Coronary artery disease is highly prevalent among patients with severe aortic stenosis who undergo transcatheter aortic valve replacement (TAVR). As indications for TAVR are now expanding to younger and lower-risk patients, the need for coronary angiography (CA) and percutaneous coronary intervention (PCI) during their lifetime is expected to increase. The objective of our study was to assess the need for CA and the feasibility of re-engaging the coronary ostia after TAVR.
MethodsWe performed a retrospective analysis of 853 consecutive patients undergoing TAVR between August 2007 and December 2020. Patients who needed CA after TAVR were selected. The primary endpoint was the rate of successful coronary ostia cannulation after TAVR.
ResultsOf a total of 31 CAs in 28 patients (3.5% of 810 patients analyzed: 57% male, age 77.8±7.0 years) performed after TAVR, 28 (90%) met the primary endpoint and in three cannulation was semi-selective. All failed selective coronary ostia cannulations occurred in patients with a self-expanding valve. Sixteen (52%) also had indication for PCI, which was successfully performed in all. The main indication for CA was non-ST-elevation acute coronary syndrome (35%, n=11). Two cases of primary PCI occurred without delay. There were no complications reported during or after the procedure.
ConclusionAlthough CA was rarely needed in patients after TAVR, selective diagnostic CA was possible in the overwhelming majority of patients. PCI was performed successfully in all cases, without complications.
A doença arterial coronária é altamente prevalente em doentes com estenose aórtica grave submetidos a substituição de válvula aórtica percutânea (VAP). Com a expansão atual das indicações de VAP para doentes mais jovens e de baixo risco, espera-se um aumento da necessidade de angiografia coronária (AC) e intervenção coronária percutânea (ICP) durante a vida desses doentes. O objetivo do nosso estudo foi avaliar a necessidade de AC e a exequibilidade de cateterizar os óstios coronários após VAP.
MétodosAnálise retrospetiva de 853 consecutivos doentes submetidos a VAP entre agosto de 2007 e dezembro de 2020. Doentes que necessitaram de AC após VAP foram selecionados para o estudo. O endpoint primário foi a taxa de cateterização seletiva dos óstios coronários após VAP.
ResultadosEntre 31 AC realizadas em 28 doentes (3,5% dos 810 doentes analisados - 57% sexo masculino; 77,8±7,0 anos) após VAP, 28 (90%) cumpriram o endpoint primário e em três a cateterização foi semiseletiva. Todas as cateterizações semiseletivas ocorreram em doentes com válvulas autoexpansíveis. Dezasseis (52%) doentes apresentaram associadamente indicação para ICP, a qual foi realizada com sucesso em todos. A principal indicação para AC foi a síndrome coronária aguda sem supradesnivelamento de ST (35%, n=11). Os dois casos de ICP primária ocorreram sem atraso. Não foram observadas complicações durante ou após o procedimento.
ConclusõesEmbora a necessidade de angiografia coronária tenha sido rara em doentes após implante de VAP, as angiografias coronárias seletivas foram possíveis na grande maioria dos doentes. A ICP foi realizada com sucesso em todos os casos, sem complicações associadas.
The advent of transcatheter aortic valve replacement (TAVR) has revolutionized the treatment of severe aortic stenosis (AS) in recent years. It is the standard of care for older patients and patients who are at high surgical risk or are not candidates for surgery.1,2 Recently, its indications have expanded to intermediate- and lower-risk patients.3–5 The prevalence of coronary artery disease (CAD) is high among patients undergoing TAVR, ranging from 30% to 75%.5–8 As TAVR indications are now expanding to younger and lower-risk patients with longer life expectancy, there is an increasing risk of developing CAD, and the need for coronary angiography (CA) and percutaneous coronary intervention (PCI) during their lifetime is expected to increase. The possible geometric interactions between the prosthetic valve and the coronary ostium have raised concerns regarding the difficulty of re-engaging the coronary ostia after valve implantation, particularly in long-frame supra-annular self-expanding transcatheter heart valves.9 A commissural post against the coronary ostium or the sealing skirt covering the ostium could hinder future access. However, available data on the incidence, feasibility and complications of CA and PCI in patients who previously underwent TAVR are still scarce. Moreover, some evidence suggests that CA feasibility may differ with different transaortic valve designs.10–12
The purpose of our study was to assess the need for CA and the feasibility of re-engaging the coronary ostia after TAVR, to describe complications, and to compare these outcomes between different valve designs.
MethodsFrom our transcatheter valve therapy registry database, we retrospectively analyzed the data of 853 consecutive patients undergoing TAVR with all commercially available devices between August 2007 and December 2020. Before TAVR, all patients underwent CA and in some cases PCI with drug-eluting stents if indicated. Patients were followed until December 2021 and time to first CA after TAVR was recorded. Deceased patients were censored at time of death. Patients with incomplete follow-up data or acute obstruction of coronary artery ostia during or immediately after TAVR were excluded.
