The field of Cardio-Oncology has grown significantly, especially during the last decade. While awareness of cardiotoxicity due to cancer disease and/or therapies has greatly increased, much of the attention has focused on myocardial systolic disfunction and heart failure. However, coronary and structural heart disease are also a common issue in cancer patients and encompass the full spectrum of cardiotoxicity. While invasive percutaneous or surgical intervention, either is often needed or considered in cancer patients, limited evidence or guidelines are available for dealing with coronary or structural heart disease. The Society for Cardiovascular Angiography and Interventions consensus document published in 2016 is the most comprehensive document regarding this particular issue, but relevant evidence has emerged since, which render some of its considerations outdated. In addition to that, the recent 2022 ESC Guidelines on Cardio-Oncology only briefly discuss this topic.
As a result, the Portuguese Association of Cardiovascular Intervention and the Cardio-Oncology Study Group of the Portuguese Society of Cardiology have partnered to produce a position paper to address the issue of cardiac intervention in cancer patients, focusing on percutaneous techniques. A brief review of available evidence is provided, followed by practical considerations. These are based both on the literature as well as accumulated experience with these types of patients, as the authors are either interventional cardiologists, cardiologists with experience in the field of Cardio-Oncology, or both.
A Cardio-Oncologia cresceu consideravelmente, particularmente ao longo da última década. Não obstante o aumento de sensibilização relativamente à cardiotoxicidade secundária ao cancro e/ou a terapêuticas oncológicas, muita da atenção tem-se focado na disfunção sistólica e insuficiência cardíaca. Todavia, a doença coronária e estrutural cardíaca representam também um desafio comum em doentes oncológicos, fazendo assim parte do abrangente conceito de Cardio-Oncologia. Apesar da necessidade de intervenção invasiva, percutânea ou cirúrgica ser frequentemente necessária ou considerada em doentes com cancro, é escassa a evidência ou recomendações relativas à doença coronária ou estrutural neste subgrupo. O documento de consenso da Society for Cardiovascular Angiography and Interventions, publicado em 2016, é a referência mais completa relativamente a esta problemática. Contudo, alguma evidência que entretanto surgiu torna necessária uma atualização. Adicionalmente, as recentes recomendações de 2022 em Cardio-Oncologia da Sociedade Europeia de Cardiologia abordam este assunto somente de forma muito sucinta.
Por este motivo, a Associação Portuguesa de Intervenção Cardiovascular e o Grupo de Estudos de Cardio-Oncologia associaram-se para produzir um Artigo de Posição sobre Intervenção Cardíaca em doentes com cancro, com foco nas técnicas percutâneas. Procedeu-se a uma breve revisão da literatura, seguida de considerações práticas. Estas baseiam-se na evidência disponível, bem como na experiência acumulada dos autores, que são Cardiologistas de Intervenção, Cardiologistas dedicados à área da Cardio-Oncologia, ou ambos.
Cardiology and oncology have both experienced dramatic improvements in patient outcomes and survival in recent decades, due to increasingly effective therapies and prevention strategies. Both fields have contributed significantly to the ever-increasing life expectancy in developed countries, including in Portugal. Because age is a dominant and common risk factor for disease in both fields, it is no surprise that cardiovascular (CV) disease and cancer are thus the prevailing causes of morbidity and mortality in our societies, closely matched in terms of relative weighting.1,2 In addition, oncologic therapies (and cancer itself) can lead to CV toxicity, further increasing the magnitude of the problem. As a result, cancer patients often face CV disease and vice-versa. We have, therefore, become unwillingly victims of our own success. The emergence of this peculiar paradigm led to the creation of the field of Cardio-Oncology.
The most widely known form of CV toxicity is heart failure due to systolic disfunction. Cardiologists and oncologists often perceive the field of Cardio-Oncology largely as pertaining solely to this issue. However, those of us who have been working in this area for some time, have clearly witnessed the involvement of all forms of CV disease. A multidisciplinary approach encompassing all fields of cardiology is warranted.
Two commonly encountered problems in Cardio-Oncology are coronary and structural heart disease, especially the former. As a result, one of the most difficult issues we face today is when, if, and how to offer interventional cardiology procedures to cancer patients. To address these issues, the Society for Cardiovascular Interventions (SCAI) published a detailed expert consensus document offering extensive advice.3 However, since the publication of this document, in January 2016, major trials and several observational studies have shed light on many issues facing Interventional Cardiology which directly and indirectly impact cancer patients. Of note are data from the Portuguese Registry of Acute Coronary Syndromes (ProACS).4 While the SCAI consensus papers remains an excellent and very helpful document, some of its considerations are now outdated. Important insights in Interventional Cardio-Oncology, which have since arisen, are not taken into consideration. The 2022 European Society of Cardiology Guidelines on Cardio-Oncology briefly address this issue,5 while ESC guidelines on coronary revascularization,6 acute coronary syndromes (ACS)7,8 and chronic coronary syndromes (CCS)9 also offer very limited guidance, if any, for this particular subset.
As a result, the Portuguese Association of Cardiovascular Intervention and the Cardio-Oncology Study Group of the Portuguese Society of Cardiology have partnered to produce this position paper. The Board of both groups appointed two Interventional Cardiologists with experience in Cardio-Oncology for the initial draft. The paper was then received further contributions by the coordinator of the Position Papers Working Group of the Portuguese Association of Cardiovascular Intervention and both Board members. The authors of this paper are either interventional cardiologists, cardiologists dedicated to the field of Cardio-Oncology, or both. We aimed to address the specificities of cardiac intervention in cancer patients, focusing on percutaneous techniques. This document focuses more extensively on coronary artery disease (CAD), while also summarily addressing some relevant issues in structural intervention. A brief review of available evidence is provided, followed by practical considerations.
Coronary artery disease: a growing problemThe number of cancer survivors in the USA and Europe is currently estimated to encompass 5–6% of the population.3,10,11 In Portugal, this would account for approximately half a million patients. In ProACS, cancer patients (active or in remission) represented about 5% of the overall population in the past decade.4 Currently, approximately 15000 percutaneous coronary intervention (PCI) procedures/year are performed nationwide, which would roughly translate to about 750 procedures/year in cancer patients, if such cases were approached as in the general population. These somewhat simplified statistics highlight the magnitude of the problem we are facing.
The use of an invasive approach in these patients is, however, consistently lower than in the general population, both in Portugal4 and abroad.10 This is understandable, as the perceived benefit of intervention for these patients was lower, while the perceived risk was higher. A generally conservative approach to these patients might have been appropriate in the past, however current cancer therapies have changed the outlook for cancer patients, so much so that this strategy is clearly no longer appropriate.
