Acute heart failure (AHF) is a heterogeneous clinical syndrome requiring urgent therapy. The prognosis is poor after the index hospitalization, with a high risk for rehospitalization and early death. The costs of managing AHF are thus increasing rapidly. A literature review was performed to gather and compare data on prevalence and treatment and to identify gaps in AHF management, based on European and Portuguese studies.
MethodsA literature search from 1995 to 2014 was conducted in selected databases (BIOSIS Previews, EMBASE and Ovid MEDLINE).
Results and DiscussionSeven Portuguese and nine European studies were analyzed. The mean age of AHF patients was ≥65 years and 30–50% were women. Coronary artery disease (42.3% vs. 61.9%) and hypertension (53.3% vs. 76.7%) were identified as primary etiologies in Europe and in Portugal. Similar proportions of heart failure with preserved ejection fraction were found in the Portuguese (19.9–44.7%) and European (32.8–39.1%) studies. Overall, all-cause mortality rates were comparable (six months: 9.3–25.5% vs. 13.5–27.4%; one year: 15.9–31% vs. 17.4–46.5%), as was in-hospital mortality (5.5–14% vs. 3.8–12%) in Portuguese and European studies, respectively. Length of stay was comparable. The studies were performed in very different hospital settings and data on treatment were scarce.
ConclusionsGaps were identified in treatment and clinical pathways of patients with AHF. Based on the results of this review, collection and investigation of data on the disease and treatment solutions, training in disease management, and improved organization of healthcare should be the subject of further investment.
A insuficiência cardíaca aguda (ICA) é uma síndrome heterogénea que requer intervenção terapêutica urgente. O prognóstico pós-hospitalização é crítico existindo risco aumentado de reospitalização e morte precoce. Consequentemente, os custos da gestão de ICA aumentam exponencialmente. De forma a comparar dados de prevalência e tratamento e identificar lacunas na gestão de ICA, foi realizada uma revisão de literatura.
MétodosRealizou-se uma pesquisa bibliográfica entre 1995-2014 recorrendo a termos específicos e bases de dados selecionadas (BIOSIS Previews, EMBASE, Ovid MEDLINE).
Resultados e discussãoSete estudos portugueses e nove europeus foram considerados. A idade média foi ≥65 anos, sendo 30-50% mulheres. A etiologia primária, na Europa e em Portugal, foram a doença coronária (42,3-61,9%) e a hipertensão (53,3-76,7%). Doentes com insuficiência cardíaca com fração de ejeção preservada nos estudos portugueses (19,9-44,7%) e europeus (32,8-39,1%) foram semelhantes. As taxas de mortalidade por todas as causas foram igualmente comparáveis (seis meses: 9,3-25,5% versus 13,5-27,4%; um ano: 15,9-31% versus 17,4-46,5%, assim como a taxa de mortalidade intra-hospitalar (5,5-14% versus 3,8-12%) nos estudos portugueses e europeus, respetivamente. A duração do internamento foi comparável. Os estudos foram realizados em realidades hospitalares distintas. Os dados da gestão farmacológica são limitados.
ConclusõesForam identificadas lacunas no tratamento e percurso clínico do doente com ICA. Com base nos resultados desta revisão, a geração e investigação de novos dados sobre a doença e soluções de tratamento, treino na gestão da doença, e melhoria na organização dos cuidados de saúde deverão ser áreas de maior investimento.
acute coronary syndrome
atrial fibrillation
acute heart failure
coronary artery disease
cardiac care unit
chronic decompensated heart failure
chronic heart failure
chronic kidney disease
chronic obstructive pulmonary disease
electrocardiogram
ejection fraction
European Society of Cardiology
European Society of Intensive Care Medicine
heart failure
heart failure with preserved ejection fraction
heart failure with reduced ejection fraction
intensive care unit
intravenous
length of stay
New York Heart Association
Heart failure (HF) is a major public health problem worldwide and is associated with high mortality, morbidity, and healthcare costs. The overall prevalence of HF in the US is 2.4% of the adult population, while the corresponding rates in Europe range between 2% and 4.3%, rising to 10–16.1% among individuals aged ≥70 years.1–3 Acute heart failure (AHF) is a complex pathological entity, defined by the European Society of Cardiology (ESC) guidelines as the rapid onset of, or change in, symptoms and signs of HF.4 In most cases, AHF arises as a result of deterioration in patients with a previous diagnosis of HF (HF with either reduced ejection fraction [HFrEF] or preserved ejection fraction [HFpEF]).4 The prognosis of patients with AHF is poor, with high rates of rehospitalization and mortality. In the US nearly 25% of patients with AHF are rehospitalized within 30 days of the index presentation.5,6 In Europe, approximately 44–50% are rehospitalized within one year of an acute episode,3,7 with significant rates of in-hospital and one-year mortality (6.7%8 and 17.4–21%,7,9 respectively). Long-term all-cause mortality is also very high: nearly 50% of patients with a diagnosis of HF will die within five years.10 A prospective analysis also shows that the rate of HF deaths can rise to 23% at 30 days.11 This has prompted medical societies, researchers, policy makers and the pharmaceutical industry to focus their efforts on consolidating and analyzing evidence and developing innovative solutions for the treatment of patients with AHF.