The primary endpoint was the rate of successful coronary ostia cannulation during follow-up after TAVR (i.e., selective cannulation). Coronary cannulations were classified as selective when it was possible to cannulate and inject contrast into both coronary ostia, semi-selective when the catheter was positioned next to the coronary ostia without obtaining complete engagement but resulting in at least partial opacification of the coronary tree, or unsuccessful when coronary arteries could only be visualized by an aortic root angiogram. The secondary endpoints were the rate of successful PCI (i.e., ability to perform balloon angioplasty or stent implantation) and complications associated with coronary catheterization after TAVR, including death, valve dislodgment, fracture and coronary ostial dissection. These endpoints were also analyzed according to the type of percutaneous aortic valve implanted. All coronary angiograms and specified endpoints were analyzed individually by an experienced interventional cardiologist. Data on CA and PCI procedures including clinical presentation, type and number of vascular access and catheters used, fluoroscopy time and volume of contrast media administered were also collected.
Continuous data were expressed as mean±standard deviation or median (interquartile range). Categorical data were expressed as percentages. Continuous variables were compared using the Student's t test. IBM SPSS version 26.0 was used for statistical analyses. A p-value <0.05 was considered statistically significant.
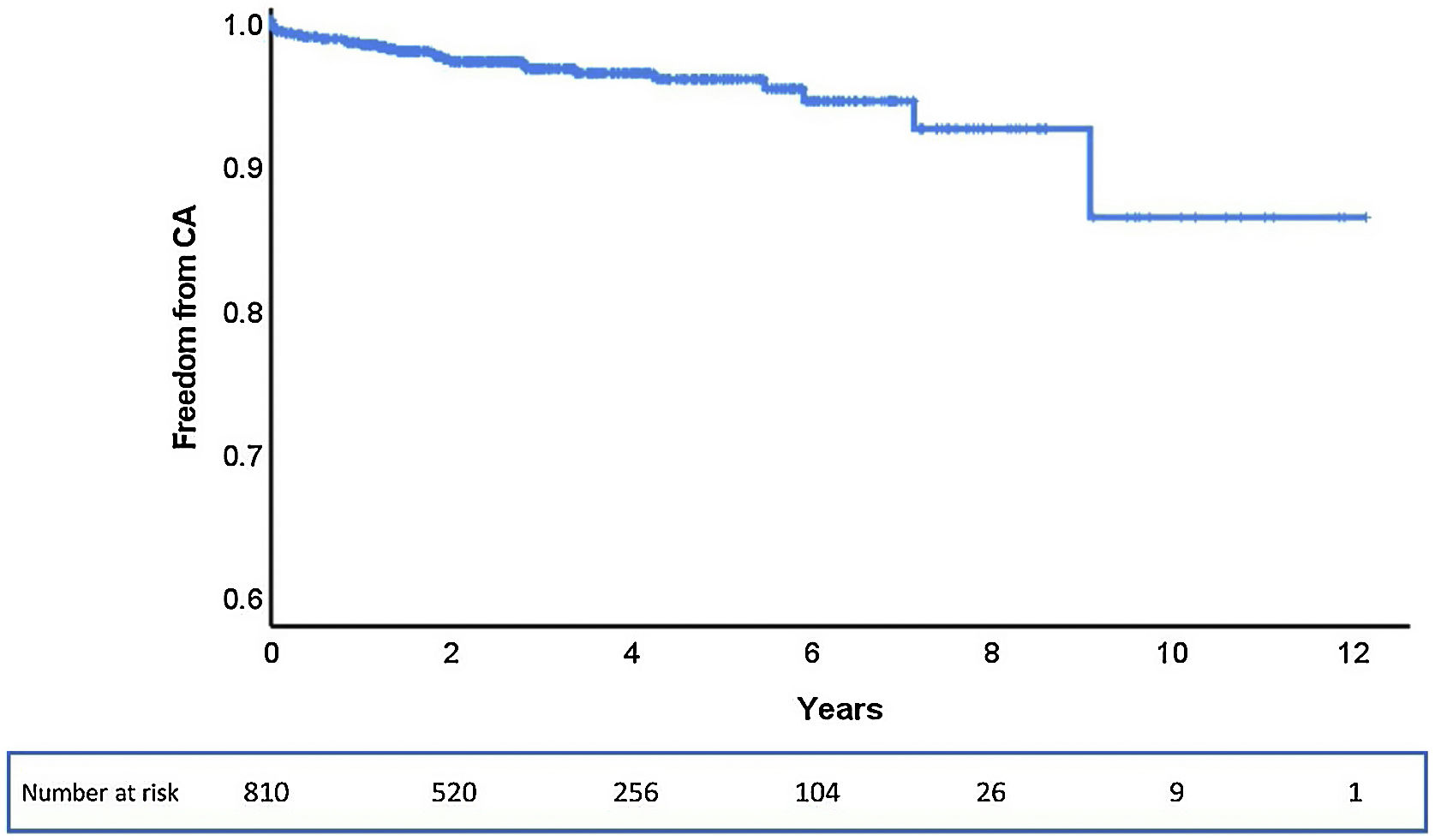
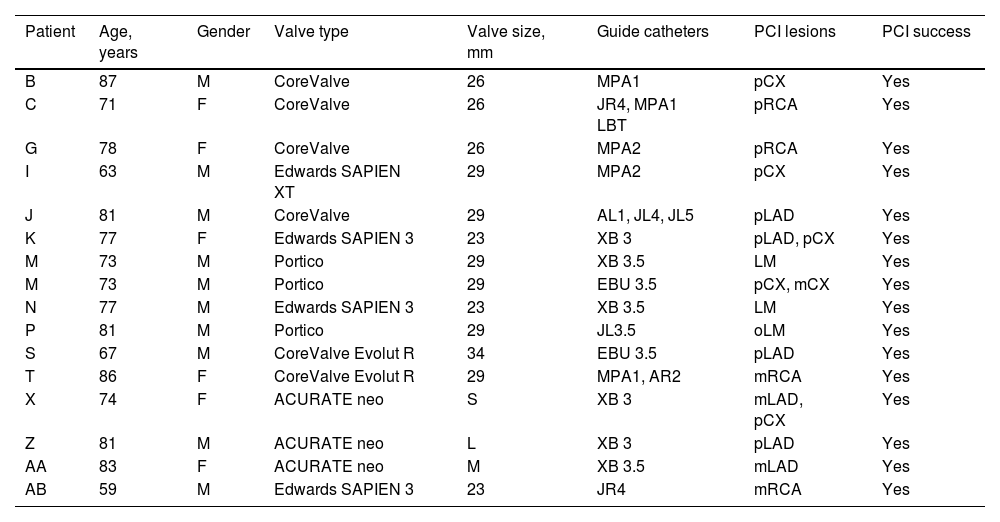