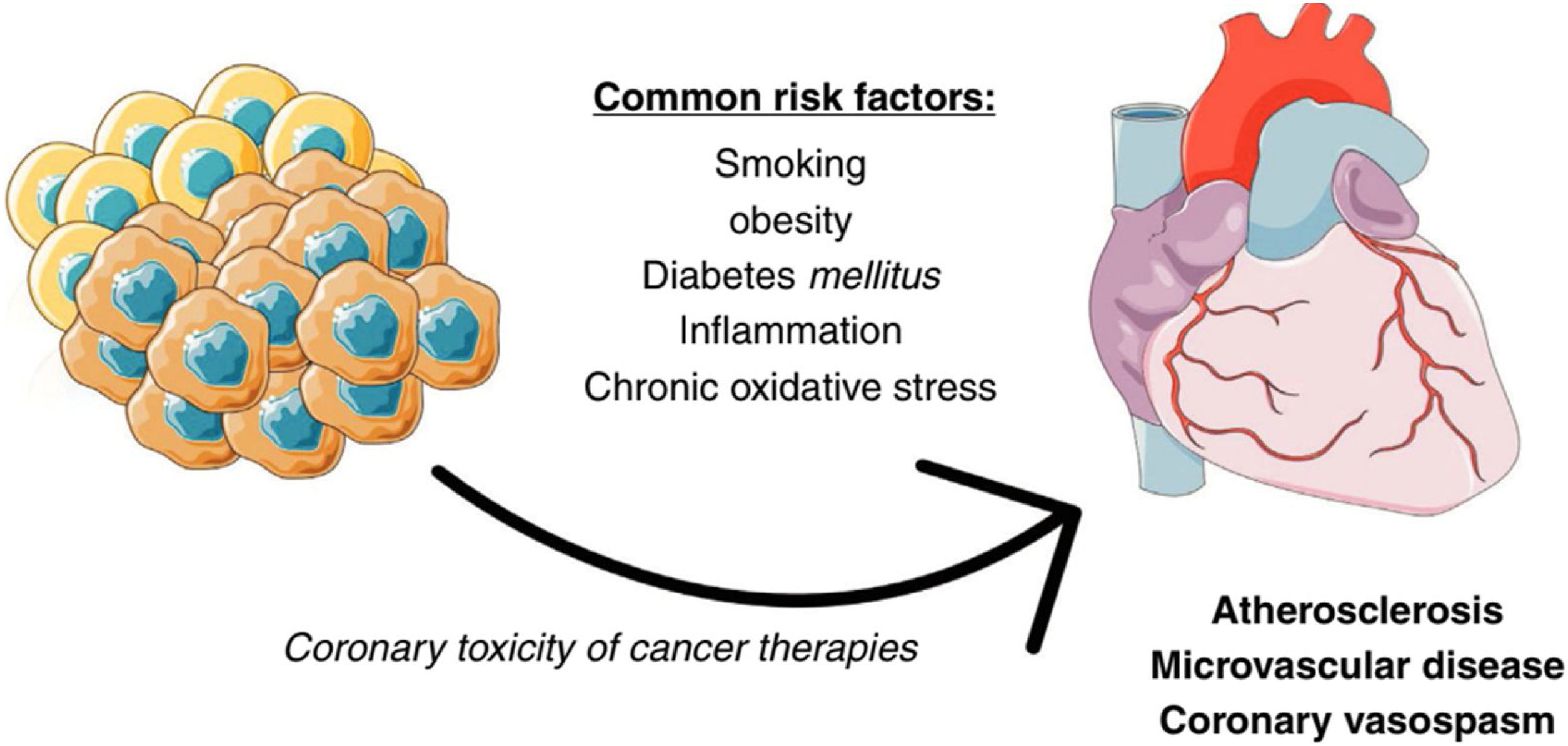
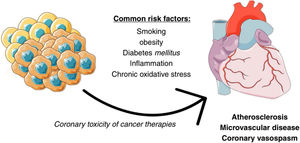
The link between cancer and coronary artery diseaseCancer and CAD share multiple pathophysiological pathways (Figure 1). Several classic risk factors, such as smoking or diabetes, play a relevant role for both. Inflammation and chronic oxidative stress are also of relevance. An interesting example is the CANTOS trial, where the Interleukin-1β inhibitor canakinumab lead to both a reduction in cardiovascular events and fatal cancer.12 Cancer therapies can also often have unwanted coronary toxicity. Another important aspect to bear in mind is that the manifestations of CAD in cancer patients are often due not only to classical atherosclerotic disease, but also due to vasospasm and microvascular disease, and perhaps to a greater extent than in the general population.10
How to assess coronary artery disease risk in cancer patientsThe general principles of cardiovascular risk assessment should, of course, also apply to cancer patients. Classic risk factors should be sought and detailed as in the general population. This is especially important as cancer patients often have a higher incidence of classic cardiovascular risk factors compared to the general population.4,13,14
Whether cancer (or specific primary tumors) is an independent risk factor for CAD, is still subject to debate, However, some studies have suggested that gastric, pancreatic, lung, urogenital and lymphoproliferative cancers may be at particular risk.15,16
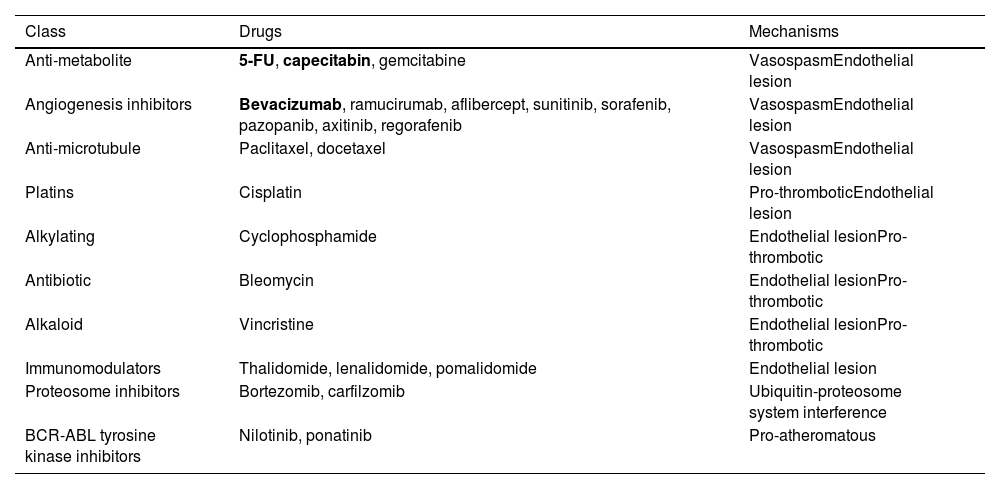
It is clear is that several cancer therapies pose a clear risk of CAD toxicity. Table 1 outlines the major therapies and their known mechanisms. The incidence of clinical manifestations, either in CCS or ACS presentation, while poorly defined, is usually reported <5% for most agents. However, for some drugs, especially antimetabolites, the incidence of coronary toxicity exceeds 10% in some case series, varying according to posology, timing and route of administration.10,15,17–19 5-Fluorouracyl (5-FU), a drug commonly used for the treatment of many gastro-intestinal cancers, as well as breast cancer, is particularly concerning, with no consensus having been reached regarding an approach. Some advocate routine anti-anginal prophylaxis for such patients, while others recommend cessation of chemotherapy with this agent and initiation of anti-anginal drugs after toxicity. Rechallenge is possible, under supervision, but should generally be avoided if possible.20 Current ESC guidelines suggest that severe CAD should first be excluded by CT or coronary angiography and once prophylactic multidrug anti-anginal therapy has been started.5
Chemotherapy agents with potential for coronary toxicity.
