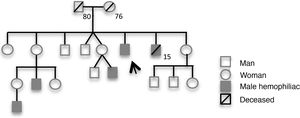
Clinical caseWe describe the case of a 48-year-old man from Cape Verde, evacuated from his country of origin due to unstable angina. The patient had a known personal history of type 2 diabetes and severe hemophilia A. In addition to oral antidiabetic therapy, he was taking acetylsalicylic acid (ASA) 100 mg/day. Despite mentioning numerous episodes of hemarthrosis and presenting chronic joint alterations, he had never been treated for this disease. Family history included three nephews and a brother (who died of intracranial hemorrhage at the age of 15) diagnosed with severe hemophilia A (Figure 1).
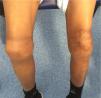
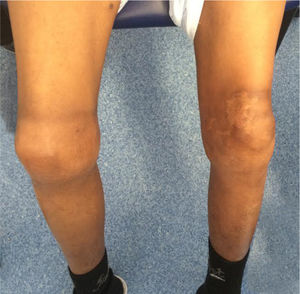
The patient complained of stress angina with an evolution of approximately six months, progressively worsening and evolving to pain at rest (Class IV of the Canadian Cardiology Society. The patient had been started on ASA 100mg/day in Cape Verde and at the time of assessment, he was asymptomatic. The objective examination showed no other alterations apart from evidence of bilateral arthropathy at the knee (Figure 2). Laboratory analyses, in particular, prothrombin time and platelet count, were normal and activated partial thromboplastin time >100 seconds, FVIII <1% and FVIII inhibitors were negative. Myocardial necrosis markers were negative, and there were no other laboratory test abnormalities except for hemoglobin of 9.1g/dL. The electrocardiogram showed sinus rhythm, Q wave in aVL, with no other repolarization changes; the echocardiogram was normal.
The clinical situation was discussed at a multidisciplinary meeting (a hematologist specializing in hemophilia, a clinical cardiologist and an interventional cardiologist) and it was decided to admit him to the cardiology department to undergo a coronary angiography.
Prior to this invasive examination, 50 IU/kg of FVIII was administered and the pre-catheterization FVIII value was 66%. Prior to coronary angiography, a platelet pool transfusion was also performed based on the platelet aggregation study performed by rotational thromboelastometry (ROTEM platelet system), which indicated marked platelet inhibition.
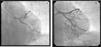
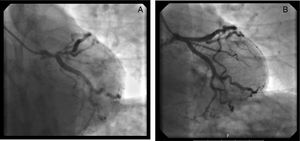
Coronary angiography revealed significant disease in the anterior descending artery - 85% ostial stenosis and 70% proximal stenosis up to the emergence of the first diagonal. No significant lesions were observed in the remaining vessels via angiography. Pre-dilatation with a balloon was performed on the proximal and ostial lesion of the anterior descending artery, and a 3.5×28mm antiproliferative stent was then implanted, with good final angiographic results (Figure 3).
A loading dose of 600 mg of clopidogrel was administered during the procedure, and afterwards dual antiplatelet therapy was maintained with clopidogrel 75mg and ASA 100 mg. In the 72 hours following the coronary angiography, FVIII therapy was maintained at intervals of eight and 12 hours and FVIII levels varied between 38 and 46%.
There were no hemorrhagic complications and hospitalization was uneventful. The patient was discharged on ASA and clopidogrel, with the indication to maintain dual therapy for three months, later interrupting clopidogrel and maintaining ASA indefinitely.
In the first 10 days, he underwent daily replacement therapy, maintaining FVIII levels at around 10%. Subsequently, according to the pharmacokinetics results and the decision to maintain FVIII levels between 3 and 5%, prophylactic therapy was started on alternate days (40 IU/kg). In this patient, and despite intensive treatment, testing for inhibitors was always negative.
DiscussionAddressing acute coronary events in hemophilic patients, as demonstrated in the present case, is a real challenge. The bleeding risk, already high in these patients, is aggravated not only by the hypocoagulation that is necessary during angioplasty, but also by the anti-platelet therapy that is indispensable in the treatment of ACS and in the prevention of stent thrombosis. On the other hand, there are no recommendations regarding the most adequate and safe factor levels in this type of intervention and during antiplatelet therapy, which require a multidisciplinary approach and strict monitoring.
According to the European Hemophilia Association, adequate correction with FVIII concentrate is recommended before percutaneous coronary intervention (PCI) and in the subsequent 48h, without specifying the minimum ideal target value. Thus, little is defined beyond the guidelines proposed by an expert consensus published in 2013,9 where it is suggested that PCI be performed as early as possible, under adequate protection with FVIII, ideally for FVIII values >80% and that in the first 24h after PCI the levels should be ∼50%. This document also proposes FVIII levels between 5 and 15% during dual antiplatelet therapy and >1% when on monotherapy.