ResultsOf the 853 patients, 43 were excluded due to incomplete follow-up data. Among the total of 810 patients analyzed, during a median follow-up of 33 months (0–145 months), 28 (3.5%) patients underwent CA after TAVR, corresponding to an estimated freedom from CA of 86% (95% confidence interval: 75–100%) at a maximum follow-up of 145 months (Figure 1).
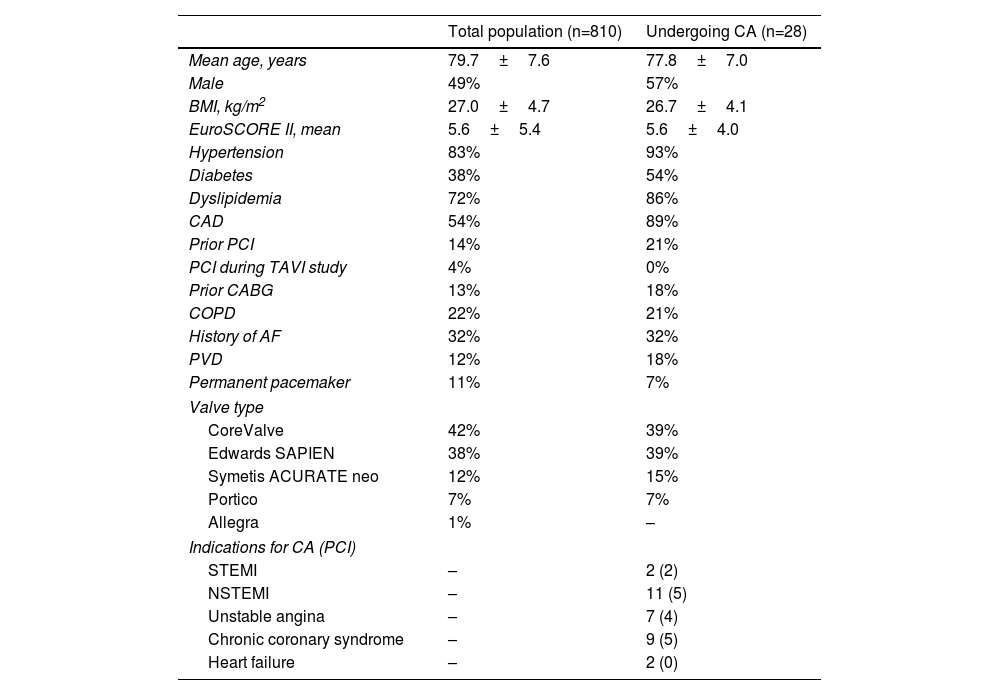
Regarding the 28 patients who needed CA, the median time between TAVR and first CA was 14.9 months (2.1–34.1 months). Six patients needed CA within 30 days of TAVR, eight between 30 days and 12 months, and the other 17 after one year. Baseline demographic and clinical characteristics of the study population are summarized in Table 1. Sixteen patients were male (57%), mean age was 77.8±7.0 years, and 10 (36%) had a history of previous coronary revascularization. Of the valve types used, 11 patients (39%) had a balloon-expandable Edwards SAPIEN valve, 11 (39%) had a self-expanding CoreValve, four (15%) had a self-expanding Symetis ACURATE neo, and two (7%) had a self-expanding Portico. A total of 31 CAs were performed. The main indication was non-ST-elevation acute coronary syndrome (n=11, 35%). Two cases occurred in the context of ST-elevation acute coronary syndrome. Transradial access was used in 17 cases (55%) and a transfemoral approach was used in 13 cases (42%). One patient crossed over from transradial to transfemoral access. There were no differences in mean fluoroscopy time or mean contrast volume used between pre- and post-TAVR CA (5.4±4.4 vs. 7.4±5.3min, p=0.105 and 113±62 vs. 95± 45ml, p=0.183, respectively). PCI was performed in 16 cases (52%). Tables 2 and 3 show baseline and procedural characteristics of individual patients undergoing CA and PCI, respectively.