| Class | Drugs | Mechanisms |
|---|---|---|
| Anti-metabolite | 5-FU, capecitabin, gemcitabine | VasospasmEndothelial lesion |
| Angiogenesis inhibitors | Bevacizumab, ramucirumab, aflibercept, sunitinib, sorafenib, pazopanib, axitinib, regorafenib | VasospasmEndothelial lesion |
| Anti-microtubule | Paclitaxel, docetaxel | VasospasmEndothelial lesion |
| Platins | Cisplatin | Pro-thromboticEndothelial lesion |
| Alkylating | Cyclophosphamide | Endothelial lesionPro-thrombotic |
| Antibiotic | Bleomycin | Endothelial lesionPro-thrombotic |
| Alkaloid | Vincristine | Endothelial lesionPro-thrombotic |
| Immunomodulators | Thalidomide, lenalidomide, pomalidomide | Endothelial lesion |
| Proteosome inhibitors | Bortezomib, carfilzomib | Ubiquitin-proteosome system interference |
| BCR-ABL tyrosine kinase inhibitors | Nilotinib, ponatinib | Pro-atheromatous |
Bold: agents approaching or exceeding 10% incidence of myocardial infarction in some case series. The true incidence of most agents is poorly defined or unknown, but usually reported as <5%.
It is also worth noting that angiogenesis inhibitors typically induce or increase hypertension, which indirectly contributes to CAD. Lastly, while CAD toxicity manifests principally during therapy, some drugs may produce late-onset disease, after cessation, such as ponatinib, nilotinib and cisplatin.19
Radiotherapy is also a very important cancer specific risk factor for CAD development. Three main clinical characteristics should be remembered: (1) the location of lesions is often specific, including ostial, posing special challenges both regarding risk and revascularization; (2) mediastinal irradiation is especially important, such as (left-sided) breast cancer21 and Hodgkin's lymphoma, and may be mitigated by the use of techniques such as breath holding; (3) CAD often presents very late, sometimes more than a decade after cessation of therapy and is especially concerning for patients who have received treatment at a young age.18,22 As a result, current ESC guidelines suggest screening for high-risk patients who previously underwent chest radiotherapy including the heart, from five years after completion of radiotherapy, ideally with imaging modalities.5
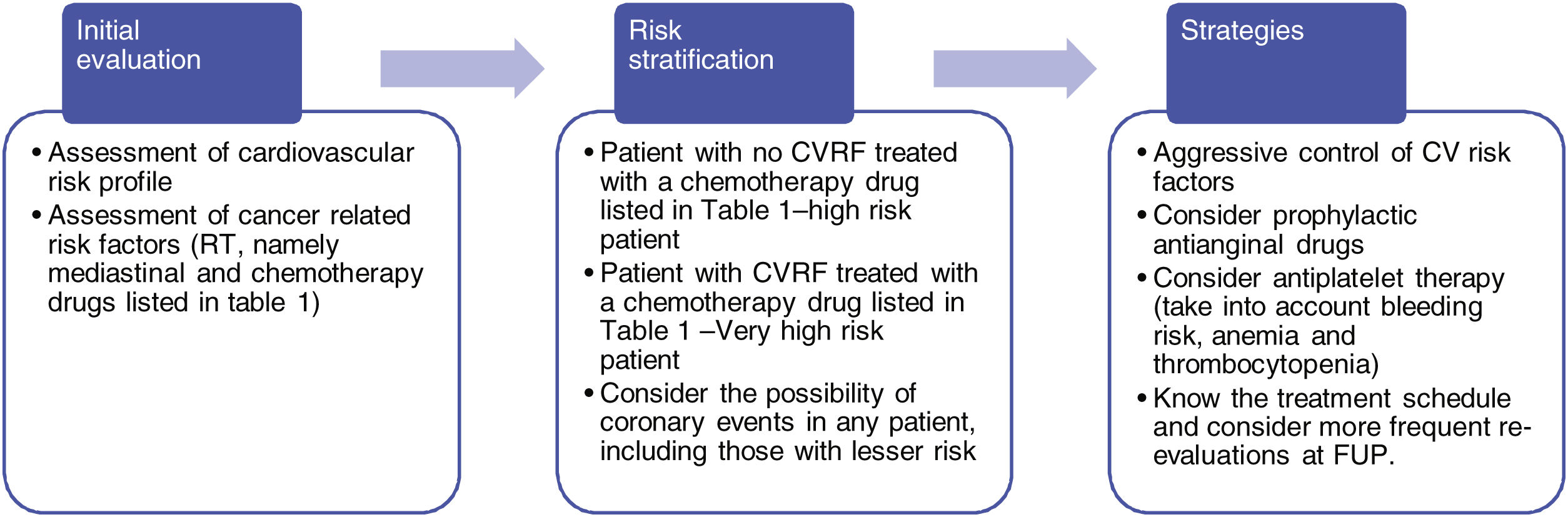
Some strategies (listed in Figure 2) can be considered to reduce CAD burden. The patient specific cardiovascular risk profile should first be assessed as usual in any other setting. Then, cancer specific risk factors, including chemotherapy agents and radiotherapy should be evaluated. Table 1 or a similar example should be routinely available and updated for those dealing with these patients.
Considering the baseline classic cardiovascular risk profile of the patient, if therapy with a potentially coronary toxic cancer agent is planned, the patient should be regarded as high risk (patients undergoing paclitaxel with no classic risk factors) or very high risk (patients undergoing therapy with 5-FU, or a less relevant agent but in combination with cardiovascular risk factors) in terms of CAD management. If so, aggressive risk factor modification should be employed and prophylactic anti-anginal drugs considered. Antiplatelet therapy may also be considered, taking into account the patients’ bleeding risk, as well as the presence of anemia or thrombocytopenia, which is an ever-present issue in these patients. Lastly, information regarding the timing of cancer therapy should be available, with shorter follow-up intervals during administration of these drugs.
