Ablation of atypical left atrial flutters (LAF) is very challenging due to the complexity of the underlying atrial substrate and diverse arrhythmia mechanisms. The interpretation of the arrhythmia mechanism is usually difficult, even using advanced three-dimensional (3D) mapping systems. SparkleMap is a novel mapping algorithm that displays each electrogram as a green dot that lights up at the point corresponding to the local activation time, superimposed either on the substrate or the local activation time 3D-maps. It is not affected by the setting of the “window of interest” and there is no need for user post-processing.
We present the case of patient with a persistent atypical LAF in whom we tested the concept of complex arrhythmia interpretation exclusively based on the analysis of the substrate and evaluation of SparkleMap-derived wavefront propagation. We describe the workflow for map collection and the systematic approach for arrhythmia interpretation that resulted in the identification of a dual loop perimitral mechanism with a common slow conducting isthmus inside a scar at the septum/anterior atrial wall. This new method of analysis enabled the use of a specifically targeted and precise approach for ablation, with restoration of sinus rhythm within five seconds of radiofrequency application. After 18 months of follow-up, the patient remains free from recurrences, without anti-arrhythmic medication. This case report exemplifies how helpful new mapping algorithms can be in the interpretation of the arrhythmia mechanism in patients with complex LAF. It also suggests an innovative workflow to integrate the SparkleMap into the mapping approach.
A ablação dos flutters atípicos da aurícula esquerda constitui frequentemente um desafio devido à complexidade do substrato auricular subjacente e à diversidade dos mecanismos arrítmicos implicados. A interpretação do mecanismo da arritmia é usualmente difícil, mesmo utilizando sistemas avançados de mapeamento eletroanatómico tridimensional. O SparkleMap é um novo algoritmo de mapeamento que representa cada eletrograma como um ponto verde que acende no momento correspondente ao momento da ativação local. Pode ser sobreposto ao mapa tridimensional de substrato ou de ativação, não é influenciado pela escolha da janela de interesse do mapa e não requer pós-processamento manual.
Apresentamos o caso de um doente com flutter atípico persistente em quem testámos a interpretação do mecanismo de arritmias complexas com base exclusivamente na análise do substrato e na avaliação da propagação das ondas eléctricas por SparkleMap. Descrevemos o protocolo para a colheita do mapa e para a análise sistemática do mecanismo da arritmia, que resultou na identificação de um mecanismo de dupla-reentrada, com um dos circuitos em rotação perimitral e localizando-se o istmo comum de condução lenta na parede anterior/septo, num canal de miocárdio viável intracicatricial. Este método de análise inovador resultou numa estratégia de ablação dirigida e precisa, que conduziu à reposição de ritmo sinusal após apenas 5 segundos de ablação. Com um seguimento de 18 meses, o doente mantém-se livre de recorrências, sem terapêutica antiarrítmica.
Este caso clínico exemplifica como os novos algoritmos de mapeamento podem ser úteis na interpretação dos mecanismos arrítmicos em doentes com arritmias auriculares complexas e propõe um protocolo inovador para a incorporação do SparkleMap na estratégia de mapeamento.
Ablation of atypical left atrial flutters (LAF) is very challenging due to the complexity of the underlying atrial substrate and diverse arrhythmia mechanisms.1–4 A large proportion of these arrhythmias, which are increasingly frequent in clinical practice, result from iatrogenic atrial fibrosis induced by ablation procedures performed for the treatment of atrial fibrillation.3–5
The difficulties of using activation algorithms with electroanatomic mapping systems in the diagnosis of LAF are widely recognized. They result from several factors, including inappropriate setting of the window of interest in relation to a reference time point and mis-annotation of signals within that window.5–8 SparkleMap is a novel algorithm incorporated into the Ensite Precision™ (St Jude Medical-Abbott) electroanatomic mapping system that displays each electrogram at the surface of the cardiac chamber as a green dot that lights up at the point corresponding to the local activation time. The spatial progression of the green dots lighting up produces the effect of a wavefront that enables the operator to evaluate the arrhythmia mechanism in a way not affected by the setting of the “window of interest” and without need of user post-processing.
Since the SparkleMap may be superimposed on the substrate voltage map, it is possible to integrate the arrhythmia mechanism into the analysis of the underlying substrate.