Baseline demographic and clinical characteristics of the study population.
| Total population (n=810) | Undergoing CA (n=28) | |
|---|---|---|
| Mean age, years | 79.7±7.6 | 77.8±7.0 |
| Male | 49% | 57% |
| BMI, kg/m2 | 27.0±4.7 | 26.7±4.1 |
| EuroSCORE II, mean | 5.6±5.4 | 5.6±4.0 |
| Hypertension | 83% | 93% |
| Diabetes | 38% | 54% |
| Dyslipidemia | 72% | 86% |
| CAD | 54% | 89% |
| Prior PCI | 14% | 21% |
| PCI during TAVI study | 4% | 0% |
| Prior CABG | 13% | 18% |
| COPD | 22% | 21% |
| History of AF | 32% | 32% |
| PVD | 12% | 18% |
| Permanent pacemaker | 11% | 7% |
| Valve type | ||
| CoreValve | 42% | 39% |
| Edwards SAPIEN | 38% | 39% |
| Symetis ACURATE neo | 12% | 15% |
| Portico | 7% | 7% |
| Allegra | 1% | – |
| Indications for CA (PCI) | ||
| STEMI | – | 2 (2) |
| NSTEMI | – | 11 (5) |
| Unstable angina | – | 7 (4) |
| Chronic coronary syndrome | – | 9 (5) |
| Heart failure | – | 2 (0) |
AF: atrial fibrillation; BMI: body mass index; CA: coronary angiography; CABG: coronary artery bypass grafting; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; PVD: peripheral vascular disease; STEMI: ST-elevation myocardial infarction.
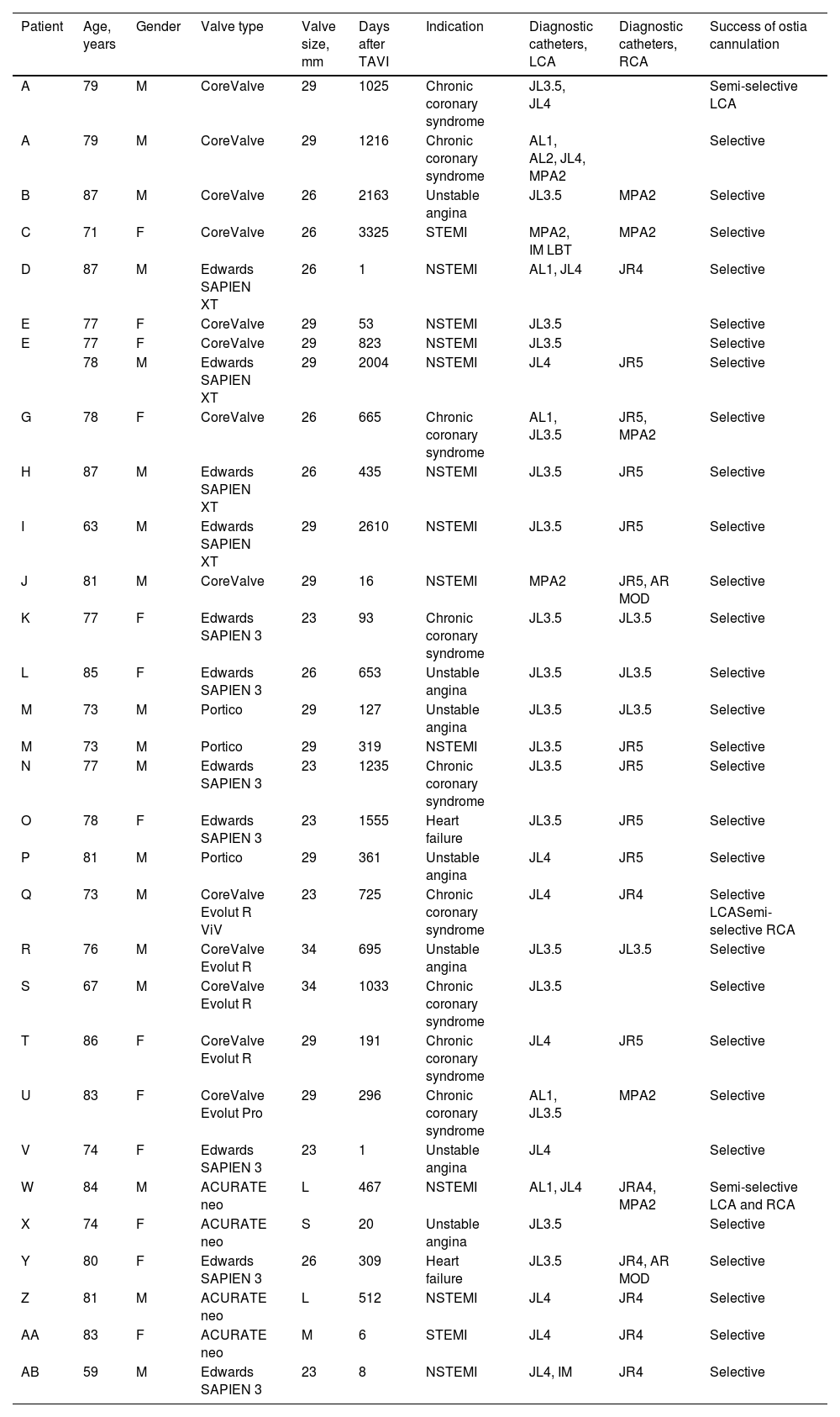
Baseline and procedural characteristics of patients undergoing coronary angiography.