Revascularization in cancer patients: what we knowCurrently available evidence for revascularization in cancer patients has grown in the past few years yet remains scarce and entirely observational.
For coronary artery bypass surgery (CABG), the largest amount of data comes from the USA National Inpatient Sample.23 A propensity score analysis encompassing over one hundred thousand patients up to 2015 found that cancer patients had higher rates of major bleeding (nearly 3 times) and stroke, highlighting the increased frailty of these patients. Interestingly, the commonly perceived issue of higher risk of mortality in these patients was not confirmed, as in-hospital mortality was similar across groups. However, in clinical practice cancer patients are often considered poor surgical candidates and often referred for PCI instead by heart teams. Thus, selection bias in this study was a likely factor. Nonetheless, the results highlight that CABG in cancer patients is possible and should not be routinely denied.
For PCI, currently available evidence is more extensive (albeit also clearly lacking) and comes from several (sometimes large-scale) observational studies. As for CABG, the largest studies derived from the USA National Inpatient Sample,24,25 which included all patients with cancer (active or not) undergoing PCI, regardless of clinical presentation (ACS vs. CCS) with the primary aim of assessing outcomes – especially mortality – in cancer patients. Other studies have focused mostly on ACS patients,26,27 while others on ischemic/bleeding outcomes13,14 and some on specific cancer types, especially non-solid tumors.28 The outcomes of cancer patients with ACS in Portugal were also evaluated in a ProACS analysis.4 The main findings of currently available studies can be summarized as follows:
- -
Cancer patients are generally older, have more CV/non-CV comorbidities than non-cancer patients and are more often managed conservatively4,13,25–27
- -
The effect of PCI in reducing ischemic events was maintained in cancer patients, without an increased risk of stent thrombosis4,13
- -
Cancer patients generally have higher rates of mortality (both in-hospital and later on),4,13,25,26 bleeding13,25–27 and early readmissions27
- -
Active cancer and advanced stage (especially the presence of metastasis) significantly worsen prognosis, with increased risk of mortality and bleeding,13,24–27 as well as possibly ischemic events and stent thrombosis29
- -
There may be an in-hospital mortality benefit with PCI for head and neck cancers and some hematologic malignancies (Hodgkin's lymphoma and leukemia patients), across all presentation subtypes (non-ACS, non-ST elevation myocardial infarction/unstable angina and STEMI), but not in the presence of metastases. Active cancer patients may derive greater benefit24
- -
Lung cancer carries the highest risk for PCI, with increased in-hospital mortality (three times higher than the general population if metastatic disease), bleeding and readmissions,24,25 followed by hematologic malignancies in general28
- -
Breast cancer is in the opposite spectrum of risk, with outcomes similar to the general population, albeit with increased risk of bleeding if metastatic disease2,4,25
- -
Prostate and colon cancer are in a somewhat intermediate spectrum of bleeding and readmissions risk, with worse results for the latter25,27
- -
Radiotherapy induced CAD patients undergoing PCI with drug-eluting stents (DES) have similar outcomes to the general population,30 but increased mortality with BMS or POBA31; for CABG, radiotherapy induced atherosclerosis of mammary arteries and impaired tissue healing after sternotomy32 are important issues to consider before surgery, as recommended in recent ESC guidelines.5
It is worth noting that the abovementioned studies have major limitations. In addition to their observational and sometimes retrospective and/or single-center nature, most patients have past rather than active cancer, antiplatelet therapy details are absent/scarce and stent/PCI technology is often not contemporary. Another very important issue is the clinical setting of PCI i.e. ACS vs CCS. While many of these studies specifically focus on ACS patients only, those that do not fail to provide detailed results according to clinical presentation. In addition, most CCS patients who undergo PCI do so in an in-patient setting, where the trigger for angiography was unclear and likely related to specific factors, such as very high CV/cancer therapy risk, severe symptoms or impaired systolic function. Therefore, the evidence for revascularization in cancer patients outside the scope of ACS is especially limited.
When to perform revascularization in cancer patientsThe main takeaway from currently available evidence is that cancer patients can still derive important benefits from revascularization, and thus a generally conservative approach is inappropriate. However, given the much larger risk of complications as well as mortality in these patients, a case-by-case evaluation should be undertaken. It is also important to bear in mind that performing a coronary angiography does not mean proceeding with revascularization – as a result, the threshold for undertaking both should be quite different.
Current ESC Guidelines recommend an invasive approach for STEMI or high risk NSTE-ACS, provided life expectancy is ≥6 months, whereas with low-risk NSTE-ACS a conservative approach may be attempted.5 While we generally agree with these recommendations, we believe more factors should be taken into consideration, as outlined below. Additionally, in the STEMI or very high risk NSTE-ACS context, information regarding life expectancy often cannot be obtained in a timely manner, given its emergency setting. As such, the application of such of the life expectancy principle may not be feasible in a considerable number of cases. For chronic coronary syndromes, current ESC guidelines recommend an initial conservative approach,5 with which we agree.