Despite the advances seen in the treatment of chronic heart failure (CHF) over the past few years, the management of patients with AHF, including classification, diagnosis and treatment, has not changed significantly.3 This can be attributed, at least in part, to the wide heterogeneity in clinical presentation and underlying pathophysiological mechanisms of AHF, which poses challenges for the timely diagnosis and individualized management of these patients. The classification of AHF in the ESC heart failure guidelines has undergone a change. The 2008 classification was based on clinical presentation, including a spectrum of conditions such as worsening or decompensated CHF, pulmonary edema, hypertensive HF, cardiogenic shock, isolated right HF, and acute coronary syndrome (ACS).3 In the latest guidelines (2012), classification is limited to the time of presentation of the acute heart failure episode, i.e., de novo (first presentation) or decompensated (deterioration of previously stable CHF).4
In the 2008 ESC guidelines, the approach to assessment of patients with AHF includes hemodynamic profiles, i.e. the presence or absence of congestion (“wet or dry”) and adequacy of perfusion (“warm or cold”).3 This approach is based on the determination of HF hemodynamics is adopted by the authors of the recently implemented Advanced Heart Failure Support Program (SAVIC), a partnership between the Portuguese and Brazilian Societies of Cardiology.12 According to the 2012 ESC guidelines, the diagnosis of AHF is mainly based on signs and symptoms at the time of presentation and various diagnostic modalities such as electrocardiogram (ECG), chest X-ray, echocardiogram, and biomarkers such as natriuretic peptides and other laboratory tests.4 Furthermore, the 2012 ESC guidelines5 recommend that diagnosis and treatment tasks be performed concomitantly.
Regarding AHF treatment, there have been few advances that have dramatically changed its management. The current treatment of AHF includes loop diuretics, vasodilators, and inotropic agents, and is largely opinion-based with very limited evidence from randomized clinical trials.4 This reflects the lower levels of recommendation for AHF therapies, ranging between IB for intravenous (IV) loop diuretics, and IIaC for inotropes.4 Also, most of these therapies focus on short-term symptomatic relief, and the evidence of their effect on long-term outcomes such as mortality is neutral or negative.
There have been numerous registries and studies on heart failure in several European countries including Portugal, but data on the prevalence and drug management of AHF continues to be scarce. In an attempt to gain a comprehensive understanding of AHF and to identify gaps in its characterization and management in Portugal, this review was performed to review the existing data on the epidemiology, clinical presentation, and therapeutic management of AHF in Portugal and to compare it with data from other European countries.
MethodsA literature search was conducted using BIOSIS Previews (1995–2014), EMBASE (1996–2014), Ovid MEDLINE® (without Revisions 1996–October 2014), and Ovid MEDLINE® In-Process & Other Non-Indexed Citations (2014). To ensure that all relevant papers were captured, data from the last 14 years were retrieved. The following text search terms were used: (Incidence OR Prevalence OR Mortality OR Length of stay OR Hospital* OR Cost OR Quality of life OR Outcomes AND Heart failure AND Portugal), (Incidence OR Prevalence OR Mortality OR Length of stay OR Hospital* OR Cost OR Quality of life OR Outcomes AND Heart failure AND Europe). The search terms were free text words rather than MeSH terms, in order to retrieve all relevant articles. The limits applied were: 2000 to October 2014; publication type: articles; humans.
The article selection criteria are illustrated in Figure 1. Out of the total of 1507 articles identified from the literature search, 1411 were rejected based on the title and abstract screening. Of the remaining 96 articles, 87 articles were retrieved for full text review, of which a further 68 were rejected as review articles or irrelevant data or due to duplication or inappropriate disease state. A total of 19 articles were finally included in this review.
Results and DiscussionSeven Portuguese studies were selected for this review (Table 1).13–19 These studies were published between 2004 and 2013 and all of them were observational, single-center studies with a follow-up duration ranging between two and 18 months. Patients in these studies were recruited over a period of six months and AHF was diagnosed on the basis of the diagnostic criteria of the ESC guidelines or the Framingham criteria for HF. A wide variation in sample sizes was observed across the seven studies (n=163 to 924) (Table 1).
Portuguese studies/registries on acute heart failure.
| Bettencourt et al.13 | Sarmento et al.14 | Fonseca et al.15 | Pimenta et al.16 | Cunha et al.17 | Pinho-Gomes et al.18 | Bettencourt et al.19 | |
|---|---|---|---|---|---|---|---|
| Study design | Single-center | Retrospective observational | Group 1 (IMW): retrospective observational Group 2 (HFU): prospective observational | Observational | Prospective | Hospital-based observational retrospective cohort | NA |
| No. of patients | 182 | 180 | 235 (153 IMW; 82 HFU) | 163 | 589 | 924 | 600 |
| No. of centers | Single | Single | Single | Single | Single | Single | Single |
| Follow-up | 6 months | NA | 18 months | 60 days | 6 months | 12 months | 6 months |
| Male (%) | 46.8 | 48 | IMW: 50.3; HFU: 62.2 | 69.9 | 44.3 | 60.7 | 44.7 |
| Guidelines followed | ESCa | ESC | ESC | ESC | ESC | ESC | NA |
ESC: European Society of Cardiology; HFU: heart failure unit; IMW: internal medicine ward; NA: not applicable.
