Current rates of permanent pacemaker implantation (PPMI) after transcatheter aortic valve implantation (TAVI) range between 3.4% and 25.9%. PPMI is associated with a worse prognosis. A lower valve implantation depth is associated with an increased risk of conduction disturbances. Theoretically, cusp-overlap projection (COP) has the potential to enable higher valve deployment.
ObjectiveTo compare the 30-day PPMI incidence post-TAVI using self-expanding valves according to the fluoroscopic guidance technique.
MethodsThis retrospective single-center study assessed consecutive patients undergoing TAVI with CoreValve™ valves between April 2019 and November 2021, grouped according to the fluoroscopic guidance technique (COP vs. coplanar implantation technique [CIT]).
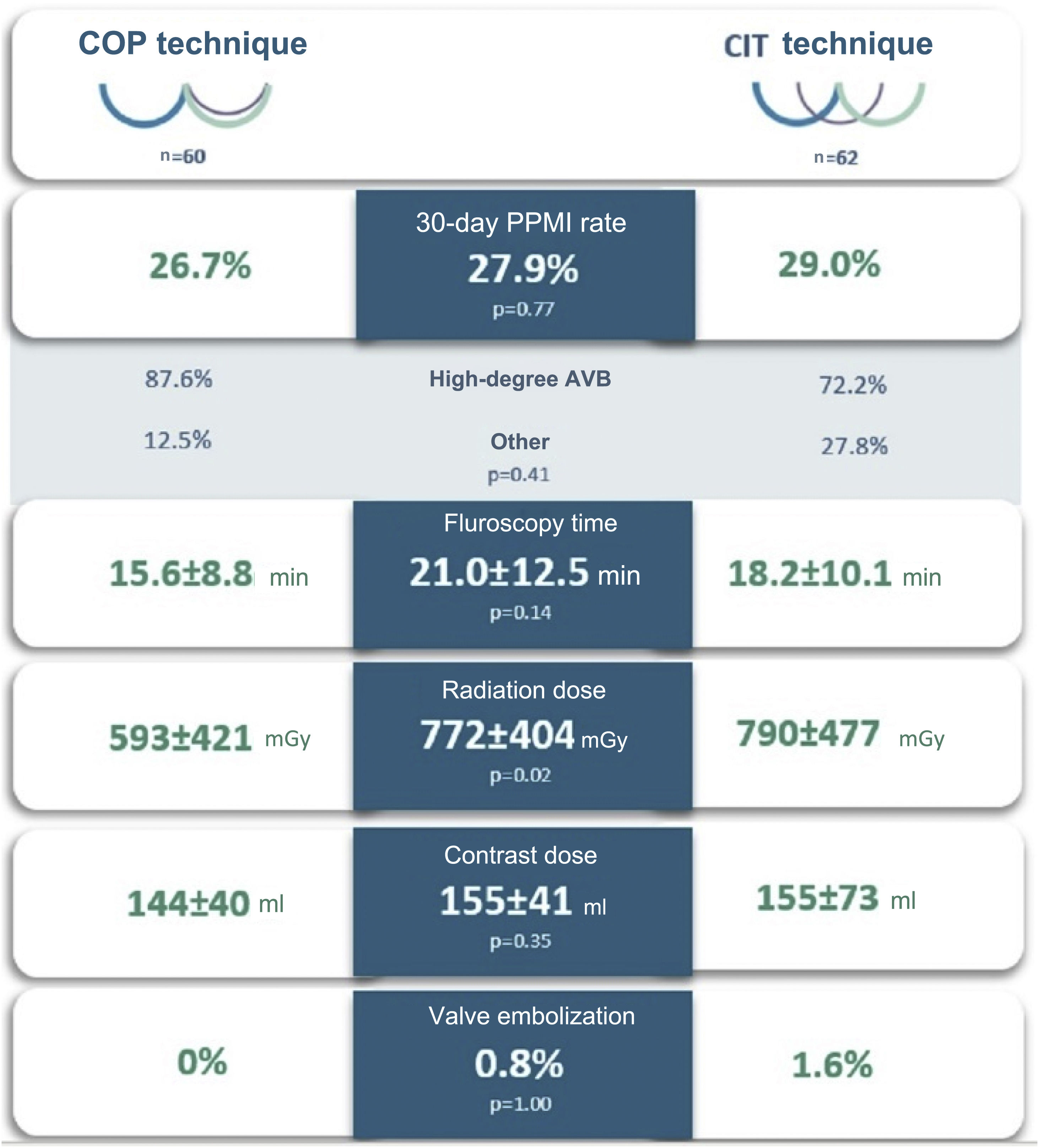
ResultsA total of 122 patients were included, predominantly women (52.5%), with a mean age of 81.6±5.5 years. COP was used in 49.2% of the sample. The CIT group had a significantly higher prevalence of previous beta-blocker use (p<0.01), lower baseline left ventricular ejection fraction (p=0.04) and a higher EuroSCORE II (p=0.02). The 30-day PPMI rate was 27.9% (n=34), with no significant difference between the COP and CIT groups (26.7% vs. 29.0%, p=0.77). Complete atrioventricular block was the main cause (38.5%). Likewise, mean fluoroscopy time (p=0.14) and contrast volume (p=0.35) used were similar between the two groups. Radiation dose was lower in the COP group (p=0.02). There was no significant difference between post-TAVI grades III and IV aortic valve regurgitation (p=0.27) and there were no cases of periprocedural acute coronary occlusion.
ConclusionsThis study shows that the COP technique, although safe and not associated with increased complexity, did not significantly reduce the 30-day PPMI rate compared to the traditional CIT view.
Atualmente, a prevalência de implantação de pacemaker permanente (PPMI) após colocação de válvulas aórticas percutâneas (VAP) varia entre 3,4-25,9%, estando tal necessidade associada a um pior prognóstico. A implantação baixa da prótese valvular determina um maior risco de desenvolvimento de distúrbios de condução. A técnica angiográfica de implantação da VAP baseada na sobreposição de cúspides (COP) possibilita teoricamente a libertação mais alta da prótese.
ObjetivoComparar a prevalência de PPMI aos 30 dias pós-VAP com válvulas autoexpansíveis, de acordo com a técnica fluoroscópica usada aquando da implantação.
MétodosEstudo retrospetivo e unicêntrico baseado na análise de doentes consecutivamente submetidos a VAP com CoreValveTM entre abril de 2019 e novembro de 2021, dicotomizados de acordo com a técnica de fluoroscópica usada aquando da implantação protésica (COP versus técnica coplanar padrão - CIT).
