Atypical atrial flutter (AFL) is a supraventricular arrhythmia that can be treated with catheter ablation. However, this strategy yields suboptimal results and the best approach is yet to be defined. Carto® electroanatomical mapping (EAM) version 7 displays a histogram of the local activation times (LAT) of the tachycardia cycle length (TCL), in addition to activation and voltage maps. Using these EAM tools, the study aimed to assess the ability of an electrophysiologic triad to identify and localize the critical isthmus in AFL.
MethodsRetrospective analysis using Carto® EAM of a single center registry of individuals who underwent left AFL ablation over one year. Subjects with non-left AFL, no high-density EAM, under 2000 points or no left atrium wall or structure mapping were excluded. Sites where arrhythmia is terminated via ablation were compared to an electrophysiologic triad comprising areas of low-voltage (0.05 to 0.3 mV), deep histogram valleys (LAT-valleys) with less than 20% density points relative to the highest density zone and a prolonged LAT-valley duration, which included 10% or more of the TCL. The longest LAT-valley was designated as the primary valley, while additional valleys were named as secondary.
ResultsA total of nine subjects (six men, median age 75, interquartile range 71-76 years) were included. All patients presented with left AFL and 66% had a history of ablation for atrial fibrillation and/or flutter. The median TCL and collected points were 254 ms (220-290) and 3300 (IQR 2410-3926) points, respectively. All individuals with AFL presented with at least one LAT-valley on the analyzed histograms, which corresponded to heterogeneous low voltage areas (0.05 to 0.3 mV) and affected more than 10% of TCL. Six of the nine patients presented with a secondary LAT-valley. All arrhythmias were terminated successfully following radiofrequency ablation at the primary LAT-valley location. After a minimum three-month follow-up all patients remained in sinus rhythm.
ConclusionAn electrophysiologic triad identified the critical isthmus in AFL for all patients. Further studies are needed to assess the usefulness of this algorithm in improving catheter ablation outcomes.
O flutter auricular atípico (FLA) é uma arritmia supraventricular que pode ser alvo de tratamento por ablação com cateter. No entanto, a melhor abordagem ainda não foi definida e esta estratégia apresenta resultados subótimos. O sistema de mapeamento eletroanatómico (MEA) Carto® versão 7.0 dispõe, para além dos mapas de voltagem e ativação, de um histograma dos tempos de ativação local (TAL) do ciclo do FLA. Usando esta tecnologia de MEA, o estudo tem como objetivo avaliar a capacidade de uma tríade eletrofisiológica para identificar e localizar o istmo crítico do FLA.
MétodosAnálise retrospetiva de um registo unicêntrico de doentes submetidos a ablação de FLA esquerdo durante um período de em ano e MEA com Carto®. Foram excluídos todos os doentes com FLA não esquerdo, ausência de MEA de alta densidade, aquisição de menos de 2000 pontos ou falta de mapeamento em qualquer parede ou estrutura da aurícula esquerda. Os locais de ablação nos quais houve supressão das arritmias foram comparados com uma tríade eletrofisiológica constituída por: zonas de baixa voltagem (0,05 até 0,3 mV), locais de vales profundos do histograma com menos de 20% da densidade de pontos do MEA relativamente à zona de maior densidade e um vale prolongado com 10% ou mais do ciclo taquicardia. O vale do TAL mais longo foi designado como vale primário, enquanto vales adicionais foram nomeados como secundários.
ResultadosForam incluídos nove doentes (seis homens, mediana de 75 anos IQR 71-76 anos) foram incluídos. Todos os doentes apresentaram FLA esquerdo e 66% tinham sido sujeitos previamente a ablação de fibrilhação e/ou flutter auricular. O ciclo mediano da taquicardia e o número mediano de pontos adquiridos foram respetivamente de 254 (220-290) milissegundos e 3300 (IQR 2410-3926) pontos. Os FLA apresentaram pelo menos um vale primário nos histogramas analisados, correspondendo a áreas heterogéneas de baixa voltagem (0,05 a 0,3 mV) e contendo mais de 10% do ciclo da taquicardia. Seis dos nove doentes apresentavam vales de TAL secundários. Os FLA foram efetivamente tratados no vale primário por ablação com radiofrequência. Após um seguimento de pelo menos três meses, os doentes permaneciam em ritmo sinusal.