| Patient | Age, years | Gender | Valve type | Valve size, mm | Days after TAVI | Indication | Diagnostic catheters, LCA | Diagnostic catheters, RCA | Success of ostia cannulation |
|---|---|---|---|---|---|---|---|---|---|
| A | 79 | M | CoreValve | 29 | 1025 | Chronic coronary syndrome | JL3.5, JL4 | Semi-selective LCA | |
| A | 79 | M | CoreValve | 29 | 1216 | Chronic coronary syndrome | AL1, AL2, JL4, MPA2 | Selective | |
| B | 87 | M | CoreValve | 26 | 2163 | Unstable angina | JL3.5 | MPA2 | Selective |
| C | 71 | F | CoreValve | 26 | 3325 | STEMI | MPA2, IM LBT | MPA2 | Selective |
| D | 87 | M | Edwards SAPIEN XT | 26 | 1 | NSTEMI | AL1, JL4 | JR4 | Selective |
| E | 77 | F | CoreValve | 29 | 53 | NSTEMI | JL3.5 | Selective | |
| E | 77 | F | CoreValve | 29 | 823 | NSTEMI | JL3.5 | Selective | |
| 78 | M | Edwards SAPIEN XT | 29 | 2004 | NSTEMI | JL4 | JR5 | Selective | |
| G | 78 | F | CoreValve | 26 | 665 | Chronic coronary syndrome | AL1, JL3.5 | JR5, MPA2 | Selective |
| H | 87 | M | Edwards SAPIEN XT | 26 | 435 | NSTEMI | JL3.5 | JR5 | Selective |
| I | 63 | M | Edwards SAPIEN XT | 29 | 2610 | NSTEMI | JL3.5 | JR5 | Selective |
| J | 81 | M | CoreValve | 29 | 16 | NSTEMI | MPA2 | JR5, AR MOD | Selective |
| K | 77 | F | Edwards SAPIEN 3 | 23 | 93 | Chronic coronary syndrome | JL3.5 | JL3.5 | Selective |
| L | 85 | F | Edwards SAPIEN 3 | 26 | 653 | Unstable angina | JL3.5 | JL3.5 | Selective |
| M | 73 | M | Portico | 29 | 127 | Unstable angina | JL3.5 | JL3.5 | Selective |
| M | 73 | M | Portico | 29 | 319 | NSTEMI | JL3.5 | JR5 | Selective |
| N | 77 | M | Edwards SAPIEN 3 | 23 | 1235 | Chronic coronary syndrome | JL3.5 | JR5 | Selective |
| O | 78 | F | Edwards SAPIEN 3 | 23 | 1555 | Heart failure | JL3.5 | JR5 | Selective |
| P | 81 | M | Portico | 29 | 361 | Unstable angina | JL4 | JR5 | Selective |
| Q | 73 | M | CoreValve Evolut R ViV | 23 | 725 | Chronic coronary syndrome | JL4 | JR4 | Selective LCASemi-selective RCA |
| R | 76 | M | CoreValve Evolut R | 34 | 695 | Unstable angina | JL3.5 | JL3.5 | Selective |
| S | 67 | M | CoreValve Evolut R | 34 | 1033 | Chronic coronary syndrome | JL3.5 | Selective | |
| T | 86 | F | CoreValve Evolut R | 29 | 191 | Chronic coronary syndrome | JL4 | JR5 | Selective |
| U | 83 | F | CoreValve Evolut Pro | 29 | 296 | Chronic coronary syndrome | AL1, JL3.5 | MPA2 | Selective |
| V | 74 | F | Edwards SAPIEN 3 | 23 | 1 | Unstable angina | JL4 | Selective | |
| W | 84 | M | ACURATE neo | L | 467 | NSTEMI | AL1, JL4 | JRA4, MPA2 | Semi-selective LCA and RCA |
| X | 74 | F | ACURATE neo | S | 20 | Unstable angina | JL3.5 | Selective | |
| Y | 80 | F | Edwards SAPIEN 3 | 26 | 309 | Heart failure | JL3.5 | JR4, AR MOD | Selective |
| Z | 81 | M | ACURATE neo | L | 512 | NSTEMI | JL4 | JR4 | Selective |
| AA | 83 | F | ACURATE neo | M | 6 | STEMI | JL4 | JR4 | Selective |
| AB | 59 | M | Edwards SAPIEN 3 | 23 | 8 | NSTEMI | JL4, IM | JR4 | Selective |
AL: Amplatz left; F: female; IM: internal mammary; JL: Judkins left; LBT: long BRITE TIP; LCA: left coronary artery; M: male; MPA: multipurpose A; NSTEMI: non-ST-elevation myocardial infarction; RCA: right coronary artery; STEMI: ST-elevation myocardial infarction; ViV: valve-in-valve.