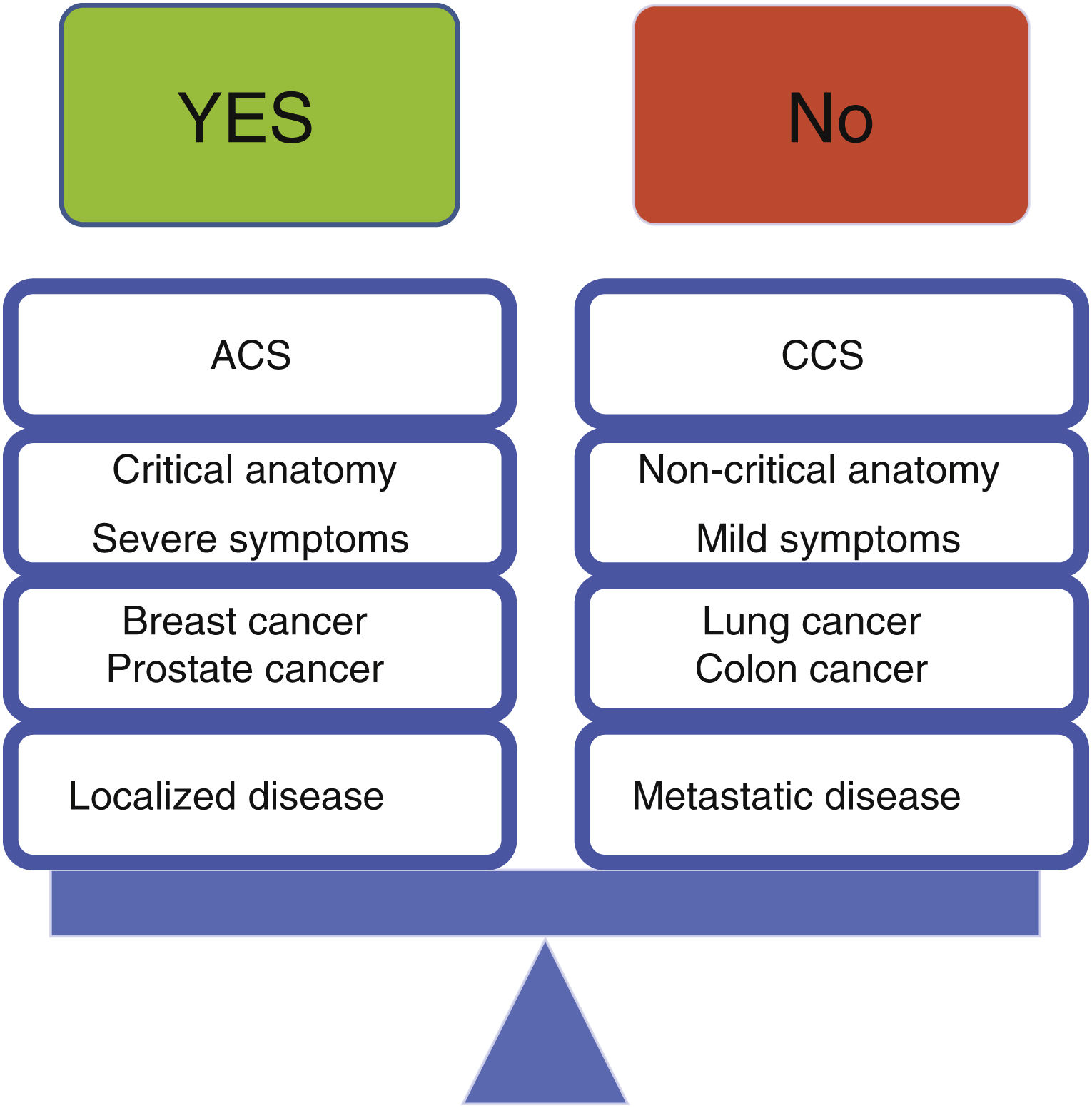
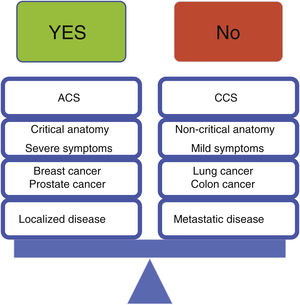
In light of the above, we believe the following principles should apply, as outlined in Figure 3:
- -
In patients with acute coronary syndrome, invasive risk stratification should generally apply according to current guidelines. Importantly, a thorough history regarding cancer status and vital prognosis should be sought before proceeding with catheterization in the setting of NSTE-ACS, favoring a conservative approach for low-risk cases or short life expectancy (<6 months). If a specific cancer therapy is clearly related to the occurrence of an ACS, temporary or permanent cessation of such therapies should be considered
- -
In patients with chronic coronary syndromes, initial conservative management should be the default, except in the presence of severe symptoms (CCS class ≥3), critical anatomy (left main) or impaired left ventricle systolic function
- -
Revascularization should preferably be undertaken with PCI, given the very scarce data available for CABG in this population, as well as the general clinical impression that surgery is often poorly tolerated in these patients; CABG should not, however, be routinely withheld from these patients, but rather evaluated individually; importantly, hybrid strategies, especially if minimally invasive approaches are feasible, should also be considered. Current ESC guidelines suggest CABG for patients with extensive CAD for whom PCI is a poor technical option, with Heart Team discussion and if prognosis exceeds 12 months.5 Furthermore, given that patients with radiation-induced CAD have in increased risk of complications after mediastinal RT,32 guidelines also suggest PCI with DES may be favored over CABG with multivessel disease and a high SYNTAX score (>22), if feasible5
- -
Low-risk primary tumor location (especially breast cancer), local and non-metastatic disease are favorable scenarios for revascularization, given their lower risk of complications
- -
High-risk primary tumor location (especially lung cancer), advanced and metastatic disease are unfavorable scenarios for revascularization. Proceeding in these patients requires judicious use of every strategy to minimize bleeding, close follow-up and realistic management of expectations
- -
Non-solid tumors and active disease are mixed scenarios regarding revascularization – while the risk of intervention is higher, the potential benefit may also be greater.
The increased risk of PCI and specificities of cancer patients warrant several special precautions. The following sections detail factors to consider.
Anemia and thrombocytopeniaSpecific evidence for exact red blood cell (RBC) and platelet levels’ thresholds regarding angiography and PCI in cancer patients is lacking.
Anemia is an almost ubiquitous problem in cancer patients. The etiology is often multifactorial and should ideally be clarified prior to the procedure. Corrective factors, such as vitamin B12, folate, iron or erythropoietin should be provided prior to the procedure whenever possible, thereby minimizing anemia, seeking help from other specialties regularly. If necessary, RBC transfusion may be administered. The general threshold is 7 g/dL in most guidelines, including those of ESMO.33 Notwithstanding, given the increased risk of bleeding and complications in cancer patients, as well as the unwanted effects of blood products administration during the procedure, higher Hb levels (closer to 9–10 g/dL) may be a more sensible option, especially if radial access is not available, complex PCI is planned or there is a specific reason for more aggressive antithrombotic therapy.
Thrombocytopenia is another challenge commonly encountered, either due to the cancer itself or chemotherapy drugs. Current SCAI recommendations3 and ESC Guidelines5 suggest a threshold of 30000 cells/μL for PCI (although 50000 cells/μL is preferable) and 50000 cells/μL for CABG, which is generally agreed upon and which we endorse. SCAI recommendations consider, however, that there is no lower limit for diagnostic angiography.3 Considering the risk of spontaneous bleeding for levels <10000 cells/μL, coronary angiogram is probably best avoided in such extreme levels, unless deemed absolutely necessary and provided radial access is easily obtainable. Platelet transfusion should be undertaken if necessary and feasible for achieving the desirable threshold. Table 2 summarizes these considerations.