ConclusãoUma tríade eletrofisiológica permitiu a identificação do istmo crítico em todos os doentes. São necessários mais estudos para avaliar o valor deste algoritmo para melhorar os resultados da ablação por cateter.
Atrial flutter (AFL) is a supraventricular tachycardia that arises from both substrate and ablation scars.1,2 While typical AFL is treated successfully with cavotricuspid isthmus ablation,3 catheter radiofrequency (RF) ablation results are suboptimal in patients with atypical AFL,4,5 especially when the macroreentrant circuit involves the left atrium. Left AFL ablation usually employs electroanatomic mapping (EAM) systems and entrainment mapping.6 Another possibility is the identification and ablation of the mid-diastolic isthmus.7,8 Nevertheless, the absence of a precise tool to identify the critical isthmus in tachycardia may be one of the reasons for the mixed outcomes using this technique.
MethodsPatient population and study designWe conducted a retrospective analysis of consecutive patients undergoing catheter ablation for left AFL in the previous year. The patients were identified from the prospective registry and they gave written informed consent.
Inclusion criteria were defined as:
- -
EAM with the Carto® system;
- -
At least 1 well distributed high-density activation and voltage map during the procedure;
- -
More than 2000 evenly collected points.
All patients were anticoagulated for at least one month before the procedure and transesophageal echocardiography or computed tomography angiography were performed before the procedure to exclude left atrial thrombus. The procedures were performed under an activated clotting time of over 300 s; an octopolar steerable catheter was placed in the coronary sinus (CS) and a 20-pole catheter (Pentaray®, Biosense Webster Inc., California, USA) was used for high-density mapping. A transseptal puncture was performed with a Brockenbrough needle and an SL 1 sheath (Abbott Laboratories, Abbott Park, IL, USA). The mapping was performed with Carto® EAM system 6.0 version with the CONFIDENSE™ Module with high-density mapping. The activation map was created with a CS fixed reference. The Wavefront algorithm was used to signal annotation. Geometry was created with fast anatomical mapping. The map was collected based on the following CONFIDENSE filters settings: Tissue Proximity Either Electrode or Internal Points Filter set to seven, position and LAT stability of four, density of 1 mm and respiratory gating. RF was delivered with a steerable 3.5 mm irrigated, contact-force catheter (Thermocool®, Biosense Webster Inc., California, USA). All patients underwent conventional mapping, entrainment, scar homogenization and/or line of conduction block. The ablation was performed during AFL rhythm. In patients who had undergone pulmonary vein isolation, residual gaps were eliminated to restore bidirectional block.
