The Ross procedure is the preferred surgical treatment for pediatric aortic valve diseases, and its long-term outcomes have been extensively documented. This article presents the results of the Ross and Ross–Konno procedures performed on pediatric patients in our center.
MethodsRoss and Ross–Konno procedures were performed on 20 patients in our center between January 2015 and January 2019.
ResultsThe patients’ mean age was 10.6 years (range: 23 days to 18 years) and mean weight was 37.6 kg (range: 3–63 kg). Thirteen had aortic valve stenosis, four had aortic valve insufficiency, and three had a mixed disease. The Ross–Konno procedure was used for four patients. The mean cardiopulmonary bypass time was 184.68±60.1 min and the mean cross time was 149±67.8 min. One neonatal patient died in the early postoperative phase due to low cardiac output. The mean follow-up time was 60.15±24.45 months. One patient later underwent reoperation due to conduit stenosis. One patient is being monitored for moderately serious conduit stenosis. At present, of those who underwent the procedure, one has moderate aortic regurgitation, two have mild or moderate aortic regurgitation, and others have minimal aortic regurgitation. No patients required intervention for left ventricular outflow tract obstruction and mortality was not observed in the long term.
Conclusion(s)The reintervention rates for autograft and conduit-related cases were low in early and medium-term follow-up, and no significant autograft insufficiency was observed. Ross or Ross–Konno surgery is preferred for aortic diseases in pediatric patients due to its low mortality and satisfactory long-term results.
O método de Ross é o tratamento cirúrgico preferencial para doenças da válvula aórtica pediátrica e os seus resultados a longo prazo têm sido amplamente documentados. Este artigo apresenta os resultados dos procedimentos de Ross e Ross–Konno realizados em pacientes pediátricos no nosso centro.
MétodosForam realizados procedimentos de Ross e Ross–Konno em 20 pacientes no nosso centro entre janeiro de 2015 e janeiro de 2019.
ResultadosA idade média dos pacientes foi de 10,6 anos (intervalo: 23 dias a 18 anos) e o peso médio foi de 37,6kg (intervalo: 3–63kg). Treze pacientes tinham estenose da válvula aórtica, quatro tinham insuficiência da válvula aórtica e três tinham patologia mista. O procedimento Ross–Konno foi utilizado em quatro pacientes. O tempo médio de circulação extracorpórea foi de 184,68±60,1 minutos e o tempo médio de pinçamento aórtico foi de 149±67,8 minutos. Um paciente neonatal faleceu na fase pós-operatória precoce devido a baixo débito cardíaco. O tempo médio de acompanhamento foi de 60,15±24,45 meses. Um paciente posteriormente passou por reoperação devido a estenose do conduto. Um paciente está a ser monitorizado devido a estenose moderadamente grave do conduto. Neste momento, um paciente apresenta regurgitação aórtica moderada, dois pacientes têm regurgitação aórtica ligeira ou moderada e os outros têm regurgitação aórtica mínima. Nenhum paciente necessitou de intervenção para obstrução do trato de saída do ventrículo esquerdo e não foi observada mortalidade a longo prazo.
ConclusãoAs taxas de reintervenção para casos relacionados com autoenxerto e com o conduto foram baixas no acompanhamento a curto e médio prazo e não foi observada insuficiência significativa no autoenxerto. A cirurgia de Ross ou Ross–Konno é preferida para patologias aórticas em pacientes pediátricos devido à baixa mortalidade e resultados satisfatórios a longo prazo.
The Ross procedure (pulmonary autograft replacement), first performed by Donald Ross in 1967, involves the placement of a pulmonary valve as an autologous graft in the aortic valve position and a right ventricular pulmonary artery conduit in the pulmonary area to correct aortic valve diseases.1 The Ross–Konno procedure is an excellent technique for the treatment of complex multilevel left ventricular outflow tract obstruction with severe annular hypoplasia and a dysplastic aortic valve.
The advantages of this procedure include good hemodynamic results, no requirement for anticoagulants, native tissue growing with the patient, a low risk of endocarditis, and fewer thromboembolic events.2,3 Progressive neoaortic valve regurgitation and the need to replace the conduit at the right ventricular outlet in growing children are the main obstacles.4,5 The Ross procedure is preferred for young patients due to the limited types of artificial aortic valves available for small children and the difficulty of adapting anticoagulant agents.6
ObjectivesIn this article, we present the early- and medium-term results of the Ross procedure that we performed on pediatric patients in our center.