Case reportWe describe the case of a 67-year-old man with hypotension who had undergone a kidney transplant in the past. He presented with a very symptomatic and recurrent atrial flutter refractory to anti-arrhythmic drugs. He had previously undergone an empirical cavotricuspid isthmus ablation. The arrhythmia recurred and he was referred for a second ablation procedure. Atrial tachycardia presented a distal-to-proximal sequence of activation at the coronary sinus, with a cycle length of 280–290 ms. Double transseptal puncture was performed and an electroanatomic map (Ensite Presision™) of the left atrium was obtained with a decapolar Advisor™ circular catheter manipulated through a deflectable Agilis™ sheath. The Automap algorithm was used for fully automated point collection and annotation, using a window of interest with the coronary sinus reference at the middle of the tachycardia cycle length. During the collection phase, only the bipolar voltage map information was displayed, and dense point collection was assured, evenly covering the entire left atrial surface with particular emphasis on the scars and their borders. The lower threshold of the bipolar voltage map was set up at 0.1 mV and the upper threshold at 0.3 mV: these values enabled the display of only dense scar areas that do not have electrical activity. In some patients, these thresholds may be further adjusted during the SparkleMap analysis, and reduced to lower values, in order to highlight isthmuses of conducting tissue inside the scar.
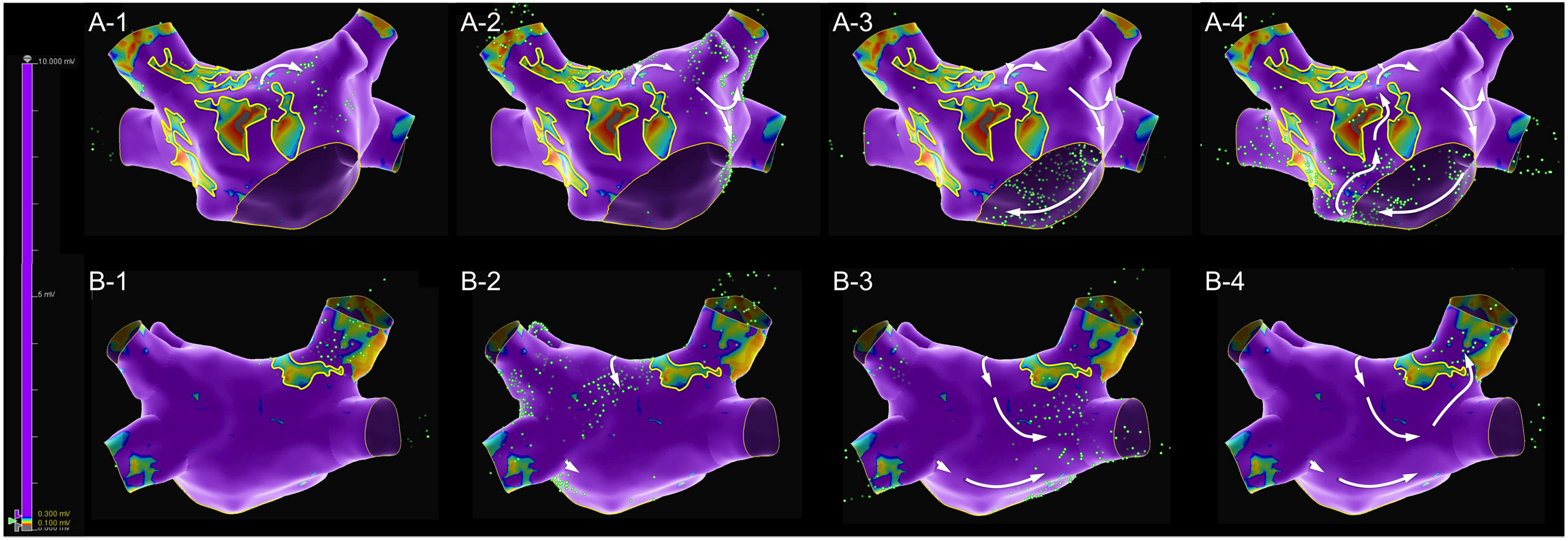
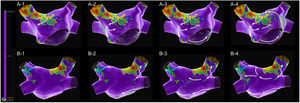
A map with 2473 electroanatomic points (Figure 1 and Supplementary Video 1) was collected in 10 min 38 s. The voltage map was analyzed, and lines were drawn surrounding the low voltage areas. An extensive scar was identified at the anterior wall, extending from the right superior pulmonary vein ostia to the anterior mitral annulus. The scar appeared to be heterogeneous and crossed by a narrow isthmus of electrically viable tissue. This scar also extended through the posterior wall, near to the right superior pulmonary vein. The mechanism of the arrhythmia was then assessed with the SparkleMap algorithm using a systematic approach, analyzing the sequence of wavefront propagation and drawing lines representing each wavefront, starting from the border of the scar. In this case, a complex double-loop macro-reentrant circuit was identified, with a common slow conducting isthmus inside the anterior wall scar, a clockwise perimitral outer-loop and an inner-loop descending the posterior wall and turning around the right veins.