ResultadosForam incluídos 122 doentes, predominantemente mulheres (52,5%), com uma idade média de 81,6±5,5 anos. A técnica COP foi utilizada em 49,2% da amostra. O grupo em que foi aplicada a CIT apresentou uma prevalência significativamente maior de uso prévio de betabloqueador (p<0,01), menor fração de ejeção ventricular esquerda basal (p=0,04) e maior EuroSCORE II (p=0,02). A prevalência de PPMI 30 dias pós-VAP foi de 27,9% (n=34), sem diferença significativa entre os grupos COP e CIT (26,7% versus 29,0%, p=0,77). O bloqueio auriculoventricular completo foi a causa subjacente mais comum (38,5%). De igual modo, não se identificaram diferenças estatisticamente significativas relativas ao tempo médio de fluoroscopia (p=0,14) e volume de contraste (p=0,35) usados entre os dois grupos. A dose de radiação usada foi significativamente mais baixa com a aplicação da técnica de COP (p=0,02). Não se verificou diferença quanto à percentagem de doentes com regurgitação aórtica graus III ou IV pós-VAP entre os dois grupos (p=0,27) e não se registaram casos de oclusão coronária aguda peri-procedimento.
ConclusõesA técnica fluoroscópica COP, embora segura e não associada a um aumento da complexidade, não reduziu significativamente a prevalência de PPMI aos 30 dias pós-VAP quando comparada à utilização da técnica angiográfica tradicional.
Transcatheter aortic valve implantation (TAVI) has become the standard procedure for the management of severe aortic stenosis in high-risk or inoperable patients.1 Recently, its use has also proved safe and effective in intermediate-2,3 and low-surgical risk4–7 elderly patients.
However, several significant periprocedural complications need to be taken into consideration, such as the occurrence of new conduction disturbances requiring permanent pacemaker implantation (PPMI), which remains frequent.8 Despite greater operator experience and technical advances in percutaneous aortic valve engineering and design, there has been little or no improvement over the years in PPMI incidence, which ranges from 3.4% to 25.9% in different series.6,9 PPMI has been associated with longer hospital stay, increased costs (accounting for 25% of TAVI procedure expenses)10 and worse long-term prognosis.11–15
Several factors have been associated with an increased risk of post-TAVI conduction disturbances,6 the most robust independent predictors being pre-existing right bundle branch block (RBBB), the use of self-expanding valves, balloon post-dilation and a lower (i.e. more ventricular) valve implantation depth (ID).6,16–19 Lower ID is associated with a greater probability of the prosthetic valve interfering with the conduction system and consequent conduction disturbances. Accordingly, it is of utmost importance to adopt an angiographic projection that can ensure the most precise positioning of the valve.
In recent years, the cusp-overlap projection (COP) implantation technique, which uses a view overlapping the right (RCC) and left (LCC) coronary cusps that isolates the noncoronary cusp (NCC), has been advocated as a preferable projection for deployment of self-expanding valves compared to the conventional three-cusp coplanar implantation technique (CIT), which aligns the RCC between the LCC and the NCC.
The advantage of the COP technique stems from the acquisition of a better nadir of the NCC, which contributes to a higher and more precise valve position to start deployment, minimizes the risk of valve embolization and enables improved orientation of the delivery system in relation to the center of the aortic annulus.20–22 These advantages have led to widespread adoption of the COP technique; however, robust evidence regarding the real impact on PPMI of its use after self-expanding valve implantation is still lacking, since no randomized controlled trials are available.
We aimed to compare the 30-day incidence of post-TAVI PPMI using a self-expanding valve (CoreValve™) according to the fluoroscopic guidance technique (COP vs. CIT).
MethodsAn observational, longitudinal, retrospective, single-center study was carried out. Demographic and clinical characteristics, in-hospital therapeutic measures and follow-up data were obtained by consulting the electronic medical record system (SClinic®, Alert®, syngo Dynamics®).
We included all consecutive patients undergoing TAVI in the context of severe native aortic valve disease with self-expanding new generation CoreValve Evolut R™ or Evolut PRO™, at a high-volume tertiary center, between April 18, 2019 and November 22, 2021. The procedure was performed using CIT until 19 January 2021 and COP thereafter.
Patients with a previously implanted pacemaker, cardiac resynchronization therapy device or implantable cardioverter-defibrillator, bicuspid aortic valve, or who underwent valve-in-valve procedures were excluded.
Center characteristicsCentro Hospitalar Vila Nova de Gaia/Espinho (CHVNG/E) is a tertiary hospital located in Porto, Portugal. The cardiology department has a team of five interventional cardiologists with wide experience (more than five years) in TAVI procedures, which were first performed in 2007. This is currently a high-volume center with a mean of 150 percutaneous valves implanted per year.
Transcatheter aortic valve implantation procedureBefore valve implantation, electrocardiogram (ECG)-gated contrast-enhanced high-resolution computed tomography (CT) images were acquired in all patients following standard recommendations.23 Images were analyzed using dedicated software (3Mensio Structural Heart, Pie Medical Imaging BV, The Netherlands) to determine anatomic dimensions and working projections. This information was then used to select the valve size and vascular approach, and to predict the angiographic projection angulations for deployment of the device throughout the procedure. Deployment was confirmed and corrected with fluoroscopic guidance.
COP was defined on CT as an overlap of the LCC and RCC, isolating the NCC; CIT was defined as the projection aligning the three coronary cusps in the same plane.
After advancing the valve through the delivery catheter, partial release was performed once the valve's height was considered optimal according to the operator, typically at the annulus level. Flaring of the valve occurred using the NCC as the first contact point followed by the LCC. This occurred under rapid pacing achieved by the delivery wire positioned inside the left ventricle. The valve was repositioned according to the operators’ discretion to obtain an implantation depth between 3 and 5 mm below the annulus. Definitive delivery occurred if the valve hemodynamics was deemed appropriate. Balloon pre- and/or post-dilation was performed at the operators’ discretion but usually in the presence of a heavily calcified native valve.