Nine European registries were included (Table 2),7,9,20–28 five of which involved multiple centers across several countries: the Europe Acute Heart Failure Global Registry of Standard Treatment (ALARM), Euro Heart Failure Surveys I and II (EHFS I and II), the European Society of Cardiology - Heart Failure (ESC-HF) LongTerm Registry, and the ESC-HF Pilot Survey. Three of these (EHFS I and II and ESC-HF LongTerm Registry)9,22,23 included patients from Portugal. The remaining registries were conducted in France (EFICA [Etude Française de l’Insuffisance Cardiaque Aigue]21 and OFICA [Observatoire Français de l’Insuffisance Cardiaque Aigue]),27 Italy (Italian Registry on Heart Failure Outcome [IN-HF])26 and one combining data from Zurich and Helsinki (Zurich Helsinki Study).28 These European observational studies were published between 2003 and 2013. The recruitment period ranged between five and 24 months, apart from the OFICA study,27 in which the recruitment period was a single day. The follow-up in these registries ranged from 12 weeks to 12 months. The ESC/European Society of Intensive Care Medicine (ESICM) guidelines and physician-based assessments were used for the diagnosis of AHF and recruitment of patients in these registries. The sample sizes in these studies ranged from 312 to 11327 patients.
European studies/registries on acute heart failure.
| Region | Study design | No. of patients | No. of centers | Follow-up period | Male (%) | Guidelines followed | |
|---|---|---|---|---|---|---|---|
| ALARM20 | France, Germany, Italy, Spain, UK, Greece, Turkey, Australia and Mexico | Retrospective in-hospital | 4953 | 666 | NA | 62.4 | ESC/ESICM (2005) |
| EFICA21 | France | Observational | 599 | 60 | 1 year | 59 | NA |
| EHFS-I22,23 | 24 EU countries including Portugal (351 patients) | Multicenter | 11327 | 115 | 12 weeks | 53 | ESC |
| EHFS-II8,9 | 30 EU countries, including Portugal | Multicenter | 3580 | 133 | NA | 61.3 | ESC |
| ESC-HF Pilot7,24 | Denmark, Norway, Sweden, Romania, Poland, Austria, France, Germany, The Netherlands, Greece, Italy, Spain | Prospective, multicenter, observational | 5118 (1892 AHF) | 136 | 1 year | 62.6 | ESC |
| ESC-HF Long-Term Registry25 | Bulgaria, Czech Republic, Hungary, Poland, Romania, Slovakia, Latvia, Lithuania, Sweden, Bosnia-Herzegovina, Greece, Italy, Portugal, Serbia, Slovenia, Spain, Turkey, Austria, France, Egypt, Israel | Pan-European, multicenter, prospective, observational | 12440 (5039 AHF hospitalized) | 211 | 12 months | 62.7 | ESC |
| IN-HF26 | Italy | Nationwide, prospective, multicenter, observational | 5610 (1855 AHF) | 61 | 1 year | 60.2 | ESC |
| OFICA27 | France | Transversal, multicenter | 1658 | 170 | NA | 54.8 | ESC |
| Zurich Helsinki Study28 | Zurich and Finland | Prospective, observational | 312 | 2 | 1 year | 56.4 | NA |
AHF: acute heart failure; ALARM: Acute Heart Failure Global Registry of Standard Treatment; ESC: European Society of Cardiology; EFICA: Etude Française de l’Insuffisance Cardiaque Aigue (the French Study of Acute Heart Failure); EHFS-I: EuroHeart Failure survey programme Part I; EHFS-II: EuroHeart Failure survey programme Part II; ESC-HFPilot: EURObservational Research Programme: The Heart Failure Pilot Survey; ESC-HF Long-Term Registry: European Society of Cardiology Heart Failure Long Term Registry; ESICM: European Society of Intensive Care Medicine; EU: European Union; IN-HF: Italian Registry on Heart Failure Outcome; NA: not applicable; OFICA: Observatoire Français de l’Insuffisance Cardiaque Aigue (the French Survey on AHF).
There are no published data on the prevalence and incidence of AHF in Portugal. Data on the incidence of AHF could be retrieved from the European registries and showed that the rate of de novo events ranged from 28% to 43%.9,20–28 In most of the Portuguese studies the mean age of patients with AHF was ≥65 years and 30–50% were women,13–19 which was similar to data from the European registries.9,20–28
Data from the Portuguese and European studies and registries revealed that the diagnosis of AHF was primarily based on the version of the ESC guidelines available at the time of the study (Tables 1 and 2).
Across all European and Portuguese registries, coronary artery disease was identified as one of the most common etiologies of AHF, with a similar range of incidence, between 38.3% and 68%. However, the Portuguese studies revealed that hypertension (53.3–76.7%) is still the most prevalent etiology of AHF in the country, as previously identified in the EPICA study.2 Diabetes and atrial fibrillation were the most frequent comorbidities reported in the Portuguese population with AHF (22–52% and 31–46%, respectively), although other comorbidities such as anemia, chronic obstructive pulmonary disease and chronic kidney disease were also frequently observed. Overall, comorbidities in AHF patients were documented at similar rates in all nine European registries, with hypertension (53–70.2%), diabetes (27–45.3%) and atrial fibrillation (24.4–44%) being the most prevalent. Stroke is known to be the leading cause of cardiovascular death in Portugal,29 but data on its incidence in AHF patients (5.2–13.3%) could be retrieved from the European registries only.