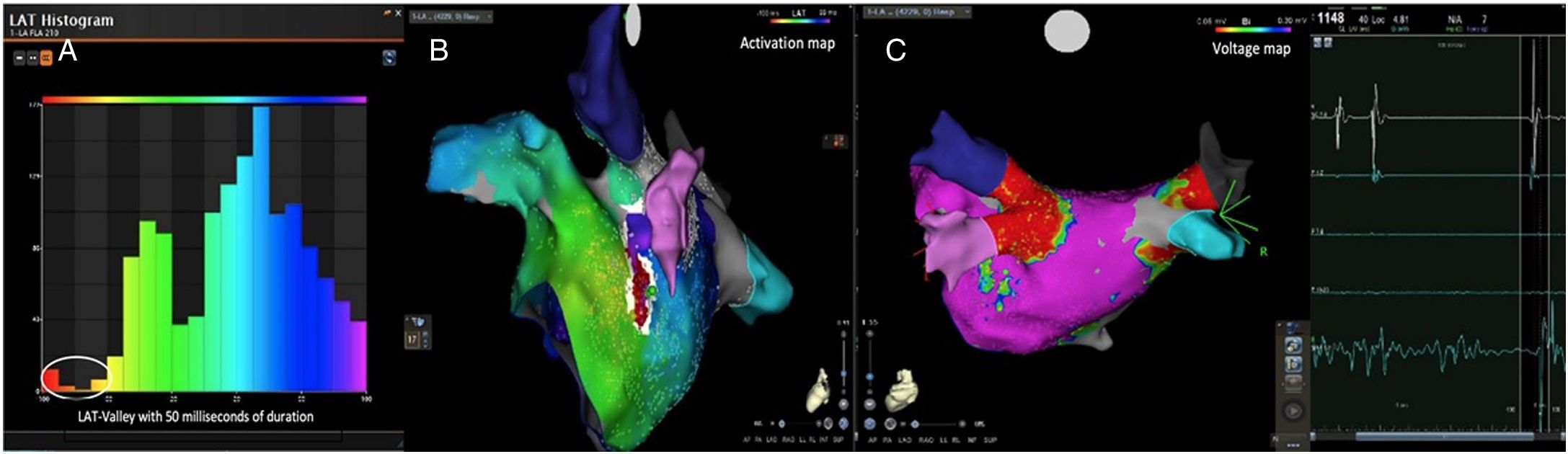
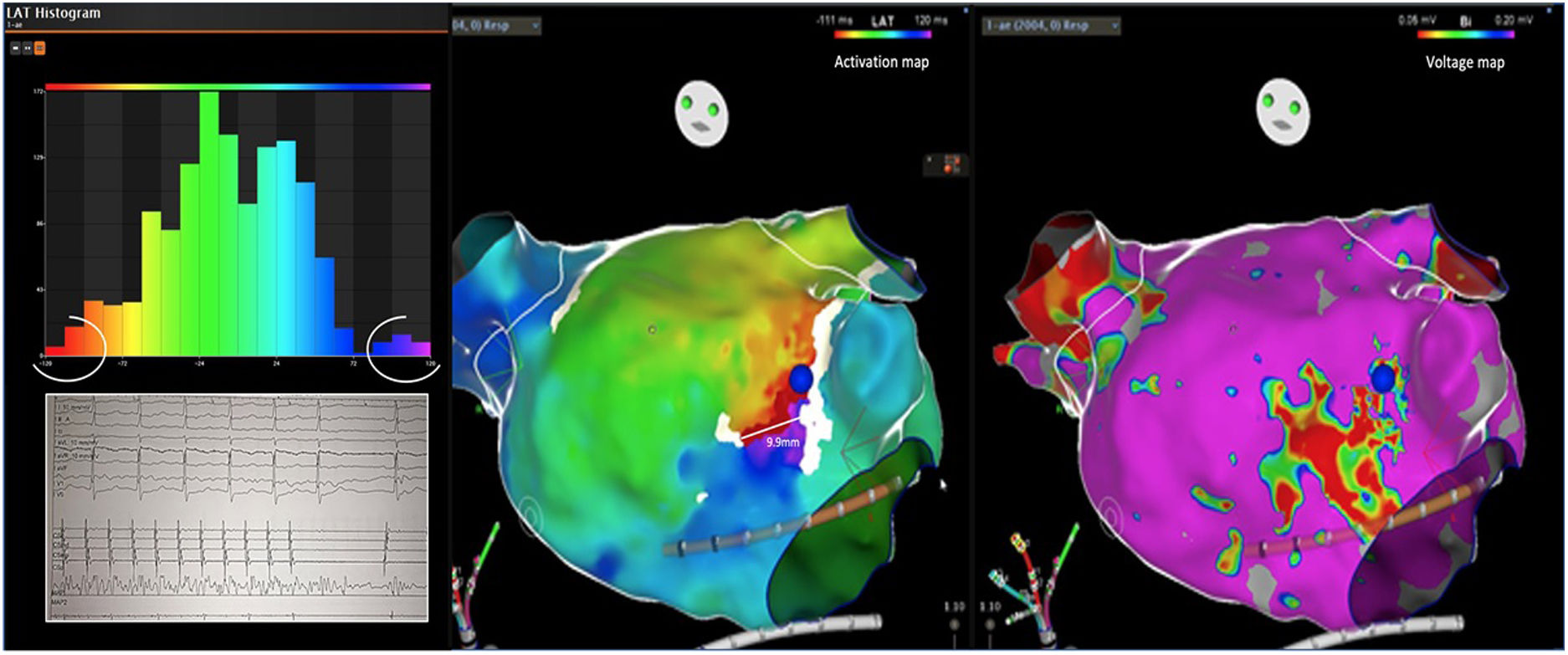
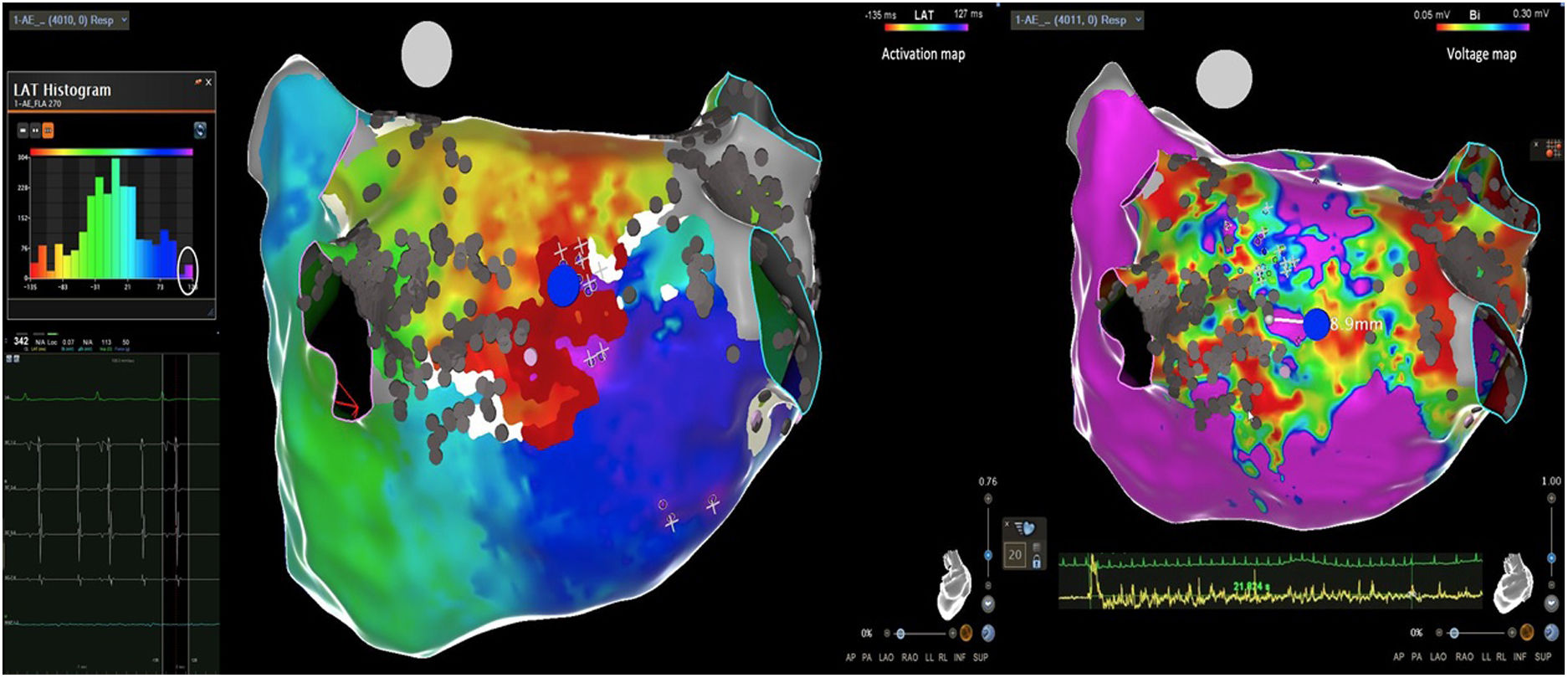
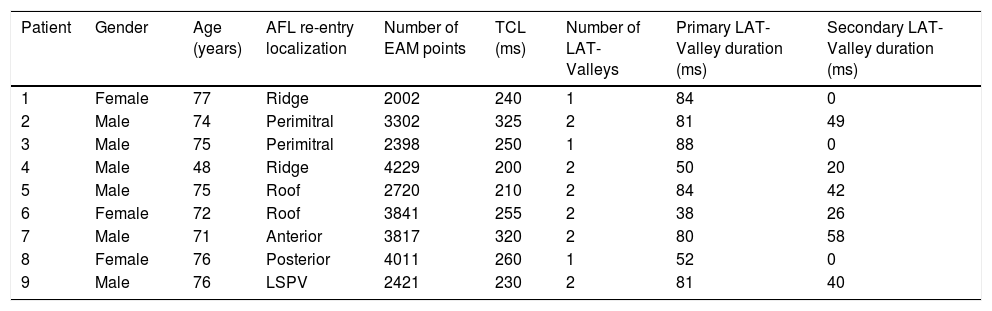
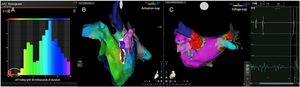
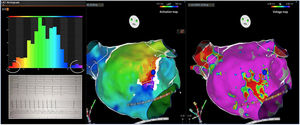
Electrophysiologic triad: EAM with LAT histogram analysisTo identify the critical isthmus in AFL, we assessed an electrophysiologic triad comprising 1) deep histogram valleys (LAT-valley), including 2) a significant portion of the TCL in 3) a heterogeneous low-voltage area. Post-hoc analysis was performed with Carto® version 7.0. This new model displays the LAT sequence in a 20-bar histogram, each one corresponding to 1/20 of the TCL. The amount of points per bar represents the segmental activated area of the left atrium with the same LAT (1/20 TCL). We classified a valley in the LAT histogram (LAT-valley) as a section of the plot with 20% or less recorded points than the maximum LAT value (highest bar).9 To accept this section as a zone of slow conduction, it should contain at least 10% of the TCL.10 The substrate of this zone should be heterogeneous scar tissue and with a local voltage in between 0.05 mV and 0.3 mV.10 When the LAT histogram presents more than 1 “valley”, we designate the area with the longest LAT duration a primary LAT-valley, and all remaining ones