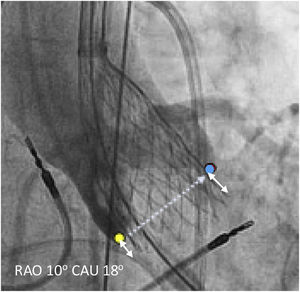
ID, defined as the distance from the aortic annulus to the distal end of the prosthetic valve, was measured retrospectively in patients in whom a final image was obtained with a projection in a perpendicular plane to the valve with a contrast bolus injection. Measurements were made using syngo Dynamics (Siemens Healthcare, USA). There is no standardized method for measuring ID, and at least three different approaches (Figure 1) have been described in previous studies24,25: (1) the distance from the NCC to the prosthetic valve; (2) the arithmetic mean of the distance from the NCC and the LCC to the valve; and (3) the deepest edge from the NCC or the LCC to the valve. Taking this into consideration we measured ID with the three methods described.
Endpoints and follow-upThe primary endpoint was 30-day new PPMI after TAVI. PPMI was performed when deemed appropriate by the medical team.
Additionally, the following secondary endpoints were recorded: Valve Academic Research Consortium 2 (VARC-2) procedural success and safety definitions26 (including incidence of new-onset high-grade atrioventricular block [AVB], periprocedural all-cause mortality, myocardial infarction, stroke, vascular complications and major bleeding defined as Bleeding Academic Research Consortium [BARC] score ≥3a); ID; fluoroscopy time; radiation dose and contrast volume during the procedure; need for surgical aortic valve replacement; more than moderate post-TAVI regurgitation; and 30-day all-cause mortality.
High-grade AVB was defined as the development of paroxysmal or permanent Mobitz II, 2:1 or complete AVB (CAVB) on postprocedural ECG monitoring.
In individuals who needed PPMI within 30 days post-TAVI, the percentage of ventricular pacing during the first post-pacemaker implantation follow-up was analyzed.
The relationship between individual first operator experience and the primary endpoint, ID and related procedural parameters (including contrast and radiation doses and fluoroscopy time) was also analyzed. Physician volume was quantified as the number of procedures performed during 2018 (the year prior to the first with patient enrollment) and analyzed as a categorical variable defined as low (1–79 cases/year) or high (≥80 cases/year).
Written informed consent was obtained for all patients.
Statistical analysisBaseline and follow-up categorical variables were compared using Pearson's chi-square test or Fisher's exact test, as appropriate.
Continuous variables were expressed as measures of central tendency and dispersion (mean and standard deviation or median and interquartile range [IQR]) and compared using the Student's parametric t test for independent variables or the Mann-Whitney test, as appropriate.
A value of p<0.05 for a confidence interval of 95% was taken to be statistically significant for all tests performed.
The statistical analysis was performed with IBM® SPSS® version 27.0 (IBM SPSS Inc., Chicago, IL).
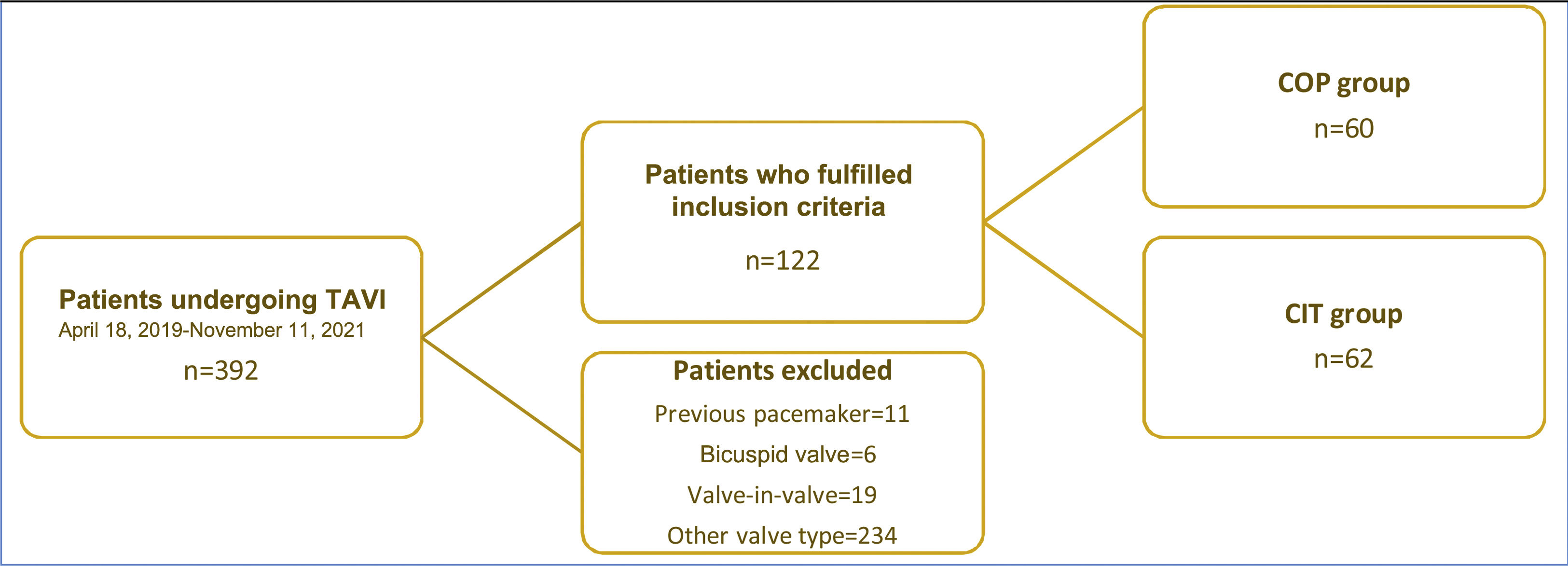
ResultsBaseline characteristics of the study populationBetween April 18, 2019 and November 22, 2021, a total of 392 patients underwent TAVI in our center. Of these, 122 were included in the current analysis; most of the others were excluded due to use of a different valve type. The COP implantation technique was used in 60 patients between January 19, 2021 and November 22, 2021, and these were compared with the previous consecutive 62 patients, in whom CIT was used (Figure 2).