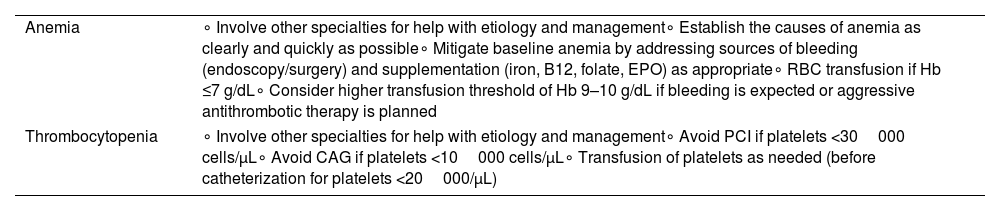
Considerations regarding anemia and thrombocytopenia.
| Anemia | ∘ Involve other specialties for help with etiology and management∘ Establish the causes of anemia as clearly and quickly as possible∘ Mitigate baseline anemia by addressing sources of bleeding (endoscopy/surgery) and supplementation (iron, B12, folate, EPO) as appropriate∘ RBC transfusion if Hb ≤7 g/dL∘ Consider higher transfusion threshold of Hb 9–10 g/dL if bleeding is expected or aggressive antithrombotic therapy is planned |
| Thrombocytopenia | ∘ Involve other specialties for help with etiology and management∘ Avoid PCI if platelets <30000 cells/μL∘ Avoid CAG if platelets <10000 cells/μL∘ Transfusion of platelets as needed (before catheterization for platelets <20000/μL) |
CAG: coronary angiography; EPO: erythropoietin; Hb: hemoglobin; PCI: percutaneous coronary intervention; RBC: red blood cell transfusion.
Radial access provides a safer choice for PCI with well-established evidence in multiple clinical trials34,35 and should be the default strategy for cancer patients, as in the general population according to current guidelines.10 If femoral access is inexorable, ultrasound guided puncture may decrease the risk of bleeding36,37 and should be sought, provided operators are familiar with the technique. Blind puncture should be avoided and if closure devices are used, especially plug-based devices, pre-closure angiography/ultrasound may be considered, also bearing in mind that endothelization may be slower in patients on chemotherapy, whose risk of infection is also greater.3 Suture devices may be preferred, if operators have the experience. The use of hydrophilic sheaths should also be considered.
Drug-eluting stents (DES) should be the default strategy while performing PCI. SCAI guidelines3 suggest the use of bare metal stents (BMS) when a minimum of 1 month of dual antiplatelet therapy (DAPT) is necessary, but ample evidence has arisen since their publication in 2016 to support DES as standard, as current ESC recommendations do.6 The rates of stent thrombosis are generally very low38,39 and even <1% in more recent trials across multiple current DES platforms.40,41 Even in patients on radiotherapy, where endothelization may be altered, there is observational data to support the use of DES.30 The improved survival of cancer patients further supports DES, as mid to long-term results should be increasingly a concern.42 Bioresorbable scaffolds should be avoided given the disappointing results of past trials43 and the still somewhat experimental nature of the platforms. Plain old balloon angioplasty (POBA) should be reserved for patients who cannot tolerate even a minimum of one month DAPT, although this technique is no more than an a priori intention, as stents may always be necessary for bailing out complications such as dissection or abrupt vessel closure.3,44
It is well known that physiology-guided PCI is generally superior to angiography guided PCI.45 The indexes with the highest level of evidence are FFR46 and iFR.47,48 The cut-off for proceeding with revascularization should follow the standard practice of use,6 although at times a lower threshold (such as the FFR 0.75 cut-off of the DEFER trial49) may be used for selected cancer patients, especially when delays in treatment caused by DAPT are especially nefarious for the oncologic outcome.3
Intravascular ultrasound (IVUS)-guided PCI has also repeatedly been shown to produce better results, with reduced risk of target vessel failure and stent thrombosis.50,51 The same has also been reported with optical coherence tomography, although evidence is less extensive.52,53
Because of the increased risk of bleeding complications in cancer patients and also target lesion revascularization29 and the improved results adjuvant technology can provide43 – i.e. physiology and intra-coronary imaging – these should always be considered, in order to achieve a technically “perfect” result, while also taking into account the added complexity of intervention as well. Table 3 summarizes these considerations.
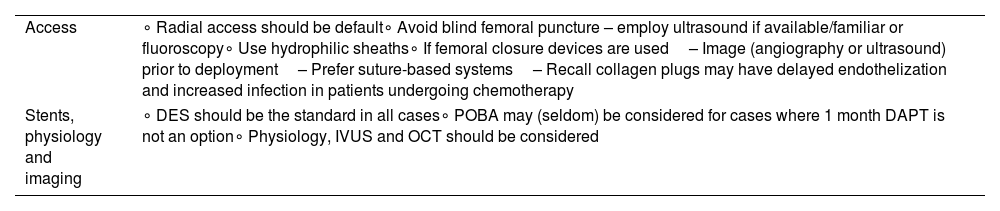
Considerations regarding technical aspects of the procedure.
| Access | ∘ Radial access should be default∘ Avoid blind femoral puncture – employ ultrasound if available/familiar or fluoroscopy∘ Use hydrophilic sheaths∘ If femoral closure devices are used– Image (angiography or ultrasound) prior to deployment– Prefer suture-based systems– Recall collagen plugs may have delayed endothelization and increased infection in patients undergoing chemotherapy |
| Stents, physiology and imaging | ∘ DES should be the standard in all cases∘ POBA may (seldom) be considered for cases where 1 month DAPT is not an option∘ Physiology, IVUS and OCT should be considered |
DES: drug-eluting stents; IVUS: intra-vascular ultrasound; OCT: optical coherence tomography; POBA: plain old balloon angioplasty.
As bleeding is the single most common complication in cancer patients, every effort should be sought to maximize safety, remembering that commonly used risk stratification scores (such as DAPT,54 PRECISE-DAPT,55 or CRUSADE56) do not take into account cancer as part of their stratification variables, although this has more recently been recognized by other authors.57 Although exact quantification by use of a specific score is lacking, bleeding risk varies according to primary tumor nature, location, stage and metastasis. Bleeding risk should therefore be considered taking these variables into account, which are unique to cancer patients. It is especially important to recall the severity of bleeding due to volume (such as GI) or critical location (such as intra-cranial). Additionally, cancer patients often require surgery on a regular basis, with minimal delays. On the order hand, chemotherapy may theoretically delay stent endothelization, perhaps contributing to the observation that target lesion revascularization is more common in cancer patients.29
Considering the above, shortening DAPT periods is by far the most common necessity in cancer patients. Fortunately, a growing number of clinical trials during the last decade have consistently shown that with current stent technology, 1 month DAPT is a safe and effective strategy, with similarly low rates of ischemic events and significant reduction of bleeding events.38–41 Importantly, the largest of these trials included malignancy other than skin (including GI or lung) as one of the high bleeding risk criteria.40,41 Longer periods of DAPT (3 months) have also been studied with good results.58 As a result, shortening DAPT to a minimum of 1 month can be generally considered whenever bleeding risk is high (such as colon, lung, metastatic cancers, or those with significant anemia or thrombocytopenia) or short-term surgery is warranted.