Data on functional class could be retrieved from both Portuguese and European studies (Table 3). The Portuguese data revealed a higher proportion of patients in New York Heart Association (NYHA) class IV (31.8–67.3%) than in class III (28.8–42.2%) (Table 3.1). On the other hand, data on functional class were limited in the European registries, with only five registries recording this information (NYHA class III/IV): EFICA21 (31%), EHFS-I22 (26%), ESC-HF Pilot24 (28%) and the ESC-HF Long-Term Registry25 and the Zurich Helsinki study,28 which revealed a higher proportion of patients with severe AHF, ranging between 86% and 94% (Table 3.2).25,28 The differences observed are mainly due to the different methods of data collection.
Summary of Portuguese studies/registries on acute heart failure.
| Bettencourt et al.13 | Sarmento et al.14 | Fonseca et al.15 | Pimenta et al.16 | Cunha et al.17 | Pinho-Gomes et al.18 | Bettencourt et al.19 | |
|---|---|---|---|---|---|---|---|
| Age, years | Mean ± SD 73.0±11.0 | Mean ± SD 74.6±14 | Mean ± SD IMW: 74.6±14.0, HFU: 73.0±12.0 | Median (IQR) 73 (61–80) | Median (IQR) 79 (72–84) | Mean ± SD 69±13 | Median (IQR) 78 (71–84) |
| Etiology, % | |||||||
| CAD | 47.4 | 42.8 | IMW: 42.8; HFU: 43.0 | NA | 39.9 | 38.3 | 40.9 |
| HTN | NA | 62.2 | IMW: 62.2; HFU: 53.3 | 76.7 | 66.2 | 76.6 | |
| Valvular disease | NA | NA | NA | 14.9 | NA | ||
| Cardiomyopathy | 7.5 | ||||||
| Comorbidities, % | |||||||
| AF | 46.2 | 43.3 | IMW: 43.3; HFU: 53.3 | NA | 46.1 | 30.8 | NA |
| Diabetes | 51.9 | 21.6 | IMW: 21.6; HFU: 27.9 | 50.8 | 37.8 | 43.0 | |
| Anemia | NA | 31.7 | NA | NA | 35.8 | NA | |
| CKD | 8.2 | 19.4 | |||||
| Obesity | NA | 31.3 | |||||
| Dyslipidemia | 51.2 | ||||||
| COPD | 27.8 | 12.4 | |||||
| Clinical presentation, % | |||||||
| CDHF | NA | NA | NA | NA | NA | 46.8 | NA |
| PE | 21.4 | ||||||
| HHF | 0.5 | ||||||
| CS | 6.0 | ||||||
| Isolated RHF | NA | 0.5 | |||||
| HF (ACS) | NA | 63.2 | |||||
| Valvular | 10.6 | NA | |||||
| Isolated AF | 3.9 | ||||||
| Functional class, % | |||||||
| NYHA class II | NA | 11 | IMW: 11.0; HFU: 4.5 | NA | NA | NA | NA |
| NYHA class III | 32.7 | 42.2 | IMW: 42.2; HFU: 28.8 | 15.3 | |||
| NYHA class IV | 67.3 | 44.8 | IMW: 31.8; HFU: 66.7 | 60.4 | |||
| LVSF (based on EF), % | |||||||
| Preserved | 19.9 | 32.8 | IMW: 32.8; HFU: 23.0 | 25.8 | 44.7 | 26.8 | NA |
| Mild LVSD | 39.7 | 42.2 | IMW: 42.2; HFU: 48.6 | 6.1 | 6.1 | NA | 55.1 |
| Moderate LVSD | 18.4 | 14.9 | 37.1 | ||||
| Severe LVSD | 23.1 | 49.7 | 34.3 | 36.1 | |||
| AHF treatment/management | |||||||
| Medication (acute phase), % | |||||||
| ACE inhibitor | 89.1 | NA | NA | NA | NA | NA | NA |
| Beta-blocker | 39.7 | ||||||
| Loop diuretic | 98.1 | ||||||
| Spironolactone | 37.8 | ||||||
| IV vasodilators | 28.2 | ||||||
| Medication (at discharge), % | |||||||
| ACE inhibitor | 87.2 | NA | IMW: 63.2; HFU: 77.9 | NA | 79.4 | NA | 78.9 |
| ARB | NA | IMW: 2.9; HFU: 2.1 | NA | NA | |||
| Beta-blocker | 39.1 | IMW: 4.6; HFU: 36.0 | 75.9 | 75.9 | |||
| Loop diuretic | 98.1 | IMW: 80.5; HFU: 82.6 | NA | NA | |||
| Spironolactone | 37.2 | IMW: 30.5; HFU: 39.5 | 31.9 | 23.4 | 23.5 | ||
| Digoxin | NA | IMW: 24.1; HFU: 24.4 | 31.9 | NA | NA | ||
| Nitrates | NA | NA | 50.0 | ||||
| Statins | 61.6 | ||||||
| Antiplatelets | IMW: 55.2; HFU: 41.9 | 66.4 | |||||
| Type of center | IM department | IM department | IMW (n=180) HFU (n=86) | IM department | IM department | Cardiology department | IM department |
| Clinical outcomes | |||||||
| Mortality (all-cause), % | |||||||
| 6 months | 17.9 | 9.3 | IMW: 25.5; HFU: 21.0 | NA | NA | 10.9 | 20.34 |
| 12 months | NA | NA | IMW: 31.0; HFU: 30.0 | 15.9 | NA | ||
| In-hospital mortality, % | 14.3 | 7.7 | IMW: 7.7; HFU: 8.5 | NA | NA | 5.5 | NA |
| Rehospitalization, % | |||||||
| 6 months | 37.2 | NA | NA | NA | NA | 20.9 | 39.5 |
| 12 months | NA | 23.9 | NA | ||||
| LOS (in days) | Mean ± SD As per NT-proBNP levels: 11.3±8.3 (≥30% decrease) 10.2±5.4 (<30% change) 13.4±10.4 (≥30% increase) | Mean 13.8 | Mean ± SD IMW: 13.8 HFU: 10.5±6.7 | NA | Median (IQR) 8.0 (6–11) | Median (IQR) 11.0 (7–16) | NA |
ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; AF: atrial fibrillation; ARB: angiotensin receptor blocker; CAD: coronary artery disease; CDHF: chronic decompensated heart failure; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; CS: cardiogenic shock; EF: ejection fraction; HF: heart failure; HHF: hypertensive heart failure; HFU: heart failure unit; HD: heart disease; HTN: hypertension; IM: internal medicine; IMW: internal medicine ward; IQR: interquartile range; IV: intravenous; LOS: length of stay; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; LVSF: left ventricular systolic function; NA: not applicable; NT-proBNP: N-terminal-pro brain natriuretic peptide; NYHA: New York Heart Association; PE: pulmonary edema; PSF: preserved systolic function; RHF: right heart failure; SD: standard deviation.