MethodsWe analyzed the data of 20 patients who underwent Ross or Ross–Konno operations in our center between 2015 and 2019 to correct aortic valve stenosis and insufficiency. The patients’ preoperative, operative, and postoperative data were documented and retrospectively evaluated, including their preoperative and postoperative routine echocardiography and angiography records. Follow-up echocardiograms were performed annually at a minimum and more frequently if necessary.
The use of the Ross and Ross–Konno procedures and the indications for subsequent valve reoperations were determined in accordance with the guidelines of the American Heart Association. Patients with symptomatic or asymptomatic mild or severe aortic stenosis (average gradient: 40 mm/Hg) and/or grade 2 or more advanced valve regurgitation progressive increase at the left ventricular end diastolic volume were recommended for surgical intervention.
Surgical techniqueHigh aortic and bicaval cannulation and cardiopulmonary bypass were performed on all patients following a median sternotomy. The cross-clamp was placed on the ascending aorta under moderate hypothermia (32°C). Cardiac arrest was achieved with antegrade hypothermic cardioplegia.
The aortic root was prepared for the ventricular–aortic junction. An aortotomy inspection was performed over 7–10 mm of the ascending aorta. The right and left coronary arteries were prepared as buttons and the aortic valves were excised. A Konno incision was performed on those patients with a small aortic root and left ventricular outflow tract obstruction. However, the ventricular portion of the incision (ventricular septal defect) was closed with a synthetic patch to provide greater stability and better sizing of the neoaortic valve annulus and to minimize damage caused to the right ventricle. The pulmonary root was extracted together with the solid adventitia and 3–5 mm of the muscular cuff to protect the support and integrity of the valve structures.
To prevent any injury to the first septal branch of the left anterior descending artery, a tangential incision was made. The pulmonary autograft was implanted with a continuous polypropylene suture in the aortic position. A second hemostasis stitch was then added. The coronary buttons were anastomosed with 6/0 polypropylene at the sinuses of Valsalva. The right ventricular outlet was reconstructed with Freestyle® SX (Medtronic, Minneapolis, MN, USA) grafts in 12 patients, Contegra® (Medtronic Inc, Minneapolis, MN, USA) grafts in six patients, and a Hancock® Bioprosthetic Valved Conduits (Medtronic Inc, Minneapolis, MN, USA) graft in one patient. Each graft was enlarged to the maximum possible size permitted by the mediastinal space (usually 1.2–1.5 times). An intraoperative transesophageal echocardiography was performed during cardiopulmonary bypass for each patient.
Statistical analysisStatistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 25.0, IBM, Armonk, NY, United States). All continuous variables were expressed in mean±standard deviation and categorical variables were expressed in median and range. A p value of <0.05 was considered statistically significant.
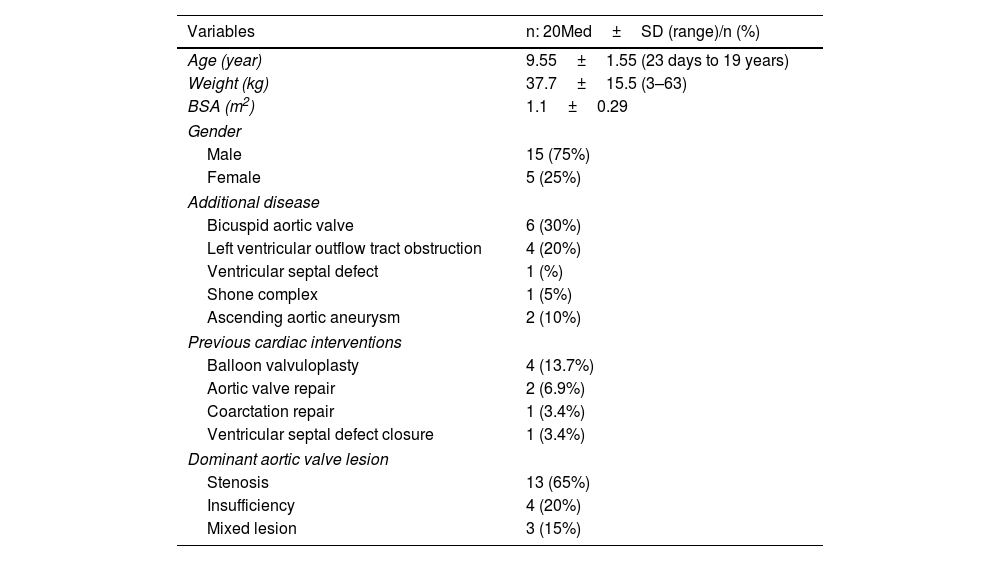
ResultsThe Ross or Ross–Konno procedures were performed on 20 patients (mean age, 10.6 years; age range, 23 days to 18 years), two (10%) of whom were in the neonatal period. Three-fourths (15) of the patients were male. Aortic valve stenosis was present in 13 (65%) patients, aortic regurgitation in 4 (20%) patients, and combined disease (aortic valve stenosis and aortic regurgitation) in three (15%) patients. Six patients had bicuspid aortic valve stenosis, four had subvalvular stenosis, and six had previously undergone ballon valvuloplasty. Ascending aortic aneurysm was present in one patient and Shone complex in one patient. Neonatal coarctation repair was performed on four patients, aortic valve repair on two patients, and ventricular septal defect closure on one patient (Table 1).