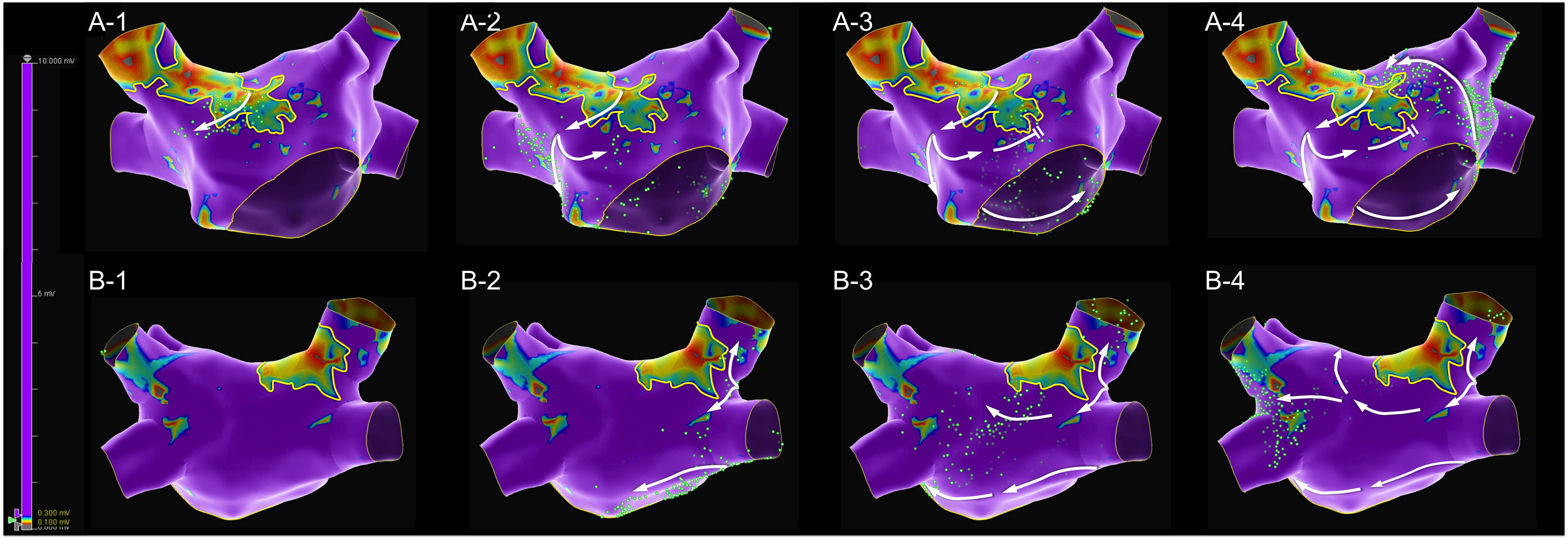
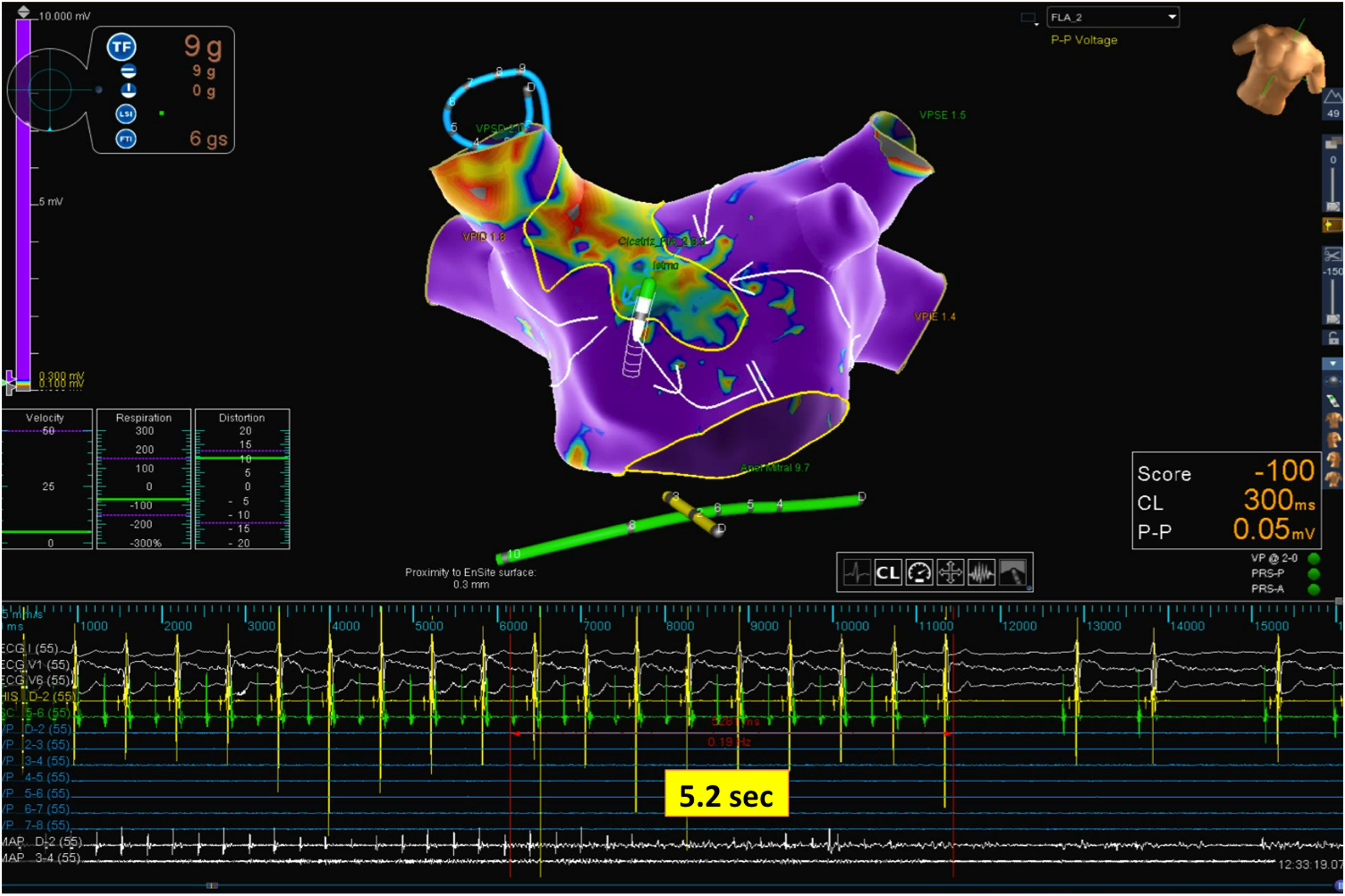
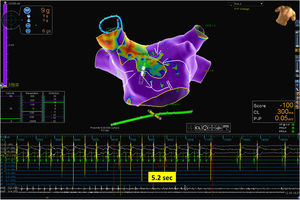
Mapping catheter mechanical contact at the scar caused destabilization of the arrhythmia, with a short period of atrial fibrillation and reorganization in a second flutter with the same cycle length but a proximal-to-distal sequence of activation at the coronary sinus, which was also mapped (Figure 2 and Supplementary Video 2). Map acquisition took 29 min 30 s and 1573 points were collected. The SparkleMap analysis revealed that the circuit of the arrhythmia was the same, but it was running in the opposite direction. Therefore, the isthmus of electrically viable tissue inside anterior wall scar was recognized as being the critical component of both flutters. The comparison of the substrate maps collected during the two flutters revealed minor disagreements in the scar area morphology, probably explained by differences in collection density of the points. Radiofrequency energy was delivered, and the flutter was immediately converted to sinus rhythm (Figure 3). This method of analysis enabled the use of a very targeted and precise approach for termination of the tachycardia. The overall procedure time, including mapping of both flutters, ablation with AT termination and remapping during pacing at the left atrial appendage, to check for bidirectional block, was less than 3 hours.