secondary LAT-valleys. We retrospectively analyzed the activation and voltage maps and compared the AFL termination site during catheter ablation with the identified LAT-valleys. The Carto® area measurement feature was used to assess the dimension of the isthmus. Normally and non-normally distributed variables were expressed as means ± standard deviation and median, respectively.
Stepwise approach to identify the critical isthmus- 1)
To identify the LAT-valleys: lowest bars on the histogram (zones with 20% or less points relative to the highest bar);
- 2)
To check whether these LAT-Valleys comprise 2 or more histogram bars (duration of 10% or more of the TCL);
- 3)
To confirm whether the selected LAT-valley corresponds to a heterogeneous low-voltage area (0.05 and 0.3 mV);
- 4)
To quantify LAT-Valley atrial surface with the Carto® area measurements feature;
- 5)
To identify whether this region corresponds to the successful ablation site;
- 6)
To assess the number of patients treated successfully in the selected area;
- 7)
To evaluate the efficacy of the treatment with a minimum three-month follow-up.
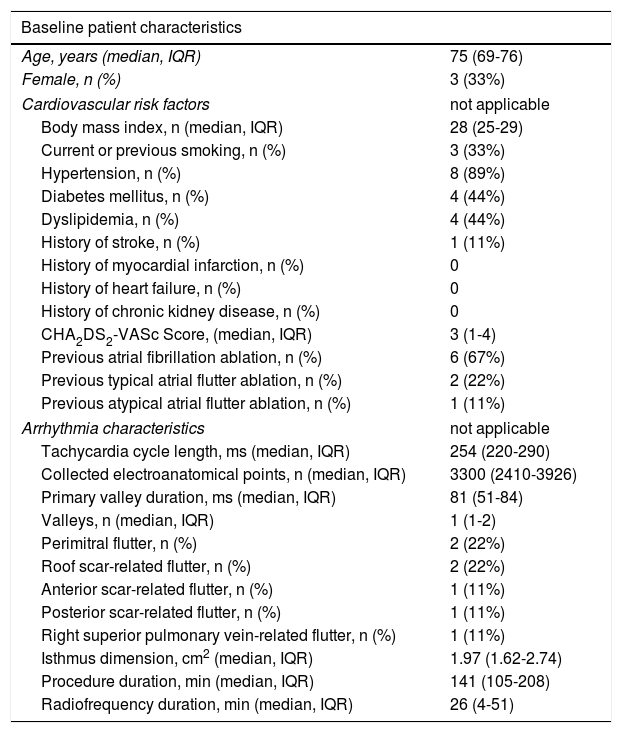
Out of 22 patients, nine met the criteria, three of whom were female, with a median age of 75, interquartile range (IQR) 71-76 years. The clinical and arrhythmic characteristics of the nine patients included in this study are presented in Tables 1 and 2.