Demographics and preoperative variables.
| Variables | n: 20Med±SD (range)/n (%) |
|---|---|
| Age (year) | 9.55±1.55 (23 days to 19 years) |
| Weight (kg) | 37.7±15.5 (3–63) |
| BSA (m2) | 1.1±0.29 |
| Gender | |
| Male | 15 (75%) |
| Female | 5 (25%) |
| Additional disease | |
| Bicuspid aortic valve | 6 (30%) |
| Left ventricular outflow tract obstruction | 4 (20%) |
| Ventricular septal defect | 1 (%) |
| Shone complex | 1 (5%) |
| Ascending aortic aneurysm | 2 (10%) |
| Previous cardiac interventions | |
| Balloon valvuloplasty | 4 (13.7%) |
| Aortic valve repair | 2 (6.9%) |
| Coarctation repair | 1 (3.4%) |
| Ventricular septal defect closure | 1 (3.4%) |
| Dominant aortic valve lesion | |
| Stenosis | 13 (65%) |
| Insufficiency | 4 (20%) |
| Mixed lesion | 3 (15%) |
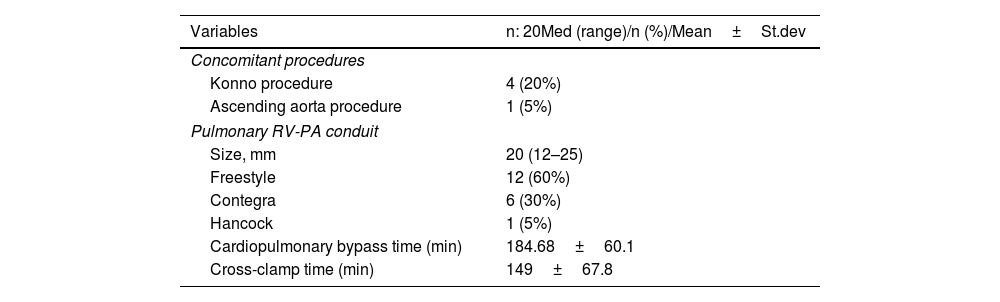
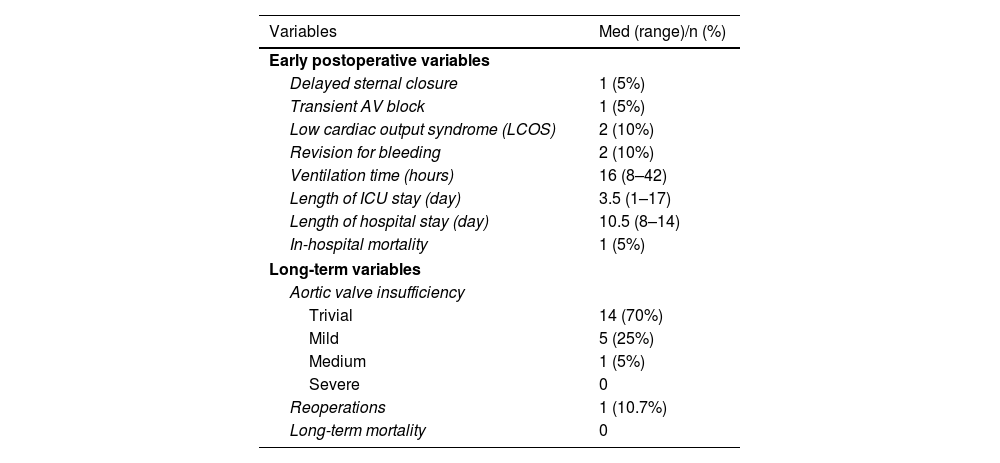
The mean cardiopulmonary bypass time was 184.68±60.1 min and the mean cross-clamp time was 149±67.8 minutes. A Konno incision was performed on four patients due to subvalvular stenosis. An ascending aortic replacement was performed on one patient. A re-exploration procedure was performed on two patients in the postoperative phase due to hemorrhage, whereas a temporary complete atrioventricular block was fully developed in one patient (Table 2). High-dose inotrope was required for three patients due to low cardiac output, and peritoneal dialysis therapy was performed on two patients. One patient (5%), a 23-day-old neonate, died due to low cardiac output during the early postoperative stage. The mean intubation time was 16 hours (8–42 hours), the average intensive care unit time was 3.5 days (1–17 days), and the mean length of hospital stay was 10.5 days (8–14 days).