Current ESC guidelines state that high potency P2Y12 inhibitors (ticagrelor, prasugrel) should be avoided in patients with a platelet count of <50000/μL, but may be considered in selected cases.6 These agents carry a higher risk of bleeding than clopidogrel as observed in both clinical trials and registries,59–61 with very limited evidence for cancer patients. As a result, while we generally agree with current guidelines, we believe clopidogrel should be the standard, with ticagrelor or prasugrel reserved for specific cancer patients with lower risk of bleeding, such as non-advanced, non-metastatic breast cancer without significant anemia or thrombocytopenia. Importantly, interactions may be of greater relevance with more potent P2Y12 inhibitors, whereas for clopidogrel it should be remembered that paclitaxel increases its concentration and therefore bleeding risks.3 Lastly, current ESC guidelines state that with a platelet count <30000 cells/μL, clopidogrel should be avoided and aspirin withheld with <10000 cells/μL.6 This is a strategy we endorse.
For patients requiring oral anticoagulation, multiple clinical trials have shown that dual therapy with OAC (warfarin or DOACs) and clopidogrel (avoiding aspirin except for a very brief period peri-procedurally) is feasible and safe following PCI.62–66 While cancer patients are mostly on anticoagulation therapy for venous thrombosis/embolism rather than atrial fibrillation, multiple clinical trials have also shown that edoxaban, rivaroxaban and apixaban can be effectively used in cancer patients.67–69 As a result, considering the very high bleeding risk of cancer patients, a combination of dual therapy with a DOAC and clopidogrel following PCI should be the default strategy. As there is limited evidence on dabigatran in cancer patients, together with the slightly increased rates of stent thrombosis observed in the RE-DUAL trial62 with the 110 mg dosage of dabigatran (which might have been preferred for cancer patients given their bleeding risk), an anti-Xa DOACs should be preferred.
Lastly, regarding intra-procedural anticoagulation, current guidelines should be followed, bearing in mind that every effort should be made to minimize bleeding. Routine use of fondaparinux for UA/NSTEMI, using heparin dosages and ACT values closer to the lower limits of target intervals (70 U/kg or 50–70 U/kg and 250 s, respectively) and avoidance of regimen crossovers and GpIIbIIIa inhibitors should be sought.3,6 A summary can be found in Table 4.
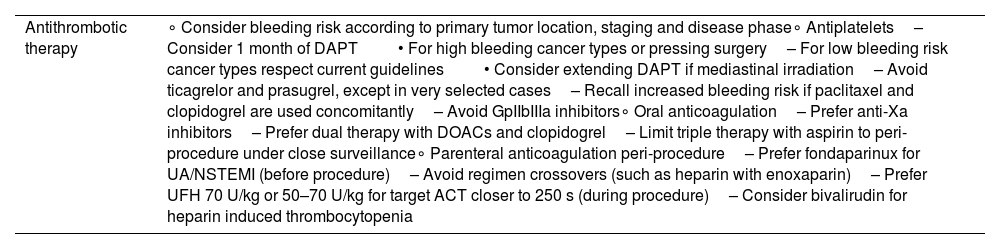
Considerations regarding antithrombotic therapy.
| Antithrombotic therapy | ∘ Consider bleeding risk according to primary tumor location, staging and disease phase∘ Antiplatelets– Consider 1 month of DAPT• For high bleeding cancer types or pressing surgery– For low bleeding risk cancer types respect current guidelines• Consider extending DAPT if mediastinal irradiation– Avoid ticagrelor and prasugrel, except in very selected cases– Recall increased bleeding risk if paclitaxel and clopidogrel are used concomitantly– Avoid GpIIbIIIa inhibitors∘ Oral anticoagulation– Prefer anti-Xa inhibitors– Prefer dual therapy with DOACs and clopidogrel– Limit triple therapy with aspirin to peri-procedure under close surveillance∘ Parenteral anticoagulation peri-procedure– Prefer fondaparinux for UA/NSTEMI (before procedure)– Avoid regimen crossovers (such as heparin with enoxaparin)– Prefer UFH 70 U/kg or 50–70 U/kg for target ACT closer to 250 s (during procedure)– Consider bivalirudin for heparin induced thrombocytopenia |
ACT: activated clotting time; DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulant; NSTEMI: on-ST elevation myocardial infarction; UA: unstable angina; UFH: unfractionated heparin.
In the setting of structural heart intervention in Cardio-Oncology, the three most commonly faced issues in clinical practice are: aortic stenosis, mitral regurgitation and left atrial appendage occlusion (LAAO). Each is dealt with in the following sections.
Aortic stenosisAortic stenosis (AS) is a common issue in cancer patients, as they share several risk factors.70 While chemotherapy, especially anthracyclines a are associated with increased risk of valvular disease,71 radiation is perhaps the single greatest cancer specific risk factor, accelerating the emergence and progression of the disease, with general screening for cardiac complications from five years after treatment cessation.70 Current available evidence suggests that surgical aortic valve replacement (SAVR) outcomes are worse for patients with past mediastinal radiotherapy72 and that transcatheter aortic valve implantation (TAVI) results in better in-hospital outcomes and survival than SAVR in patients at intermediate or high risk for heart surgery.73
Current ESC guidelines recommend TAVI for patients at intermediate surgical risk or greater, while also highlighting that STS or EuroSCORE stratification tools may not fully appreciate the surgery-related risk of these patients.5 Hence, patients with past radiotherapy should always undergo a thorough evaluation, as radiation may often induce pulmonary or ascending aortic fibrosis (favoring TAVI), pericardial fibrosis (potentially requiring heart surgery and thereby favoring SAVR) and CAD (which may warrant PCI or CABG, thereby influencing AS treatment modality).74
Regarding patients without past chest radiation, TAVI is currently recommended as the default choice for the treatment of symptomatic severe aortic stenosis in older or more frail patients, despite the fact that age cut-offs vary between American75 and European76 guidelines. Considering the good outcomes of TAVI vs. SAVR across patients with high,77,78 intermediate79,80 and low surgical risk81,82 and the increased frailty of cancer patients, TAVI seems the logical choice for most of these patients. However, cancer patients have generally been excluded from the major trials. Currently available evidence is entirely observational and focuses mostly on TAVI. Comparative studies of TAVI vs. SAVR suggest that the while outcomes (namely mortality) may be generally similar between SAVR and TAVI, the latter enables earlier administration or resumption of cancer therapies. Effective comparisons are limited, as groups are generally rather heterogeneous, with TAVI patients being older and with more comorbidities. No differences across cancer type, staging or active vs. past cancer were found.83,84 With regards to TAVI specifically, one meta-analysis focused on TAVI outcomes in patients with active cancer: short-term outcomes were generally similar between patients with cancer vs. those without, with a trend toward increased pacemaker and bleeding rates in cancer patients. One-year mortality, however, was significantly higher in cancer patients, suggesting that the bulk of mortality was derived from the oncologic rather than the CV disease.70 Another larger meta-analysis,85 partially overlapping with the former,70 found no differences of outcomes after TAVI at any time point between cancer vs. no cancer patients, but also included patients with past rather than active cancer, suggesting that active disease has a relevant contribution for prognosis, as would be expected.