Summary of European studies/registries on acute heart failure.
| ALARM20 | EFICA21 | EHFS-I22,23 | EHFS-II8,9 | ESC-HF Pilot7,24 | ESC-HF Long-Term Registry 25 | IN-HF26 | OFICA27 | Zurich Helsinki Study28 | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | Median 66–70 | Mean ± SD 73±13 | Mean 71.0 | Mean ± SD 69.9±12.5 | Mean ± SD 70±13 | Median (IQR) 71 (61–79) | Mean ± SD 72±12 | Median 79.3 | Mean ± SD 73 |
| Etiology, % | |||||||||
| ACS | 36.9 | NA | MI: 39 | 30.2 | NA | NA | NA | STEMI: 4.6; NSTEMI: 8.8 | NA |
| Arrhythmia | 26.9 | NA | 32.4 | 23.7 | |||||
| Infection | 16.3 | 17.6 | 27.2 | ||||||
| Cardiomyopathy | NA | 15 | 6 | NA | NA | 24.4 | |||
| Valvular disease | 21 | 29 | 26.8 | NA | NA | ||||
| Angina | NA | 51 | NA | ||||||
| HTN | NA | 6.2 | |||||||
| CAD | 61 | 68 | NA | 50.7 | 54 | 42.3 | 43.6 | 61.9 | |
| Comorbidities, % | |||||||||
| Hypertension | 70.2 | 60.0 | 53.0 | 62.5 | 61.8 | 64.5 | 57.8 | 61.7 | 53.8 |
| Diabetes | 45.3 | 27.0 | 27.0 | 32.8 | 35.1 | 38.9 | 40.4 | 31.0 | 32.1 |
| Stroke | NA | NA | 9.0 | 13.3 | NA | 13 | 5.2 | NA | NA |
| AF | 24.4 | 42.0 | 38.7 | 43.7 | 44 | 37.7 | 38.0 | 29.2 | |
| CAD | 30.7 | NA | NA | NA | NA | NA | NA | NA | |
| COPD | 24.8 | NA | 19.3 | NA | 20.2 | 30.1 | 21.0 | NA | |
| CKD | 21.4 | 17.0 | 16.8 | 26.0 | 26.4 | 32.5 | 15.0 | 41.0 | |
| Cardiomyopathy | 12.6 | NA | 19.3 | NA | NA | NA | NA | NA | |
| Comorbiditiesa | 51.0 | NA | NA | NA | NA | NA | NA | NA | |
| Clinical presentation, % | |||||||||
| ADHF | 38.6 | NA | NA | 65.4 | 75.0 | NA | 43.9 | 48.0 | NA |
| Acute breathlessness | NA | 40.0 | NA | NA | NA | NA | |||
| Dyspnea | NA | 94.1 | |||||||
| Orthopnea | 71.8 | ||||||||
| Chest pain | 36.4 | ||||||||
| PE/congestion | 36.7 | 82.0 | 16.2 | 13.3 | 85.0 | 27.0 | 38.0 | NA | |
| CS | 11.7 | 29.0 | <0.01 | 3.9 | 2.3 | NA | 2.3 | 6.0 | |
| Hypertensive HF | 7.4 | NA | NA | 11.4 | 4.7 | 5.1 | 2.0 | ||
| RVHF | 4.5 | NA | NA | 3.2 | 4.7 | NA | 8.8 | 6.0 | NA |
| Peripheral edema | NA | 27.0 | NA | NA | NA | NA | |||
| Angina | 14.0 | ||||||||
| Hepatomegaly | 20.0 | ||||||||
| Syncope | 4.0 | ||||||||
| Arrhythmia | 23.0 | 2.0 | |||||||
| Stroke | 1.0 | NA | |||||||
| ACS | NA | 19.0 | 12.9 | ||||||
| AF | 9.0 | NA | |||||||
| NYHA functional class, % | |||||||||
| I | NA | 36.0 | 36.0 | NA | 72.0 | NA | NA | NA | NA |
| II | 37.0 | ||||||||
| III | 22.0 | 26.0 | 28.0 | 85.9 | 94.1 | ||||
| IV | 9.0 | ||||||||
| LVSF (based on EF) | |||||||||
| Mean LVEF ± SD | 40±15% | 38±15% | 63.0% | 38±15% | NA | NA | 38±14% | NA | NA |
| Median LVEF (IQR) | NA | NA | NA | NA | 38 (27–52)% | 38 (30–51)% | NA | 40 (30–55)% | 34.9%, 31.9% |
| HFpEF, % | NA | NA | NA | 34.3(EF ≥45%) | 39.1 (EF>40%) | 32.8 (EF >45%) | 35.0 (EF>40%) | 36.2 (EF >50%) | 33.2 (EF ≥50%) |
| AHF treatment/management | |||||||||
| Medication (acute phase), % | |||||||||
| IV/oral diuretic | 89.7/60.5 | NA | NA | 92.9 | NR: 34.3% ER: 65.2% WR: 32.1% SR: 45.3% | 81.5 | 15.7 | 86.2 | NA |
| Loop diuretic | NA | 87.0 | 86.9 | NA | NA | NA | 99.4 | NA | |
| Spironolactone | 27.5 | 18.0 | 20.5 | NA | |||||
| ACE inhibitor | NA | 42.0 | 61.8 | NR: 74.3% ER: 79.6% WR: 79.4% SR: 70.3% | |||||
| Nitrates (IV) | 41.1 | 50.0 | 32.1 | 37.8 | NA | 20.4 | 29.9 | ||
| Beta-blocker | 37.8 | 13.0 | 36.9 | 10.1 | NR: 79.3% ER: 85.2% WR: 82.1% SR: 66.4% | NA | NA | NA | NA |
| Inotropes (IV) | NA | 7.2 | NA | NA | 11.9 | 19.4 | 13.2 | ||
| Adrenaline | 3.6 | NA | 1.8 | NA | NA | NA | |||
| Dobutamine | 22.3 | 10.2 | 7.7 | ||||||
| Dopamine | 13.0 | 11.3 | 13.9 | ||||||
| Levosimendan | 6.4 | 3.9 | 3.9 | ||||||
| Noradrenaline | 4.2 | 2.6 | NA | ||||||
| Amiodarone | 2.6 | 27.0 | 17.5 | ||||||
| ARB | NA | 3.0 | 4.5 | NA | |||||