Baseline and procedural characteristics of patients undergoing percutaneous coronary intervention.
| Patient | Age, years | Gender | Valve type | Valve size, mm | Guide catheters | PCI lesions | PCI success |
|---|---|---|---|---|---|---|---|
| B | 87 | M | CoreValve | 26 | MPA1 | pCX | Yes |
| C | 71 | F | CoreValve | 26 | JR4, MPA1 LBT | pRCA | Yes |
| G | 78 | F | CoreValve | 26 | MPA2 | pRCA | Yes |
| I | 63 | M | Edwards SAPIEN XT | 29 | MPA2 | pCX | Yes |
| J | 81 | M | CoreValve | 29 | AL1, JL4, JL5 | pLAD | Yes |
| K | 77 | F | Edwards SAPIEN 3 | 23 | XB 3 | pLAD, pCX | Yes |
| M | 73 | M | Portico | 29 | XB 3.5 | LM | Yes |
| M | 73 | M | Portico | 29 | EBU 3.5 | pCX, mCX | Yes |
| N | 77 | M | Edwards SAPIEN 3 | 23 | XB 3.5 | LM | Yes |
| P | 81 | M | Portico | 29 | JL3.5 | oLM | Yes |
| S | 67 | M | CoreValve Evolut R | 34 | EBU 3.5 | pLAD | Yes |
| T | 86 | F | CoreValve Evolut R | 29 | MPA1, AR2 | mRCA | Yes |
| X | 74 | F | ACURATE neo | S | XB 3 | mLAD, pCX | Yes |
| Z | 81 | M | ACURATE neo | L | XB 3 | pLAD | Yes |
| AA | 83 | F | ACURATE neo | M | XB 3.5 | mLAD | Yes |
| AB | 59 | M | Edwards SAPIEN 3 | 23 | JR4 | mRCA | Yes |
AL: Amplatz left; AR: Amplatz right; CX: circumflex artery; F: female; JL: Judkins left; JR: Judkins right; LAD: left anterior descending artery; LBT: long BRITE TIP; LCA: left coronary artery; LM: left main artery; m: mid; M: male; MPA: multipurpose A; o: ostial; p: proximal; PCI: percutaneous coronary intervention; RCA: right coronary artery.
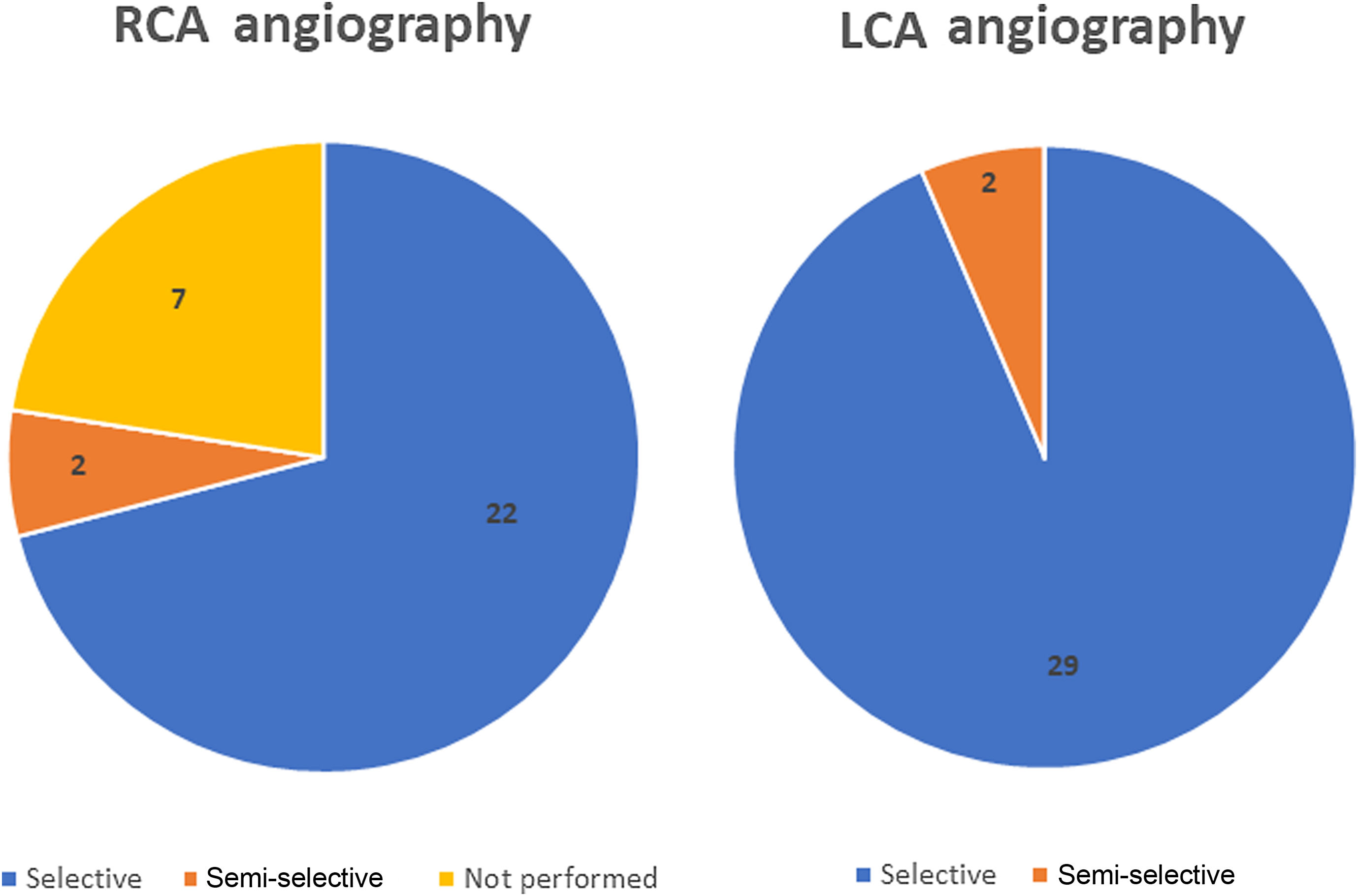
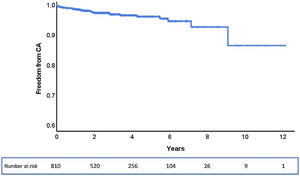
Selective CA was successfully performed in 90% of the patients (28/31 cases) (Figure 2). Regarding anatomic distribution, the left coronary artery (LCA) was selectively engaged in 94% of cases (29/31). In the two unsuccessful attempts at selective LCA cannulation, only a semi-selective contrast injection was possible. A self-expanding supra-annular valve (ACURATE neo L and CoreValve 29) was present in both cases. Right coronary artery (RCA) cannulation was not attempted in seven cases due to known chronic occlusion. Selective engagement of the RCA was achieved in 92% (22/24 cases). In two patients, who also previously had a self-expanding valve implanted (valve-in-valve with a CoreValve Evolut R 23 and an ACURATE neo L), only a semi-selective injection was possible.