Operative variables.
| Variables | n: 20Med (range)/n (%)/Mean±St.dev |
|---|---|
| Concomitant procedures | |
| Konno procedure | 4 (20%) |
| Ascending aorta procedure | 1 (5%) |
| Pulmonary RV-PA conduit | |
| Size, mm | 20 (12–25) |
| Freestyle | 12 (60%) |
| Contegra | 6 (30%) |
| Hancock | 1 (5%) |
| Cardiopulmonary bypass time (min) | 184.68±60.1 |
| Cross-clamp time (min) | 149±67.8 |
The mean follow-up time was 60.15±24.45 months. Mortality was not observed in the long term. One patient underwent reoperation due to stenosis of the Contegra conduit. Another patient was monitored for mild–moderate stenosis in the Hancock conduit. One patient has moderate aortic regurgitation, two patients have mild or moderate aortic regurgitation, and the remaining 16 patients have minimal or trivial aortic regurgitation. None of the patients required left ventricular outflow tract intervention (Table 3).
Postoperative early and long-term variables.
| Variables | Med (range)/n (%) |
|---|---|
| Early postoperative variables | |
| Delayed sternal closure | 1 (5%) |
| Transient AV block | 1 (5%) |
| Low cardiac output syndrome (LCOS) | 2 (10%) |
| Revision for bleeding | 2 (10%) |
| Ventilation time (hours) | 16 (8–42) |
| Length of ICU stay (day) | 3.5 (1–17) |
| Length of hospital stay (day) | 10.5 (8–14) |
| In-hospital mortality | 1 (5%) |
| Long-term variables | |
| Aortic valve insufficiency | |
| Trivial | 14 (70%) |
| Mild | 5 (25%) |
| Medium | 1 (5%) |
| Severe | 0 |
| Reoperations | 1 (10.7%) |
| Long-term mortality | 0 |
Surgical treatments for aortic valve diseases in pediatric patients are still under development. The results of surgical procedures performed in patients in this age group are less satisfactory than they are in adult patients. Alternative techniques are used for pediatric patients due to the technical difficulty of aortic valve repair and its poor long-term durability.
The durability of complex valve repair procedures is not properly defined and the risk of requiring reoperation remains high, even in leading centers. The Ozaki procedure and its modifications are now being used in pediatric patients, but there is insufficient long-term clinical follow-up data to determine the valve failure rate and reoperation risk.7
The Ross procedure has proven a successful method of treating pediatric patients. The long-term outcomes of this procedure are well known for adult patients.8 One of the advantages of the Ross procedure is that it offers a functional, hemodynamically ideal valve with growth potential. The surgical indications concerning the Ross procedure are now gradually being expanded to include patients in younger age groups. One of its major advantages is its low mortality rate, which ranges from 1% to 6% in serious cases.
There is no age limitation for the Ross procedure in the pediatric population. The Ross operation is increasingly being performed, especially in adolescent women who are expecting pregnancy. In a recent study published by Donald et al., the Ross procedure was applied to 140 patients over 23 years, and mortality was observed in only seven patients. Furthermore, all of the patients were under one year of age (four neonates and three infants).9 The major factors affecting mortality in patients who undergo the Ross procedure are young age, low weight, preoperative endocarditis, long cardiopulmonary bypass and cross-clamp times, and adverse preoperative status.10
As cardiac valves continue to mature after birth, we believe the Ross procedure will adapt to hemodynamic changes in systemic circulation during infancy.11 However, the current data collected from a meta-analysis dated 2022 showed that Ross and Ross–Konno procedures still pose a serious risk to neonatal patients in terms of early/late mortality and autograft reintervention. The high variability of results from different centers confirms the need for surgical expertise and patient selection.12 A Ross operation was performed on two neonatal patients at our center. Mortality was observed in one patient weighing 3.1 kg following an unsuccessful balloon valvuloplasty performed on the third day of life due to severe aortic stenosis after birth. This patient died due to low cardiac output syndrome, which developed during the postoperative phase.