| Digitalis | 16.0 | NA | |||||||
| CCB | NA | 21.2 | |||||||
| Dihydropyridine | NA | 6.0 | NA | NA | NA | NA | NA | NA | NA |
| Non-dihydropyridine | 4.0 | ||||||||
| Opioids | NA | 19.4 | |||||||
| Cardiac glycosides | 35.7 | NA | |||||||
| Antiplatelets | 43.0 | NA | |||||||
| Medication (at discharge), % | |||||||||
| ACE inhibitor | NA | 57.0 | NA | 71.1 | NA | 77 | NA | 55 | NA |
| ARB | 3.0 | 10.4 | NA | 13 | |||||
| Beta-blocker | 21.0 | 61.4 | 71.8 | 60 | |||||
| Loop diuretic | 79.0 | 90.1 | 83.6 | 84.8 | |||||
| Spironolactone | 19.0 | 47.5 | NA | 18 | |||||
| Digitalis | 17.0 | 31.0 | 26.4 | 10 | |||||
| Inotropes (amiodarone) | 27.0 | NA | 13.7 | 20.7 | |||||
| Antiplatelets | 45.0 | Aspirin: 49.4, clopidogrel: 13.4 | 61.9 | NA | |||||
| Nitrates | NA | 35.0 | NA | 32.9 | NA | 32 | NA | NA | NA |
| CCB | 14.6 | 15.9 | 18 | ||||||
| Dihydropyridine | 9.0 | NA | NA | NA | |||||
| Non-dihydropyridine | 5.0 | ||||||||
| Types of center (% patients) | ICU (45.4), CCU (29.8), wards (24.8) | ICU (49), CCU (51) | GMW (50), cardiology wards (43), geriatric wards (5) | ICU/CCU (51), emergency area, IM and cardiology wards | Cardiology | Cardiology | Cardiology | ICU (5), CCU (35), cardiology ward (42), IM (18) | Emergency room, ICU, ward |
| Clinical outcomes | |||||||||
| Mortality (All-cause), % | 10.7b | 27.4 (1 month) 46.5 (1 year) | 13.5 (12 weeks) | NA | 17.4% (1 year) | NA | NA | NA | 29 (1 year) |
| In hospital mortality, % | 12.0 | NA | 6.9 (12 weeks) | 6.7 | 3.8 | NA | 6.4 | 8.2 | NA |
| Rehospitalization, % | NA | NA | 34.9 (12 weeks) | NA | 43.9% (1 year) | NA | NA | NA | NA |
| LOS, days | Median (IQR) 6 (4–10) | Mean 15.1±23.8 vs. 14.5±15.1 (with vs. without cardiogenic shock) | Mean 11.0 | Median (IQR) 9 (6–14) (overall), 3 (2–5) (ICU/CCU) | Median (IQR) 8 (5–11) | NA | Median 10.0 | 13 days (8–20) | Center 1: 11.5 (2–77) days; center 2: 8 (1–50) days; ICU stay: 3 days |
ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; ADHF: acute decompensated heart failure; AF: atrial fibrillation; ALARM: Acute Heart Failure Global Registry of Standard Treatment; CAD: coronary artery disease; CCBs: calcium channel blockers; CCU: cardiac care unit; CDHF: congestive decompensated heart failure; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CS: cardiogenic shock; EFICA: Etude Française de l’Insuffisance Cardiaque Aigue (the French Study of Acute Heart Failure); EHFS-I: EuroHeart Failure survey programme Part I; EHFS-II: EuroHeart Failure survey programme Part II; ER: eastern region; ESC-HFPilot: EURObservational Research Programme: The Heart Failure Pilot Survey; ESC-HF Long-Term Registry: European Society of Cardiology Heart Failure Long Term Registry; GMW: general medical ward; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFU: heart failure unit; HTN: hypertension; ICU: intensive care unit; IM: internal medicine; IMW: internal medicine ward; IN-HF: Italian Registry on Heart Failure Outcome;IQR: interquartile range; IV: intravenous; LOS: length of stay; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; MI: myocardial infarction; NA: not applicable; NR: northern region; NSTEMI: non-ST-segment elevation myocardial infarction; NYHA: New York Heart Association; OFICA: Observatoire Français de l’Insuffisance Cardiaque Aigue; PE: pulmonary edema; RVHF: right ventricular heart failure; SD: standard deviation; SR: southern region; STEMI: ST-segment elevation myocardial infarction; WR: western region.
Based on the available data, Portuguese13–19 and European9,20–28 patients were found to have comparable ejection fraction (EF) measurements. However, it is important to point out that this classification was based on different cut-offs of EF across all retrieved studies (preserved EF could be defined as an EF >40%,14,24,26 >45%,9,13,15,25 or >50%17,18,27,28). Regarding EF classification, a higher proportions of patients were found to have HFrEF (42.2–74.2%) than HFpEF (19.9–44.7%).9,13–28