A diagnosis of arrhythmia in this patient was obtained without any analysis of the classic local activation time (LAT) map or post-pacing intervals to detect concealed entrainment. Supplementary Videos 3 and 4 present the LAT maps of the first and second flutters, both with mapped cycles comprising almost the full tachycardia cycle length, thus consistent with macro re-entrant mechanisms. The appearance of the LAT color-coded map respecting to the second flutter would have been very clear, even with minimal post-processing; only 19 electroanatomic points were manually re-annotated. In contrast, the LAT map of the first flutter could have been misleading, with two different areas of early activation too far apart, one at the border of the anterior wall scar (extending from the right superior pulmonary vein to the anterior mitral annulus) and the other at the posterior aspect of the right superior pulmonary vein. This appearance is justified by the very delayed activation of the right superior pulmonary vein, which in the color codification was classified as an area of early activation.
DiscussionRecent improvements in electroanatomic mapping systems have made enabled the automated collection of very detailed, high-density and accurate maps that provide high quality information on the substrate and on the mechanisms of complex atrial tachycardia. The reason behind the analysis of arrhythmia mechanism on the SparkleMap is to overcome the limitations of the LAT analysis in patients with complex atrial tachycardia.
The appearance of the LAT map is completely dependent on the window of interest empirically chosen and might direct the operator to irrelevant areas.5–9
In addition, the majority of patients with LAF present scar areas which are either idiopathic or resultant from prior ablation procedures. Such fibrotic regions may act not only as localized zones of slow conduction but also as electrical obstacles.1–3 Therefore, these patients may present bystander areas, which may be activated so late that the local activation time occurs during the next tachycardia cycle, and therefore will appear as early activation (color-coded in red).9 This explains why the LAT maps of patients with LAF, even with correct annotation of the electrograms, often present several areas of early activation too far apart. In clinical practice, entrainment maneuvers are often performed as complementary tools to clarify the arrhythmia mechanism,5 but their use may be problematic. LAF circuits often include slow conduction zones with conducting properties critically influenced by the tachycardia cycle and stimulation cycle. Therefore, entrainment maneuvers may modify the circuit causing transformation into a different flutter, atrial fibrillation or terminate the arrhythmia, and therefore be an obstacle to successful ablation.
Since SparkleMap has no interpolation between points and is played in a continuous loop, the appearance of the map is not influenced by the window of interest applied and the areas of ultra-delayed activation would not be a cause of mistaken interpretation. In addition, SparkleMap-based analysis almost avoids the need for manual re-annotation of electrograms. When analyzing the arrhythmia mechanism, the attention of the operator is spatially focused on the electrical wavefront, where green dots are lighting up. Any wrongly annotated electrograms appear as scarce green dots lighting up out-of-phase of their surrounding electroanatomic points. This is intuitively ignored by the operator. By avoiding the need for user post-processing and manual re-annotation, SparkleMap based analysis has the potential to be a faster and more efficient tool for complex atrial tachycardia mapping. SparkleMap appears very different from conventional mapping methods, but the adoption of a systematic approach, starting with the analysis of the underlying substrate and then drawing lines representing the wavefront propagation over the different atrial walls, facilitates not only the recognition of the arrhythmia mechanism, but also the identification of the most appropriate site for ablation.
In conclusion, SparkleMap superimposed on the bipolar voltage map enabled an easy identification of the arrhythmia mechanisms in a patient with complex atrial substrate and two distinctive LAF. The integrated analysis of the arrhythmia mechanism with the evaluation of underlying substrate guided us to the appropriate target for successful tachycardia termination.
Conflicts of interestThe author has no conflicts of interest to declare.