The Ross–Konno procedure is another reliable option for patients with aortic and subaortic stenosis. It increases therapeutic options for neonates and infants diagnosed with critical aortic valve stenosis who have unsuccessful results following balloon valvuloplasty. Studies have demonstrated that the decrease in the left ventricular outflow tract gradient (LVOT) is significant after surgery. A sustainable decrease was observed in the LVOT gradient in these patients during long-term follow-up.6,13 In our center, Ross–Konno surgeries were performed on four patients with subaortic stenosis, and the patients had no further issue.
The transformation of single-valve disease into dual-valve disease and the need for reoperation in the long term are two of the major disadvantages of the Ross procedure. Aortic regurgitation caused by autograft dilatation and, to a lesser extent, valvular and subvalvular stenosis are the most common pulmonary autograft-related reoperations. Whether the postoperative increase in aortic annulus root size is caused by somatic growth or pathological reformation with unbalanced dilatation is still being debated. Advancing age is the most critical risk factor for autograft dilatation surgery: Matsuzaki et al. reported better long-term results when the Ross procedure was performed in children under 8.6 years.14
Dilated autografts are also a risk factor. Aortic root stabilization is performed in most adult patients to avoid autograft dilatation, but preventing this in growing children can be problematic. Tan Tanny et al. used absorbable poly-(p-dioxane) filaments to stabilize autograft and sinotubular junctions in pediatric patients and found that it reduced autograft dilation.15 No further procedures were performed for root stabilization purposes in our study and no reoperation was required for autograft. No serious aortic regurgitation or autograft dilatation requiring intervention was observed during follow-up. Five patients with mild aortic regurgitation and one patient with moderate aortic regurgitation continue to be monitored without issue.
Other reasons for reoperation include interventions to implant homografts or conduits used to repair the right ventricular outflow tract. Studies included in a systematic review reported a rate of 0.34–4.76% of patients per year undergoing reoperation due to right ventricular outflow tract stenosis or failure. In this study, a single-variable analysis indicated that the risk factors for needing any reoperation included young age, low surgical weight, and smaller diameters of implanted conduits. Although the entire mechanism underpinning this correlation has yet to be revealed, differences in calcium metabolism, immunity activity, somatic growth, and age groups are considered to play key roles in hemodynamics.16
Implanting a conduit with good durability in the right ventricular outflow tract reduces the burden of reinterventions after a Ross procedure. Homografts and bovine jugular vein grafts are frequently used conduits.17 Homografts are considered the gold standard for right ventricular outflow tract reconstruction, but their availability is limited, especially those of smaller sizes. Bovine jugular vein grafts, on the other hand, are a readily available and widely used alternative, although their high cost has induced the search for alternatives in some Asian countries, especially Japan. Miyazaki et al. used a 0.1 mm thick expanded polytetrafluoroethylene membrane to create a brand-new, handmade, three-valve conduit with a fan-shaped structure that can be constructed in the operating room.18 A recently published study compared handmade polytetrafluoroethylene grafts to bovine jugular vein grafts and found no significant differences between them in terms of early mortality, cross-clamp time, length of stay, right ventricular outflow tract graft thrombosis, or endocarditis.19
Although it has yet to be confirmed whether stent-free xenografts such as Freestyle SX demonstrate similar hemodynamic performance and long-term stability, the use of such xenografts is increasing gradually. The Freestyle SX has been used to treat right ventricular outflow tract lesions in congenital heart diseases for a long time now with positive outcomes, as evidenced by a 100% exemption from pulmonary valve replacement at 8 years in a group of patients with a wide range of illnesses.20 A recently published meta-analysis examined 156 patients across six studies and found no significant differences, particularly in the early period. In conclusion, it is agreed that the wide availability and customized valve size option would be beneficial.21
We did not use pulmonary homografts due to accessibility and cost issues. We used Freestyle SX grafts in 12 patients, Contegra grafts in seven patients, and a Hancock graft in one patient. We performed a reoperation on one patient with Contegra due to serious conduit stenosis. One patient had medium to serious stenosis. The patients in which Freestyle was used had no issue.
The Ross procedure is a definitive solution for congenital aortic stenosis in pediatric patients, and we believe that the required right ventricle to pulmonary artery conduit replacements will involve minimal risk in the upcoming years.
This study had several limitations. First, it is a retrospective, single-center study. The limited number of relevant cases and follow-up times may not be an objective reflection of reintervention rates objectively for left and right ventricular outflow tract surgeries. The age disparities among the patients may also have been a disadvantage for the study.
ConclusionRoss and Ross–Konno surgeries are performed with low mortality rates and positive medium-term morbidity results in aortic pathological cases, especially in mature pediatric patients. In the medium-term follow-up of the cases we reviewed, the rates of reintervention due to autograft and conduit were low and no significant autograft insufficiency developed.
Conflicts of interestThe authors have no conflicts of interest to declare.