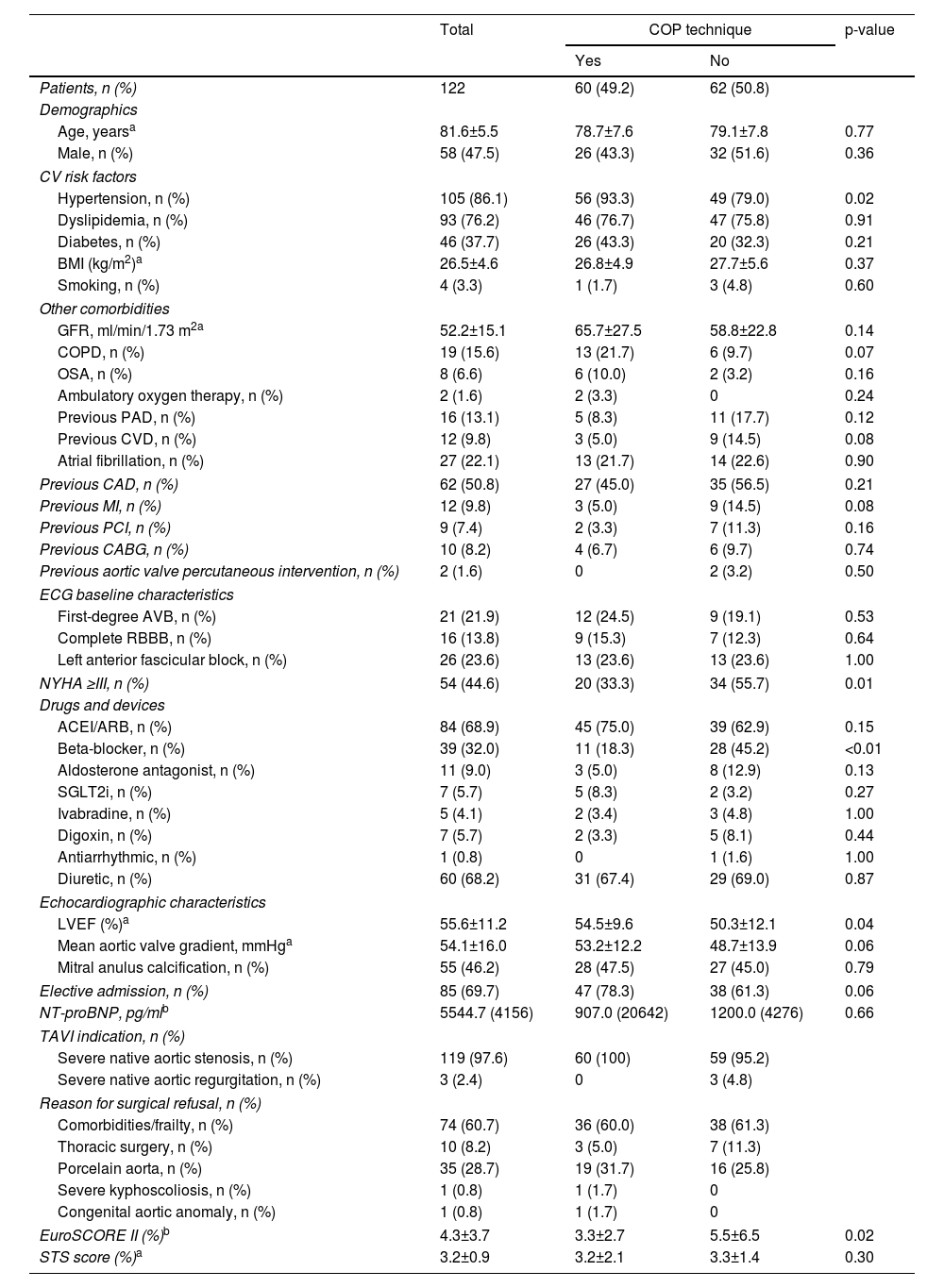
Baseline characteristics of the study population are shown in Table 1. Patients’ mean age was 81.6±5.5 years; most were electively admitted (69.7%) and had intermediate surgical risk (mean EuroSCORE II 4.3±3.7 and Society of Thoracic Surgeons score 3.2±0.9).
Baseline characteristics of the study population.
| Total | COP technique | p-value | ||
|---|---|---|---|---|
| Yes | No | |||
| Patients, n (%) | 122 | 60 (49.2) | 62 (50.8) | |
| Demographics | ||||
| Age, yearsa | 81.6±5.5 | 78.7±7.6 | 79.1±7.8 | 0.77 |
| Male, n (%) | 58 (47.5) | 26 (43.3) | 32 (51.6) | 0.36 |
| CV risk factors | ||||
| Hypertension, n (%) | 105 (86.1) | 56 (93.3) | 49 (79.0) | 0.02 |
| Dyslipidemia, n (%) | 93 (76.2) | 46 (76.7) | 47 (75.8) | 0.91 |
| Diabetes, n (%) | 46 (37.7) | 26 (43.3) | 20 (32.3) | 0.21 |
| BMI (kg/m2)a | 26.5±4.6 | 26.8±4.9 | 27.7±5.6 | 0.37 |
| Smoking, n (%) | 4 (3.3) | 1 (1.7) | 3 (4.8) | 0.60 |
| Other comorbidities | ||||
| GFR, ml/min/1.73 m2a | 52.2±15.1 | 65.7±27.5 | 58.8±22.8 | 0.14 |
| COPD, n (%) | 19 (15.6) | 13 (21.7) | 6 (9.7) | 0.07 |
| OSA, n (%) | 8 (6.6) | 6 (10.0) | 2 (3.2) | 0.16 |
| Ambulatory oxygen therapy, n (%) | 2 (1.6) | 2 (3.3) | 0 | 0.24 |
| Previous PAD, n (%) | 16 (13.1) | 5 (8.3) | 11 (17.7) | 0.12 |
| Previous CVD, n (%) | 12 (9.8) | 3 (5.0) | 9 (14.5) | 0.08 |
| Atrial fibrillation, n (%) | 27 (22.1) | 13 (21.7) | 14 (22.6) | 0.90 |
| Previous CAD, n (%) | 62 (50.8) | 27 (45.0) | 35 (56.5) | 0.21 |
| Previous MI, n (%) | 12 (9.8) | 3 (5.0) | 9 (14.5) | 0.08 |
| Previous PCI, n (%) | 9 (7.4) | 2 (3.3) | 7 (11.3) | 0.16 |
| Previous CABG, n (%) | 10 (8.2) | 4 (6.7) | 6 (9.7) | 0.74 |
| Previous aortic valve percutaneous intervention, n (%) | 2 (1.6) | 0 | 2 (3.2) | 0.50 |
| ECG baseline characteristics | ||||
| First-degree AVB, n (%) | 21 (21.9) | 12 (24.5) | 9 (19.1) | 0.53 |
| Complete RBBB, n (%) | 16 (13.8) | 9 (15.3) | 7 (12.3) | 0.64 |
| Left anterior fascicular block, n (%) | 26 (23.6) | 13 (23.6) | 13 (23.6) | 1.00 |
| NYHA ≥III, n (%) | 54 (44.6) | 20 (33.3) | 34 (55.7) | 0.01 |
| Drugs and devices | ||||
| ACEI/ARB, n (%) | 84 (68.9) | 45 (75.0) | 39 (62.9) | 0.15 |
| Beta-blocker, n (%) | 39 (32.0) | 11 (18.3) | 28 (45.2) | <0.01 |
| Aldosterone antagonist, n (%) | 11 (9.0) | 3 (5.0) | 8 (12.9) | 0.13 |
| SGLT2i, n (%) | 7 (5.7) | 5 (8.3) | 2 (3.2) | 0.27 |
| Ivabradine, n (%) | 5 (4.1) | 2 (3.4) | 3 (4.8) | 1.00 |
| Digoxin, n (%) | 7 (5.7) | 2 (3.3) | 5 (8.1) | 0.44 |
| Antiarrhythmic, n (%) | 1 (0.8) | 0 | 1 (1.6) | 1.00 |
| Diuretic, n (%) | 60 (68.2) | 31 (67.4) | 29 (69.0) | 0.87 |
| Echocardiographic characteristics | ||||
| LVEF (%)a | 55.6±11.2 | 54.5±9.6 | 50.3±12.1 | 0.04 |
| Mean aortic valve gradient, mmHga | 54.1±16.0 | 53.2±12.2 | 48.7±13.9 | 0.06 |
| Mitral anulus calcification, n (%) | 55 (46.2) | 28 (47.5) | 27 (45.0) | 0.79 |