Data on the clinical presentation of patients with AHF were scarce in the Portuguese studies. In the study by Pinho Gomes et al., 18 reflecting HF in a cardiology department setting, ACS was the most frequent clinical presentation (63.2%), followed by chronic decompensated heart failure (46.8%), and pulmonary edema (21.4%). Among the Portuguese studies, most of which were performed in internal medicine departments, the most common etiology reported for HF was hypertension (53.3–76.7%). In contrast, the European registries captured data from a broad spectrum of clinical settings including cardiology departments. Furthermore, according to the available data, more than 40% of patients were admitted to the intensive care unit (ICU), and the most common clinical presentation was decompensated HF (38.6–75%) followed by pulmonary edema (16.2–82%). Other clinical presentations included cardiogenic shock, arrhythmia, right ventricular HF, hypertensive HF and ACS.9,20–28
Overall, the demographic data in the Portuguese and European studies provide similar evidence.
Treatment of acute heart failureData on the pharmacological management of AHF (from the hemodynamic stabilization phase to discharge) are limited in Portuguese and European studies and are not uniformly captured.
Hemodynamic stabilization stageOnly one Portuguese study presented data on the medication administered during the hemodynamic stabilization phase. Bettencourt et al.13 reported that 98.1% of patients with AHF received loop diuretics. During this phase other drugs administered were ACE inhibitors (89.1%), beta-blockers (39.7%), spironolactone (37.8%), and IV vasodilators (28.2%). Data on acute phase treatment could also be retrieved from the majority of the European studies. Diuretics (including loop diuretics) were reported to be the most commonly administered medications (60.5–99.4%) during this phase, followed by ACE inhibitors (42–79.6%), IV nitrates (20.4–50%), and beta-blockers (10.1–85.2%).7,9,20,21,23,25–27
Of all the European registries analyzed in this review, the ALARM-HF registry20 provided the most detailed data on drug treatment and therapeutic measures at admission, both in the overall patient population and in different country subgroups of patients. IV diuretics were the most frequently used drugs for initial symptomatic treatment of AHF, followed by nitrates; IV diuretics were used in more than 80% in the European patients (France, Germany, Italy, Spain, UK, Greece, and Turkey), while IV nitrates (mainly nitroglycerine) were administered in 41.1% of patients. Marked geographical variability was observed in the use of both IV inotropic agents (26–51%) and IV vasodilators across participating countries of the ALARM-HF registry (data not shown).20
Therapeutic optimization stageData on medications administered at discharge were captured in five Portuguese studies and four European registries.9,13,15–17,19,21,25,27 In the Portuguese studies, commonly used medications at discharge included ACE inhibitors (78–87%), beta-blockers (4.6–75.9%) and spironolactone (23.4–39.5%), which was similar to the European registries (Tables 3.1 and 3.2).9,13,15–17,19,21,25,27 In two of the Portuguese studies, use of loop diuretics at discharge was particularly high (83–98%).13,15 It is noteworthy that the variation observed with respect to the study setting was more noticeable in the European registry data than in the Portuguese studies (Tables 3.1 and 3.2).