Baseline patient characteristics; *including pulmonary vein isolation.
| Baseline patient characteristics | |
|---|---|
| Age, years (median, IQR) | 75 (69-76) |
| Female, n (%) | 3 (33%) |
| Cardiovascular risk factors | not applicable |
| Body mass index, n (median, IQR) | 28 (25-29) |
| Current or previous smoking, n (%) | 3 (33%) |
| Hypertension, n (%) | 8 (89%) |
| Diabetes mellitus, n (%) | 4 (44%) |
| Dyslipidemia, n (%) | 4 (44%) |
| History of stroke, n (%) | 1 (11%) |
| History of myocardial infarction, n (%) | 0 |
| History of heart failure, n (%) | 0 |
| History of chronic kidney disease, n (%) | 0 |
| CHA2DS2-VASc Score, (median, IQR) | 3 (1-4) |
| Previous atrial fibrillation ablation, n (%) | 6 (67%) |
| Previous typical atrial flutter ablation, n (%) | 2 (22%) |
| Previous atypical atrial flutter ablation, n (%) | 1 (11%) |
| Arrhythmia characteristics | not applicable |
| Tachycardia cycle length, ms (median, IQR) | 254 (220-290) |
| Collected electroanatomical points, n (median, IQR) | 3300 (2410-3926) |
| Primary valley duration, ms (median, IQR) | 81 (51-84) |
| Valleys, n (median, IQR) | 1 (1-2) |
| Perimitral flutter, n (%) | 2 (22%) |
| Roof scar-related flutter, n (%) | 2 (22%) |
| Anterior scar-related flutter, n (%) | 1 (11%) |
| Posterior scar-related flutter, n (%) | 1 (11%) |
| Right superior pulmonary vein-related flutter, n (%) | 1 (11%) |
| Isthmus dimension, cm2 (median, IQR) | 1.97 (1.62-2.74) |
| Procedure duration, min (median, IQR) | 141 (105-208) |
| Radiofrequency duration, min (median, IQR) | 26 (4-51) |
IQR: interquartile range.
Characteristics of patients’ arrhythmias.
| Patient | Gender | Age (years) | AFL re-entry localization | Number of EAM points | TCL (ms) | Number of LAT-Valleys | Primary LAT-Valley duration (ms) | Secondary LAT-Valley duration (ms) |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 77 | Ridge | 2002 | 240 | 1 | 84 | 0 |
| 2 | Male | 74 | Perimitral | 3302 | 325 | 2 | 81 | 49 |
| 3 | Male | 75 | Perimitral | 2398 | 250 | 1 | 88 | 0 |
| 4 | Male | 48 | Ridge | 4229 | 200 | 2 | 50 | 20 |
| 5 | Male | 75 | Roof | 2720 | 210 | 2 | 84 | 42 |
| 6 | Female | 72 | Roof | 3841 | 255 | 2 | 38 | 26 |
| 7 | Male | 71 | Anterior | 3817 | 320 | 2 | 80 | 58 |
| 8 | Female | 76 | Posterior | 4011 | 260 | 1 | 52 | 0 |
| 9 | Male | 76 | LSPV | 2421 | 230 | 2 | 81 | 40 |
AFL: atrial flutter; EAM: electroanatomical mapping; LAT: local activation times; TCL: tachycardia cycle length.
Six of the nine patients already had a previous pulmonary vein isolation, 2 of them underwent a typical AFL ablation and 1 a mitral AFL ablation (Table 2). Three of the patients had never undergone a catheter ablation procedure. The AFL were perimitral (n=2), roof scar-related (n=2), anterior scar-related (n=1), posterior scar-related (n=1) and right superior pulmonary vein-related (n=1). The median TCL was 254 ms (IQR 220-290). The EAM had a median of 3300 (IQR 2410-3926) points and presented a median of 2 (IQR 1-2) LAT-valleys. The median duration of the primary valleys was 81 ms (IQR 51-84). In the first seven cases, the exact locations for ablation were defined in a standard fashion, whereas in the last two cases the ablation sites were selected taking in to account the cycle length duration and heterogenous scar (two out of the three elements defined in the triad). In both these cases a single RF application suppressed the AFL.