| Elective admission, n (%) | 85 (69.7) | 47 (78.3) | 38 (61.3) | 0.06 |
| NT-proBNP, pg/mlb | 5544.7 (4156) | 907.0 (20642) | 1200.0 (4276) | 0.66 |
| TAVI indication, n (%) | ||||
| Severe native aortic stenosis, n (%) | 119 (97.6) | 60 (100) | 59 (95.2) | |
| Severe native aortic regurgitation, n (%) | 3 (2.4) | 0 | 3 (4.8) | |
| Reason for surgical refusal, n (%) | ||||
| Comorbidities/frailty, n (%) | 74 (60.7) | 36 (60.0) | 38 (61.3) | |
| Thoracic surgery, n (%) | 10 (8.2) | 3 (5.0) | 7 (11.3) | |
| Porcelain aorta, n (%) | 35 (28.7) | 19 (31.7) | 16 (25.8) | |
| Severe kyphoscoliosis, n (%) | 1 (0.8) | 1 (1.7) | 0 | |
| Congenital aortic anomaly, n (%) | 1 (0.8) | 1 (1.7) | 0 | |
| EuroSCORE II (%)b | 4.3±3.7 | 3.3±2.7 | 5.5±6.5 | 0.02 |
| STS score (%)a | 3.2±0.9 | 3.2±2.1 | 3.3±1.4 | 0.30 |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; AVB: atrioventricular block; BMI: body mass index; CABG: coronary artery bypass graft; CAD: coronary artery disease; COP: cusp-overlap projection; COPD: chronic obstructive pulmonary disease; CV: cardiovascular; CVD: cerebrovascular disease; ECG: electrocardiogram; GFR: glomerular filtration rate; iSGLT2: sodium-glucose cotransporter-2 inhibitor; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NT-proBNP: N-terminal pro-brain-type natriuretic peptide; NYHA: New York Heart Association functional class; OSA: obstructive sleep apnea; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; RBBB: right bundle branch block; STS: Society of Thoracic Surgeons.
The CIT group had a significantly lower prevalence of hypertension (79.0% vs. 93.3%, p=0.02), a higher incidence of beta-blocker use (45.2% vs. 18.3%, p<0.01), more frequent New York Heart Association functional class III or IV (55.7% vs. 33.3%, p<0.01), lower baseline left ventricular ejection fraction (50.3±12.1% vs. 54.5±9.6%, p=0.04) and a higher EuroSCORE II (5.5±6.5% vs. 3.3±2.7). All other variables were similar between the two groups.
Severe native aortic valve stenosis (97.6%) was the most prevalent underlying reason for TAVI (with a mean aortic valve gradient of 54.1±16.0 mmHg).
Left anterior fascicular block was the most prevalent relevant pre-existing conduction disturbance (23.6%), followed by first-degree AVB (21.9%) and RBBB (13.8%).
Procedural dataTAVI implantation using the COP technique was achieved in all patients included in that subgroup.
Femoral access was the most frequently used vascular approach (98.4%) and ProGlide™ (Abbott Vascular) alone (48.8%) or in conjunction with Angio-Seal™ VIP (Terumo) (36.2%) were the most commonly applied closure devices. The Evolut Pro™ valve was used in 99 patients (76.2%).
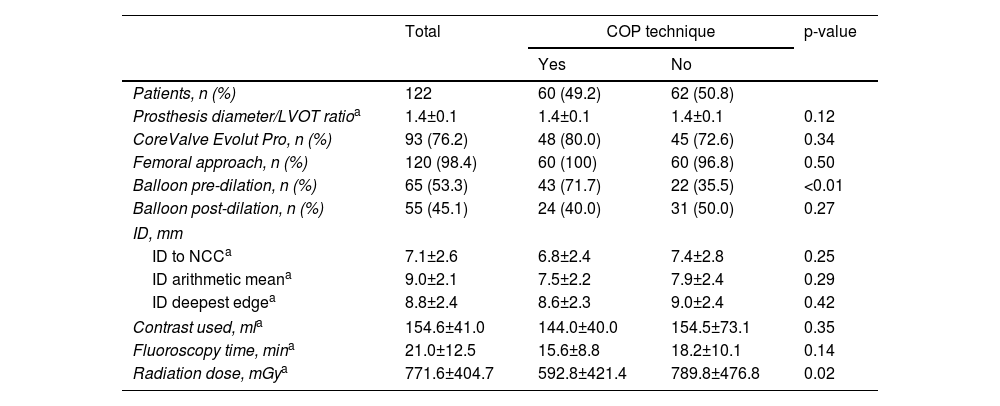
Balloon pre-dilation was more frequently performed in the COP group (71.7% vs. 35.5%, p<0.01) but there was no difference in the use of post-dilation (Table 2).