The three cases of unsuccessful selective cannulation occurred in patients with a self-expanding supra-annular valve: one CoreValve 29 in which it was not possible to selectively cannulate the LCA and the RCA was not attempted; one valve-in-valve procedure using a CoreValve Evolut R 23 in which engagement of the right coronary ostium was impossible; and one ACURATE neo L in which none of the coronary arteries were selectively catheterized. The first two cases were performed in the context of chronic coronary syndrome and the latter was due to non-ST-segment elevation myocardial infarction. Although these CAs were only semi-selective, the coronary anatomy could be adequately observed and none of the patients had additional significant lesions requiring revascularization. Two cases of semi-selective CA occurred with radial access, while the other was in a patient who crossed over from transradial to transfemoral access. The success rate for radial access was 83%, while for femoral access it was 93%. The mean number of diagnostic catheters used for the LCA was 1.3 (minimum 1; maximum 4) and for the RCA it was 1.2 (minimum 1; maximum 2). The Judkins Left 3.5 was the most used diagnostic catheter for the LCA and the Judkins Right 5 was the most used in the RCA.
PCI was performed successfully in all patients in whom it was attempted (100%; n=16). Of these, four patients had an Edwards SAPIEN (27%), six had a CoreValve (40%), three had an ACURATE neo (20%) and two had a Portico (13%). PCI after TAVR was performed in significant de novo lesions as a result of CAD progression. The left anterior descending (32%, n=6) and circumflex arteries (32%, n=6) were the most frequently treated vessels. No arterial or venous graft PCI was performed. Rotational atherectomy was needed and successfully performed in three patients. The only two cases of primary PCI were performed on an occluded RCA through a previously implanted 26-mm CoreValve and on an occluded left descending artery through an ACURATE neo M; electrocardiogram-to-balloon time was 79min and 56min, respectively. The mean number of guide catheters used in PCI procedures was 1.2 (minimum 1; maximum 3).
There were no complications reported during or after the procedure.
DiscussionAS and CAD often coexist. The expected increase in TAVR in younger and lower-risk patients may lead to a rise in the number of TAVR patients requiring CA or PCI. Therefore, it is crucial to obtain real data on the feasibility of coronary cannulation after TAVR in order to safeguard future coronary access.