Clinical outcomesMost of the Portuguese studies captured data on clinical outcomes such as all-cause long-term and in-hospital mortality, rehospitalizations and length of stay (LOS). Overall, all-cause mortality rates were comparable in the Portuguese and European studies (at six months: 9.3–25.5% in Portuguese studies and 13.5–27.4% in European studies; at one year: 15.9–31% in Portuguese studies and 17.4–46.5% in European studies). A similar trend was observed for in-hospital mortality (5.5–14% in Portuguese studies and 3.8–12% in European registries). Only three Portuguese studies13,18,19 and two European registries7,22 reported data on rehospitalization; the rates varied from 20.9% to 39.5% at six months, and 23.9–43.9% at one year.
Hospital LOS was captured in five out of seven Portuguese studies and ranged between eight and 13.8 days.13–15,17,18 This is in line with data from the European studies, which reported a range of LOS between six and 15.1 days. One Portuguese study also collected and compared LOS data between two specific settings, a heart failure unit (10.5 days) and an internal medicine ward (13.8 days).15 Two European registries also collected data on the overall LOS in specific settings, showing an overall LOS of 8–11.5 days, of which three days were spent in the ICU/cardiac care unit (CCU).9,28 There were no other data captured on worsening HF; however, end-organ damage was assessed through biomarker data in some Portuguese13,16–19 and European20,26,27 studies.
Broadly, the data were similar in the Portuguese and European studies in terms of baseline demographics, patient characteristics, comorbidities, and treatment patterns at discharge. There were limited data on medication administered during the hemodynamic stabilization stage in Portuguese studies and thus no comparisons with the European registries could be made. However, some European registries, such as ALARM-HF,20 revealed considerable variability in the use of certain drug classes such as IV inotropes and vasodilators. This variability may be attributed to the lack of evidence-based (gold standard) therapies for AHF in the guidelines. In fact, while many drugs have been shown to reduce long-term mortality and morbidity in CHF, data in the acute setting have been negative or neutral. Consequently, the guidelines’ recommendations are mainly class C. In addition, considerable heterogeneity was observed in patients flows between specialties and treatment centers (ICU, CCU, HF units, cardiology and internal medicine wards, and emergency rooms) involved in the management of patients with AHF, which in turn may be partly responsible for the variations observed in AHF treatment and outcome patterns. Moreover single center studies and even real-world registries predominantly conducted in cardiology departments may not reflect the actual management of this syndrome.
ConclusionThis review aimed to gather and compare data on the prevalence and management of AHF from European and Portuguese studies. Gaps were identified in the data on epidemiology, treatment and clinical outcomes in patients with AHF in both the Portuguese studies and European registries. The key limitation of this review is the existence of studies with a small sample size, short follow-up period, lack of comprehensive assessment of clinical outcomes and treatment patterns. When the available data could be compared, the evidence provided by Portuguese and European studies was similar. High mortality and hospitalization rates were observed for AHF in all the studies analyzed, which will presumably be reflected in a proportionally higher impact on costs in all healthcare systems. Acute heart failure should be the focus of the scientific community and public entities so that policies may be generated to help patients and physicians to manage this disease.
Future implicationsBased on the results of this review, three areas of further investment can be identified: collection of data and investigation of the disease and treatment, training in disease management, and improved organization of healthcare.
Regarding the scarcity of data, there is a clear need to implement well-defined studies incorporating larger samples, longer follow-up periods, and better defined clinical endpoints. From a national perspective, in practice this means the need for a Portuguese registry that captures accurate data on acute and chronic HF. Steps have been taken by the Portuguese Society of Cardiology to adopt the design of the ESC-HF Long-Term Registry aiming to include various types of centers: cardiology, internal medicine, and intensive care departments, so that more patients than those included in the ESC-HF Long-Term Registry can be captured. Furthermore, uniformity in reporting the treatment of patients with AHF is crucial to identify gaps in disease management. In terms of research and development of innovative solutions for the treatment of AHF, there is a need for new trials with appropriate endpoints that can capture robust acute phase data.
Identification of challenges and a focus on both management strategies and research needs are required to significantly decrease the burden of AHF. The Portuguese medical community is aware of the need for training in the management of AHF, and to address this, the Portuguese Society of Cardiology is implementing the Advanced Heart Failure Support program, a Portuguese Society of Cardiology/Brazilian Society of Cardiology partnership.
Finally, there is also an urgent need to improve healthcare organization for patients with AHF. Consensual patient flows based on different healthcare levels and specialties involved in AHF management should be implemented to improve patients’ quality of life and survival, and to reduce the burden on the healthcare system.
Conflicts of interestDaniel Brás is an employee of Novartis Farma S.A., Porto Salvo, Portugal.
The authors thank Dr. Shilpi Dasgupta (Novartis Healthcare Pvt. Ltd, Hyderabad, India) for medical writing and editorial support.









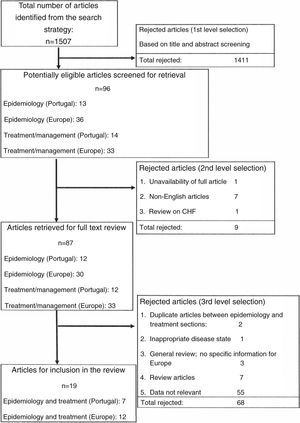 CHF: chronic heart failure.'/>
CHF: chronic heart failure.'/>