Transcatheter aortic valve replacement procedural characteristics.
| Total | COP technique | p-value | ||
|---|---|---|---|---|
| Yes | No | |||
| Patients, n (%) | 122 | 60 (49.2) | 62 (50.8) | |
| Prosthesis diameter/LVOT ratioa | 1.4±0.1 | 1.4±0.1 | 1.4±0.1 | 0.12 |
| CoreValve Evolut Pro, n (%) | 93 (76.2) | 48 (80.0) | 45 (72.6) | 0.34 |
| Femoral approach, n (%) | 120 (98.4) | 60 (100) | 60 (96.8) | 0.50 |
| Balloon pre-dilation, n (%) | 65 (53.3) | 43 (71.7) | 22 (35.5) | <0.01 |
| Balloon post-dilation, n (%) | 55 (45.1) | 24 (40.0) | 31 (50.0) | 0.27 |
| ID, mm | ||||
| ID to NCCa | 7.1±2.6 | 6.8±2.4 | 7.4±2.8 | 0.25 |
| ID arithmetic meana | 9.0±2.1 | 7.5±2.2 | 7.9±2.4 | 0.29 |
| ID deepest edgea | 8.8±2.4 | 8.6±2.3 | 9.0±2.4 | 0.42 |
| Contrast used, mla | 154.6±41.0 | 144.0±40.0 | 154.5±73.1 | 0.35 |
| Fluoroscopy time, mina | 21.0±12.5 | 15.6±8.8 | 18.2±10.1 | 0.14 |
| Radiation dose, mGya | 771.6±404.7 | 592.8±421.4 | 789.8±476.8 | 0.02 |
COP: cusp-overlap projection; ID: implantation depth; LVOT: left ventricular outflow tract; NCC: noncoronary cusp.
Although the difference did not reach statistical significance, ID (assessed in a total of 110 patients) was lower in the CIT group regardless of the method used for its measurement (Table 2).
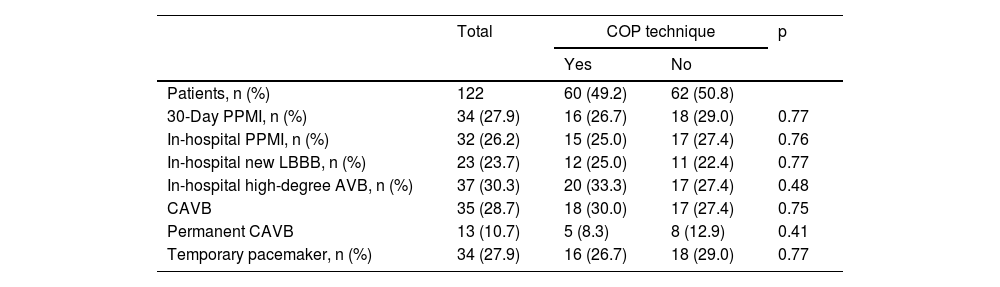
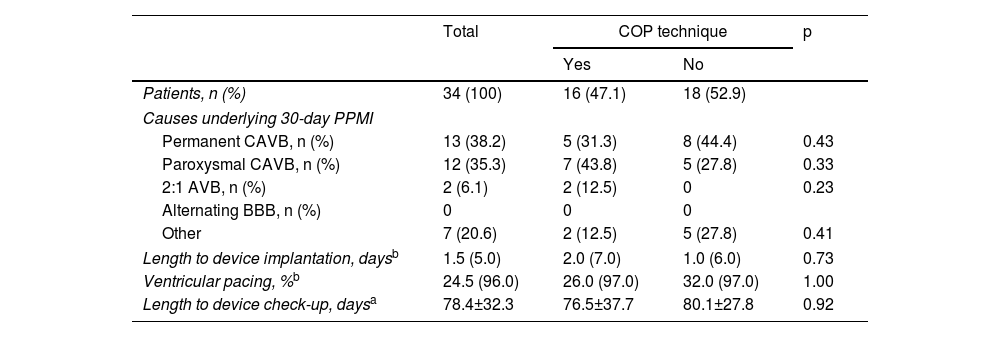
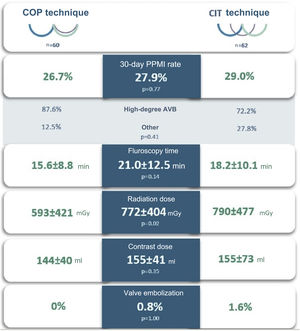
Study endpointsThe 30-day PPMI rate (Table 3) was 27.9% (n=34), with no significant difference between the COP and CIT groups (26.7% vs. 29.0%, p=0.77). The median time to device implantation was 1.5 (IQR: 5.0) days and permanent CAVB after 24 h post-TAVI was the main underlying reason (38.2%). The median percentage of ventricular pacing (Table 4) at the first post-pacemaker implantation follow-up (mean length 78.4±32.3 days) was 24.5% (IQR: 96.0), with no significant difference between the two groups (p=1.00).
Post-transcatheter aortic valve implantation rhythm complications.
| Total | COP technique | p | ||
|---|---|---|---|---|
| Yes | No | |||
| Patients, n (%) | 122 | 60 (49.2) | 62 (50.8) | |
| 30-Day PPMI, n (%) | 34 (27.9) | 16 (26.7) | 18 (29.0) | 0.77 |
| In-hospital PPMI, n (%) | 32 (26.2) | 15 (25.0) | 17 (27.4) | 0.76 |
| In-hospital new LBBB, n (%) | 23 (23.7) | 12 (25.0) | 11 (22.4) | 0.77 |
| In-hospital high-degree AVB, n (%) | 37 (30.3) | 20 (33.3) | 17 (27.4) | 0.48 |
| CAVB | 35 (28.7) | 18 (30.0) | 17 (27.4) | 0.75 |
| Permanent CAVB | 13 (10.7) | 5 (8.3) | 8 (12.9) | 0.41 |
| Temporary pacemaker, n (%) | 34 (27.9) | 16 (26.7) | 18 (29.0) | 0.77 |
AVB: atrioventricular block; CAVB: complete atrioventricular block; COP: cusp-overlap projection; LBBB: left bundle branch block; PPMI: permanent pacemaker implantation.