In the case of this patient, the pre-PCI values were not achieved, but despite this, there were no hemorrhagic complications, and the levels of FVIII >60% proved to be sufficient and safe. The decision to maintain daily therapy for only 10 days, and subsequently on alternate days, was due to various reasons. If, on the one hand, despite being an adult, venous accesses proved to be an unexpected difficulty, the risk of developing inhibitors was a complication to be considered, in this patient. Experience related to antiplatelet and anticoagulation in hemophilia, apart from being scarce, concerns patients treated many years ago with factor concentrates and in whom the risk of developing inhibitors is practically zero.10 In this patient's case, the risk of the appearance of inhibitors was high, as the first exposure to exogenous FVIII was in a period of intensive treatment, where the risk of antibody development is at a maximum.5,11
Despite the absence of recommendations to carry out a platelet aggregation test in these situations, given the high risk of hemorrhage and the fact that the patient was already previously medicated with antiplatelets, we decided to perform a thromboelastometry, which showed significant platelet inhibition and led to the choice to give treatment with platelet support.
During catheterization, 10500 IU of unfractionated heparin was administered. Although there are some reports in the literature in which bivalirudin was preferred in hemophilia patients,12,13 since it is associated with a lower rate of bleeding complications, unfractionated heparin is still the preferred anticoagulant in view of its relatively short half-life and the possibility of reversibility, if applicable.
Dual antiplatelet therapy is recommended after angioplasty to prevent stent thrombosis. Regarding the choice of antiplatelet drugs, ticagrelor and prasugrel have shown superiority in reducing ischemic complications after ACS when compared to clopidogrel, but they are also associated with higher rates of spontaneous bleeding.14,15 These drugs are strongly recommended in European Cardiology Guidelines for the treatment of ACS in the general population. However, there are no studies in hemophilia patients and therefore they should not be used in these patients. In this case we opted for dual therapy with ASA and clopidogrel for three months, after which the patient remained on ASA alone.
In the past, bare metal stents (BMS) required dual antiplatelet therapy for four to six weeks while first generation drug eluting stents (DES) required dual antiplatelet therapy for six to 12 months. Thus, in patients at a high bleeding risk, BMS were always preferred.16 However, currently, for new generation DES, the duration of dual antiplatelet therapy is similar to that required for BMS. In the RESET17 and OPTIMIZE18 studies, the use of antiplatelet therapy for three months was shown not to be inferior in terms of safety during a follow-up period of one year.
In the present case the use of DES was chosen, despite there being few existing reports of its use in hemophiliac patients, after setting minimum levels for FVIII safety. No bleeding complications were observed, probably due to strict laboratory monitoring and multidisciplinary surveillance.
According to European recommendations, in patients with high bleeding risk, the duration of dual antiplatelet therapy should be kept to a minimum.19 However, there is no evidence regarding patients with bleeding dyscrasias, as these are excluded from most studies. The expert consensus published by the ADVANCE Working Group suggests maintaining dual antiplatelet therapy for one month, but only mentions the use of BMS.9 The use of dual antiplatelet therapy for one month with DES has also been assessed, but only with specific stents: Endeavor Sprint and BioFreedom.20,21 Although these studies included patients with ACS, European guidelines consider that dual therapy for one month should be restricted to patients with stable coronary disease and high bleeding risk (class IIb).19 Thus, in this case, as it was ACS, there was significant risk of stent thrombosis associated with a critical lesion of the descending artery. In addition to the unavailability of the aforementioned stents, and since the patient would maintain strict follow-up in the immunohemotherapy day hospital, we decided to maintain dual antiplatelet therapy for three months.
As for adjuvant therapy, besides the usual medication to control risk factors and prognostic modifying drugs, the addition of a proton pump inhibitor is also recommended in these patients.9,19
Although most angioplasty surgery described in hemophilia patients has been safely performed using a femoral approach, in this patient the coronary angiography was performed using a right radial approach. This is the recommended access, if technically possible, as the vessel is smaller-caliber and peripheral, allowing easier hemostasis and minimizing bleeding complications, without compromising the success of the procedure.
In conclusion, the authors describe the clinical case of a patient with unstable angina and diagnosis of severe hemophilia A, who underwent a successful PCI of the anterior descending artery, under therapy with FVIII, having taken conventional medical therapy with dual antiplatelet therapy without any hemorrhagic complications.
Although the recommendations published by the European Society of Cardiology are based on studies that excluded patients with bleeding dyscrasias, this case is an example that hemophilia patients, if adequately assessed, treated and monitored, and despite the high bleeding risk, can have access to the same therapeutic interventions as non-hemophilia patients and according to the state of the art for ACS.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Aguiar-Ricardo I, Agostinho J, Pereira A, Rodrigues F, Brito D, Pinto FJ, et al. Síndrome coronária aguda em doente com hemofilia A grave: decisões difíceis. Rev Port Cardiol. 2021;40:985–989.