AFL was terminated successfully (100%) in all nine patients during RF application and the arrhythmia termination zone was tagged in a three-dimensional map (Figures 1, 2 and 3). Post-hoc analysis showed that all these areas corresponded to the primary LAT-valley identified in the global histogram analysis. These areas corresponded to small atrial surfaces of 1 to 2 cm2 with heterogenous low-voltage tissue (between 0.05 and 0.3 mV). We also identified a secondary LAT-valley in six of the nine patients (67%). After three months all patients presented sinus rhythm without documented episodes of arrhythmia recurrence.
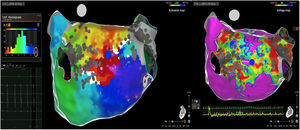
Posterior wall-related flutter, with a valley in the local activation time histogram of the global activation (A) which corresponded to an area of delayed conduction on the activation map (B), and a scar surrounding a channel of healthier tissue on the voltage mapping (C); the arrhythmia ended during radiofrequency applications in this area.
LAT: local activation time; RF: radiofrequency.
Previous approaches to atypical AFL ablation include heterogeneous catheter ablation techniques.10,11 Most centers combine EAM with entrainment maneuvers to identify critical areas of the reentrant circuit.6,10,12 Some authors also suggest the identification of the mid-diastolic isthmus as a potential ablation target.7,8 We used these conventional techniques for mapping and ablation of these patients. However, the procedures are highly complex and require extensive ablation lesions. The results are suboptimal,6 indicating that such practices are not sufficient to identify the critical isthmus. In our post-hoc analysis, we put forward an electrophysiologic triad to recognize a critical isthmus. This triad identified the critical isthmus in all patients in a limited left atrial surface of 1 to 2 cm2. In fact, deep and large LAT-valleys corresponded to critical isthmi and in these locations the heterogenous low-voltage areas were effective targets for ablation. In a previous report9 with another mapping system (Rhythmia®), there was a correlation between a valley in the global late activation histogram and slow conduction areas. However, one in four of the identified valleys did not correspond to delayed conduction. A possible limitation could be incorrect and insufficient point collection which induces false positive results. In this situation, scar tissue with delayed conduction would not be present. In this study, the triad bypasses this possibility since heterogeneous low-voltage (0.05 to 0.3 mV) and conduction delay (≥10% of TCL included) need to present in the LAT-valley, increasing its specificity and accuracy. Current software tools,6 especially Carto® ripple mapping, only show the global left atrium arrhythmia activation wave front. This novel purposed approach presents an important advantage because it can pinpoint the effective ablation area (1 to 2 cm2). The analysis of the activation maps also identified a secondary LAT-valley in most of the patients. They may be related to additional slow conduction areas which are present in more complex AFL, usually associated with previous iatrogenic scars (e.g. post atrial fibrillation ablation). In this regard, the secondary LAT-valleys are potential therapeutic targets if ablation of the primary valley is not sufficient, especially in patients with more than one reentrant circuit. The electrophysiologic triad is potentially useful for assessing any scar-related tachycardia. In the future, the critical isthmus may be easily targeted in the three-dimensional EAM, to improve and simplify the ablation procedure.
Study limitationsThe limitations of this study need to be acknowledged. This was a single-center retrospective study of patients with atypical AFL. Our analysis only assessed patients with effective termination of the arrhythmia, which may have induced survival bias. Also, the small study sample should be taken into consideration in the generalization of our findings. In addition, we lack long-term follow up for relapse of arrhythmia. A randomized study should be performed to clarify whether this tool can improve catheter ablation outcomes.
ConclusionsAn electrophysiologic triad identified the critical isthmus in AFL for all patients. Further studies are needed to assess the usefulness of this tool for improving catheter ablation outcomes.
Conflicts of interestThe authors have no conflicts of interest to declare.