Subanalyses of the population with post-transcatheter aortic valve implantation permanent pacemaker implantation.
| Total | COP technique | p | ||
|---|---|---|---|---|
| Yes | No | |||
| Patients, n (%) | 34 (100) | 16 (47.1) | 18 (52.9) | |
| Causes underlying 30-day PPMI | ||||
| Permanent CAVB, n (%) | 13 (38.2) | 5 (31.3) | 8 (44.4) | 0.43 |
| Paroxysmal CAVB, n (%) | 12 (35.3) | 7 (43.8) | 5 (27.8) | 0.33 |
| 2:1 AVB, n (%) | 2 (6.1) | 2 (12.5) | 0 | 0.23 |
| Alternating BBB, n (%) | 0 | 0 | 0 | |
| Other | 7 (20.6) | 2 (12.5) | 5 (27.8) | 0.41 |
| Length to device implantation, daysb | 1.5 (5.0) | 2.0 (7.0) | 1.0 (6.0) | 0.73 |
| Ventricular pacing, %b | 24.5 (96.0) | 26.0 (97.0) | 32.0 (97.0) | 1.00 |
| Length to device check-up, daysa | 78.4±32.3 | 76.5±37.7 | 80.1±27.8 | 0.92 |
AVB: atrioventricular block; BBB: bundle branch block; CAVB: complete atrioventricular block; COP: cusp-overlap projection; PPMI: permanent pacemaker implantation.
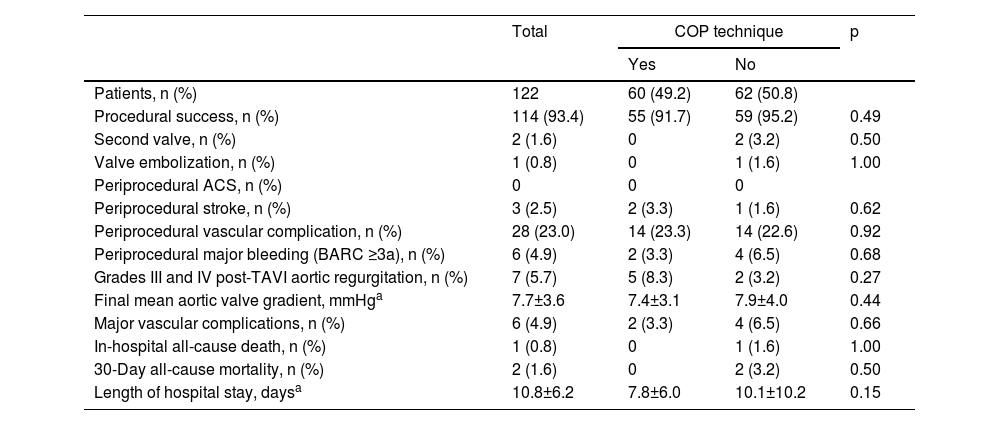
Procedural success according to the VARC-2 definition (Table 5) was achieved in 93.4% of patients.
Procedural outcomes.
| Total | COP technique | p | ||
|---|---|---|---|---|
| Yes | No | |||
| Patients, n (%) | 122 | 60 (49.2) | 62 (50.8) | |
| Procedural success, n (%) | 114 (93.4) | 55 (91.7) | 59 (95.2) | 0.49 |
| Second valve, n (%) | 2 (1.6) | 0 | 2 (3.2) | 0.50 |
| Valve embolization, n (%) | 1 (0.8) | 0 | 1 (1.6) | 1.00 |
| Periprocedural ACS, n (%) | 0 | 0 | 0 | |
| Periprocedural stroke, n (%) | 3 (2.5) | 2 (3.3) | 1 (1.6) | 0.62 |
| Periprocedural vascular complication, n (%) | 28 (23.0) | 14 (23.3) | 14 (22.6) | 0.92 |
| Periprocedural major bleeding (BARC ≥3a), n (%) | 6 (4.9) | 2 (3.3) | 4 (6.5) | 0.68 |
| Grades III and IV post-TAVI aortic regurgitation, n (%) | 7 (5.7) | 5 (8.3) | 2 (3.2) | 0.27 |
| Final mean aortic valve gradient, mmHga | 7.7±3.6 | 7.4±3.1 | 7.9±4.0 | 0.44 |
| Major vascular complications, n (%) | 6 (4.9) | 2 (3.3) | 4 (6.5) | 0.66 |
| In-hospital all-cause death, n (%) | 1 (0.8) | 0 | 1 (1.6) | 1.00 |
| 30-Day all-cause mortality, n (%) | 2 (1.6) | 0 | 2 (3.2) | 0.50 |
| Length of hospital stay, daysa | 10.8±6.2 | 7.8±6.0 | 10.1±10.2 | 0.15 |
ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium criteria; COP: cusp-overlap projection; TAVI: transcatheter aortic valve replacement.
There were two procedures in which valve implantation failed (both in the CIT group), one due to embolization needing emergent cardiac surgery, and the other due to valve entrapment in the femoral artery requiring surgical vascular intervention.
New complete left bundle branch block (LBBB) was observed in 23 (23.7%) patients and high-degree AVB occurred in 37 (30.3%) patients, with no significant differences between groups regarding the occurrence of these variables (25.0% vs. 22.4%, p=0.77 for new LBBB and 33.3% vs. 27.4%, p=0.48 for high-degree AVB).
There were 28 (23.0%) cases of minor and major vascular complications, the majority related to vascular access hematoma, with only six cases (4.9%) of in-hospital major bleeding (BARC ≥3a). There was no significant difference between the two groups regarding the occurrence of grades III and IV post-TAVI aortic valve regurgitation (p=0.27). None of the patients had periprocedural acute coronary occlusion.
After exclusion of patients with simultaneous coronary or peripheral angioplasty during the TAVI procedure, there were no differences between the groups in mean fluoroscopy time or contrast volume used. The radiation dose was significantly lower in the COP group (592.8±421.4 vs. 789.8±476.8 mGy, p=0.02).
There were two deaths (1.6%) during the 30-day post-TAVI period: one during the index admission that occurred in a patient with aortic embolization of the prosthetic valve, and the other of unknown cause after hospital discharge.
Operator experience did not significantly influence the 30-day PPMI rate, the occurrence of post-TAVI conduction disturbances or ID, regardless of the method used for its measurement (Table 6).