Another important issue is the incidental detection of cancer in CT taken for TAVI planning. The diagnosis of cancer in these patients is estimated in 2.8% according to a recent study and may have implications in the treatment of AS with delay or even cancellation of the TAVI procedure. However, these cases should be discussed with Oncology to establish the prognosis of the disease and decide the best approach to both diseases, in a similar way as other patients with already diagnosed with active cancer.86
The aggregate of currently available evidence therefore suggests that:
- (1)
Cancer patients can derive benefit from aortic valve intervention
- (2)
Active cancer patients may have a worse outcome and may be at increased risk of complications
- (3)
Evidence supporting intervention is greater and more rapidly expanding for TAVI
As a result, we believe that TAVI should be the default strategy for cancer patients, if feasible, especially those who previously underwent chest radiotherapy. It should neither be routinely denied nor offered, but rather decided on a case-by-case basis. Currently available evidence suggests that most patients probably benefit from intervention, provided expected survival is likely to exceed >1 year, as suggested in current guidelines.75,76Table 5 summarizes these considerations.
Summary of considerations regarding aortic stenosis.
| Aortic stenosis | ∘ Screening from 5 years after cessation of local radiotherapy onwards∘ Consider intervention for patients with life expectancy >1 year∘ TAVI should be the default choice for most cases∘ Balloon valvuloplasty may be used:– As a bridge to definitive therapy– As a means of stabilizing heart failure or permitting non-heart cancer surgery– Backup TAVI should be available∘ SAVR should be considered when:– Other reasons for heart surgery (coronary, pericardial or multiple valves disease)– Poor technical candidate for TAVI– Young patients with acceptable surgical risk and very good long-term cancer prognosis |
SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve intervention.
Cancer patients are at increased risk of mitral regurgitation, as radiotherapy and myocardial cardiotoxicity induced by anthracyclines, trastuzumab or other agents may contribute to development of primary (organic) or secondary (functional) regurgitation.3,18,71 Percutaneous treatment of mitral valve disease is rapidly expanding, with fewer durable results in primary regurgitation87 and mixed results for secondary regurgitation, although available evidence suggests a relevant prognostic benefit in patients where the severity of mitral regurgitation has not progressed too far.88,89 Little evidence concerning cancer patients is available, although a history of cancer seems to adversely affect prognosis in patients undergoing percutaneous edge-to-edge mitral repair.90 Given the frailty of cancer patients, percutaneous repair may be of use as definitive therapy or bridging therapy for surgery for improving functional status enabling cancer therapies. For surgical intervention, minimally invasive techniques should be considered. Table 6 summarizes these considerations.
Summary of considerations regarding mitral regurgitation.
| Mitral regurgitation | ∘ Intervention should be considered in selected cases with life expectancy >1 year∘ Percutaneous repair may be considered in selected cases either as bridging or definitive approach, both for primary (organic) or secondary (functional) regurgitation∘ For surgical intervention, minimally invasive techniques should be considered, if feasible |
The added bleeding risk of cancer patients, together with anemia and thrombocytopenia, render OAC sometimes challenging, especially for cases with GI cancer, where bleeding is especially common, including with DOACs.67–69,91 As a result, LAAO would theoretically be a good option for such cases. While still there is still no agreement, initial evidence comparing LAAO with warfarin, as well as LAAO outcomes in a large real-world registry92,93 has recently been complemented by the PRAGUE-17 trial, where LAAO has shown non-inferiority to treatment with DOACs, with significantly reduced bleeding.94 Notwithstanding the scarcity of evidence in cancer patients, with the exclusion of cancer patients from LAAO clinical trials, observational studies have suggested increased risk of in-hospital mortality (regardless of cancer status)95 and in-hospital stroke (for active cancer).96 The authors speculate on a possible relation between the hypercoagulable state in cancer and additional challenges of antithrombotic therapies peri-procedurally, as patients undergoing LAAO usually require at the very least a short period of mono antiplatelet therapy (often with an initial period of DAPT or DOAC). Thus, while LAAO may be considered for cancer patients intolerant to OAC on a case-by-case decision, extra-caution should be exercised peri-procedurally. Table 7 summarizes these considerations.
Summary of considerations regarding left atrial appendage occlusion.
| Left atrial appendage occlusion | ∘ Intervention may be considered in selected cases, especially:– DOAC intolerance– Embolic events while on DOAC∘ The intra and peri-procedural period should occur under strict regular surveillance– Higher risk of complications or events than in the general population |
DOAC: direct oral anticoagulant.
The use of multidisciplinary teams (MDT) is commonplace in Oncology. In Cardiovascular disease, the role of an MDT, the so-called heart team, has also been widely advocated for and implemented.
Given the complexity of cancer patients, it seems quite clear that an MDT approach is fundamental in caring for this subset. As a result, a Cardio-Oncology heart team should be sought for these patients, especially for those with active disease.
To achieve an effective decision-making process, the following issues should be considered:
- -
The existing Cardio-Oncology team should ideally include cardiologists from multiple fields i.e., Interventional Cardiology, Arrhythmology and Imaging, rather than only clinical cardiologists or heart failure physicians
- -
For heart team meetings:
- ∘
If cancer patients are discussed, the existing team should include at least one cardiologist with expertise in Cardio-Oncology
- ∘
An oncologist or hematologist (depending on the types of cancer) should be present at heart team meetings, if possible
- ∘
Specific heart team meetings/slots may be organized for cancer patients.
- ∘
- -
For MDT Oncology/Hematology meetings:
- ∘
A cardiologist with expertise in Cardio-Oncology can be present in selected cases, depending on the baseline CV risk (especially if high or very high) or there is suspected/existing heart disease.
- ∘
The exact framework will vary widely depending on available resources. However, with the current possibilities of remote meetings, an effective network, with multiple institutions, should be implemented.
Conflicts of interestThe authors have no conflicts of interest to declare.