In our study with an unselected real-world cohort of TAVR patients, CA was required in only a small percentage of patients (3.5%). The low prevalence of CA is similar to that reported in other studies.9,13–20 However, as in our study, these were mostly intermediate-high surgical risk populations with multiple comorbidities. This percentage is likely to rise considerably in the medium term as the TAVR procedure is extended to younger and lower-risk patients. Additionally, in 33 (4.1%) patients, PCI was performed before TAVR due to significant coronary artery stenosis in proximal lesions, and none required CA in follow-up. This may have contributed to the low rate of CA. The main indications for cardiac catheterization (non-ST-elevation acute coronary syndrome and progression of angina pectoris) are also similar to those reported in the literature.16,18
We observed high success rates of selective coronary ostium re-engagement and PCI after TAVR, without significant complications. Previous studies have assessed the feasibility of CA after TAVR. Although there are a few reports of low success rates, especially in patients receiving self-expanding valves or when accessing the right coronary ostium,9,14,19 most of these studies report success rates of coronary cannulation of over 90%,15–18,20 which is consistent with our results.
No significant differences in mean fluoroscopy time or mean contrast volume used were found between pre- and post-TAVR CA. These data support the feasibility of coronary engagement without significant delay. This is particularly important in management of ST-elevation myocardial infarction, in which access to the coronary arteries must be rapidly achieved. Our cases of primary PCI occurred without delay in two patients with a supra-annular prosthesis (CoreValve and ACURATE neo). The first case was a patient with an emergency medical system out-of-hospital first contact and primary PCI was achieved in 79min. The second occurred in a hospitalized patient and primary PCI was performed in 56min. However, a recent multicenter study including 118 TAVR patients presenting with ST-elevation myocardial infarction showed longer door-to-balloon times and a higher PCI failure rate, partially related to coronary access. There was very high in-hospital and mid-term mortality in these patients.21 Although our cases were successful, our sample is too small to make comparisons or draw conclusions.
It is also important to note the high number of CAs performed by femoral access compared to the usual radial route. It is possible that the operators frequently used femoral access as an initial strategy in order to forestall possible difficulties in engagement of the coronary arteries. Additionally, the three cases of non-selective CA occurred with an initial radial approach (one case of crossover to femoral access). However, the low number of total CAs does not allow us to give a robust and conclusive recommendation.
Unsuccessful selective cannulation was rare in our population, being observed in only three patients, all with a self-expanding supra-annular valve in place (ACURATE neo L, CoreValve 29 and CoreValve Evolut R vale-in-valve). Although this number is too low to draw definitive conclusions, this observation is in line with the literature.9,14 The supra-annular position, asymmetrical skirt and closed-cell frame design of these valves may explain the greater challenge in achieving coronary engagement, particularly when the valve is deployed high.10,17,22,23 By contrast, all CAs in patients with balloon-expandable intra-annular valves (Edwards SAPIEN and Portico) were performed successfully. A shorter frame and a subcoronary implantation position may account for these results.17,22 The more recent and widely used SAPIEN 3 was placed in six of our patients. Although this has a larger frame than previous Edwards SAPIEN valves, it does not appear to interfere with coronary ostial access. In addition to valve design, another possible concern regarding the difficulty in coronary re-access is related to narrow coronary sinuses and low coronary heights. Although these data were not analyzed in our study, which is a limitation, extra caution should be exercised in patients with these anatomical characteristics.15,22
CA was rarely needed in our population. However, this finding may not be extendable to younger and lower-risk patients. With the expansion of TAVR indications to patients with longer life expectancy, an increase in the need for CA, including in the context of acute coronary syndromes, may be expected. The type and size of valve should therefore be taken into account, particularly in younger patients with non-obstructive CAD. Although not studied in our work, pre-procedure computed tomography, new valve designs with commissural tabs identifiable on fluoroscopy, and TAVR with commissural alignment could help to optimize valve placement in relation to the coronary arteries.11,12,23,24 In addition, computed tomography could be used in planning elective CA in patients with TAVR.10 Although CA and PCI have been shown to be effective and safe in TAVR patients, integrating these different strategies into an individualized approach to the patient in the pre-TAVR assessment could be important.
The present study has several limitations. This is a retrospective single-center study, so caution should be exercised before generalizing the results. Prior knowledge of coronary anatomy may have led to the decision not to catheterize some patients, such as those with diffuse CAD not amenable to revascularization, leading to fewer CAs being performed in this specific population. Additionally, the small number of post-TAVR CAs performed may limit the statistical power of the analysis. Nevertheless, the results are in line with previous studies.
ConclusionAlthough CAD frequently coexists with AS, CA after TAVR seems to be rarely needed. Selective CA was feasible in the overwhelming majority of patients, as was PCI, which was successfully performed in all patients in whom it was indicated, without any reported complications. Valve types could however impact the feasibility of CA after TAVR, and further prospective studies are needed to clarify this. A standardized and effective approach in this field is of growing importance, as TAVR is being extended to younger and lower-risk patients, in whom the need for CA is expected to increase.
Conflicts of interestThe authors have no conflicts of interest to declare.