Subanalysis of study outcomes according to first operator experience.
| Total | Physician volume ≥80 cases/year | p | ||
|---|---|---|---|---|
| Yes | No | |||
| Patients, n (%) | 122 (100) | 84 (68.9) | 38 (31.1) | |
| 30-Day PPMI, n (%) | 34 (27.9) | 24 (28.6) | 10 (26.3) | 0.80 |
| In-hospital PPMI, n (%) | 32 (26.2) | 24 (28.6) | 8 (21.1) | 0.38 |
| In-hospital new LBBB, n (%) | 23 (23.7) | 14 (21.2) | 9 (29.0) | 0.40 |
| In-hospital high-degree AVB, n (%) | 37 (30.3) | 26 (31.0) | 11 (28.9) | 0.82 |
| ID, mm | ||||
| ID to NCCa | 7.1±2.6 | 7.2±2.6 | 6.9±2.7 | 0.61 |
| ID arithmetic meana | 7.7±2.3 | 7.6±2.3 | 7.6±2.4 | 0.77 |
| ID deepest edgea | 8.9±2.4 | 8.8±2.4 | 8.8±2.3 | 0.98 |
| Contrast used, mla | 149.0±58.0 | 146.7±46.3 | 153.5±76.8 | 0.57 |
| Fluoroscopy time, mina | 16.9±9.5 | 17.1±9.7 | 16.4±9.1 | 0.74 |
| Radiation dose, mGya | 692.1±459.1 | 703.5±425.3 | 667.2±530.9 | 0.69 |
AVB: atrioventricular block; COP: cusp-overlap projection; ID: implantation depth; LBBB: left bundle branch block; NCC: noncoronary cusp; PPMI: permanent pacemaker implantation.
We found that, compared to the standard angiographic approach, the COP implantation technique (Figure 3) did not reduce the development of conduction disturbances or the 30-day PPMI rate. It was also safe, without increased risk of other complications, and was feasible without adding complexity to the procedure.
As a greater ID is associated with a higher probability of post-TAVI PPMI,27–29 a new angiographic method such as COP that enables higher valve deployment would represent a significant advantage. This would be even more valuable considering that ID is one of the few modifiable predictors of post-TAVI conduction disturbances.
In a literature review, we found only four retrospective observational studies addressing the effect of this fluoroscopic technique on post-TAVI conduction disturbances that found a significant reduction of PPMI with the use of COP.30–33 Among these, only two also analyzed the impact of the COP technique on ID (using the same three different methods as in our study), with inconsistent results. One study32 showed a significant reduction in ID compared to the standard fluoroscopic approach only when using NCC distance, while another33 found a significantly higher ID in the COP group when applying NCC or arithmetic mean ID measures. These discrepancies raise questions about the ability of the COP technique to enable higher valve deployment. Additionally, it should be noted that we found no studies on this topic with the same conclusion as ours, which could raise the possibility of bias in preferential publication of positive results.
We found no significant difference between the CIT and COP groups regarding 30-day PPMI incidence. The failure to achieve a significant reduction in ID in the COP group could have significantly influenced this result. It should also be pointed out that, in our study, ID was somewhat lower in both groups compared to the defined target (3–5 mm) and the mean ID achieved in other studies,33 which could also have had a significant impact on our results. Although first operator experience was not associated with significant differences regarding the PPMI rate and ID, the influence of the technique's learning curve on the failure to reduce ID with the COP technique cannot be excluded.
The management of conduction disturbances in the context of TAVI was revised in the 2021 European Society of Cardiology guidelines on cardiac pacing and cardiac resynchronization therapy.34 Until then, recommendations were limited to expert consensus.8
Like ours, previous studies on this subject analyzed two consecutive cohorts of patients. However, these were performed at an earlier time and patient recruitment was longer. Since the evidence regarding post-TAVI conduction disturbances that would benefit from pacemaker implantation was limited in the past, there was a lower threshold for device implantation even with minor conduction disturbances. This could have resulted in a higher PPMI rate in the first years of these studies (during which the CIT technique was applied) and an improvement in terms of 30-day PPMI post-TAVI in the COP group (which was recruited later) that was not attributable to the technique. This tendency was observed in our study, although it did not reach statistical significance, with no high-degree AVB or alternating BBB underlying pacemaker implantation decisions in 12.5% of the COP group vs. 27.8% of the CIT group (p=0.41). None of the above-mentioned observational studies identified the underlying causes for device implantation, which makes it impossible to confirm this potential bias.
Several independent predictors of conduction disturbances and PPMI after TAVI have been described: patient-related (advanced age, male gender and higher body mass index); anatomic (mitral annular, aorta and/or left ventricular outflow tract [LVOT] calcifications; short membranous septum); electrocardiographic (previous RBBB, left anterior hemiblock and first-degree AVB); and procedural factors (self-expanding valve, lower ID, larger valve/LVOT diameter ratio, balloon post-dilation).34 With the exception of membranous septum length, which was not measured, all non-ID variables had a similar distribution between the two groups in our study. Accordingly, it is unlikely that these could have influenced our results regarding PPMI incidence. Nevertheless, special note should be taken of the fact that, although there was no statistically significant difference between the two study groups in the incidence of pre-existing RBBB (which is the most consistent and powerful predictor for post-TAVI conduction disturbances), the number of patients with pre-existing RBBB was slightly higher in the COP group. In a relatively small sample like ours this could have had some effect on the final result, leading to overestimation of the need for a pacemaker in patients undergoing TAVI using COP fluoroscopic guidance (the incidence of PPMI was 47.1% in patients with pre-existing RBBB compared to 22.9% in those without, p=0.04).
While a higher valve deployment may confer protection against conduction disturbances, it can also increase the risk of valve embolization, paravalvular leak and coronary occlusion (especially when ID is less than 3 mm).35 However, in our sample there was only one case of valve embolization in the CIT group (1.6%) and none in the COP group (p=1.00). This is consistent with other observational studies that reported few cases of valve embolization, with more cases reported in the CIT groups.32,33 There were no cases of acute coronary occlusion and no significant difference between the two groups in the percentage of grade III or IV post-TAVI aortic regurgitation.
The distribution of other VARC-2 safety criteria was also comparable between the two groups, which confirms the safety of the COP technique.
As in previous studies,20 implementation of the COP technique did not add complexity to the standardized TAVI protocol, as shown by similar fluoroscopy time and iodinated contrast volume used in the two groups; and lower radiation dose used in the COP group.
LimitationsThe main limitations of our study are related to its small sample size and retrospective observational design. Although consecutive series of patients were compared, selection bias cannot be excluded. We analyzed only one type of self-expanding valve implanted in a high-volume center; therefore, the results may not be generalizable to other settings.
The decision to implant a pacemaker was ultimately at the discretion of the local heart team; however, since this is a single-center study over a short period, the adoption of different thresholds for PPMI in the two groups is unlikely.
ConclusionOur study suggests that the COP technique is a safe and simple modification of the conventional three-cusp view for self-expanding valve implantation during the TAVI procedure. However, in contrast to previous studies, it did not decrease the incidence of high-degree AVB or of the 30-day PPMI rate. Dedicated larger randomized trials are needed to assess the impact of this strategy.
FundingNone declared.
Conflicts of interestThe authors have no conflicts of interest to declare.