Decompensated heart failure (HF) is associated with poor short- and long-term prognosis. Remote invasive monitoring of pulmonary artery pressures (PAP) enables early detection of HF decompensation before symptoms occur and may improve clinical outcomes. We aimed to describe our initial experience with the use of the CardioMEMS™ remote monitoring system in patients with HF, including its safety and effectiveness.
Methods and resultsFive patients with HF in New York Heart Association class III and at least one hospitalization due to decompensated HF in last 12 months, who underwent invasive remote monitoring of PAP, were included in this prospective registry. The median age was 66.0 years (interquartile range [IQR] 50.5-77.5 years), 80.0% were men and all had HF with reduced ejection fraction. The pulmonary artery (PA) sensor was placed in a left PA branch in all patients and no major procedural complications occurred. In median follow-up of 40 days (IQR 40-61 days), a total of 271 pressure readings were transmitted, patient compliance was 100% and freedom from sensor failure 98.1%. In three patients, PAP remained within the goal during follow-up. Two patients presented an increase in PAP to values above the targets, despite the absence of symptom worsening. These required dietary and diuretic dose adjustment, without the need for outpatient clinic visits, which reduced PAP. No hospitalizations for HF or deaths occurred during follow-up.
ConclusionHemodynamic-guided HF monitoring was safe and effective and may be a useful adjunctive tool to the standard-of-care management in selected HF patients, particularly in the context of the COVID-19 pandemic, where a reduction in the number of health care visits may be desirable.
A insuficiência cardíaca (IC) descompensada está associada a mau prognóstico em curto e longo prazo. A monitoração remota invasiva da pressão na artéria pulmonar (PAP) possibilita a deteção precoce da descompensação da IC, previamente ao início dos sintomas, pode melhorar os resultados clínicos. Descrevemos a experiência inicial com o uso do sistema de monitoração remota CardioMEMS™ em doentes com IC, inclusive a sua segurança e eficácia.
Métodos e resultadosForam incluídos prospetivamente cinco doentes com IC em classe III da New York Heart Association, com pelo menos uma hospitalização por IC descompensada nos últimos 12 meses, submetidos a implantação de sistema de monitoração remota invasiva da PAP. A mediana de idade foi de 66 anos (intervalo interquartil [IIQ] 50,5-77,5 anos), 80% eram do sexo masculino e todos apresentavam IC com fração de ejeção reduzida. O sensor de PAP foi colocado num ramo da artéria pulmonar esquerda em todos os doentes, não ocorreram complicações major. Durante o follow-up mediano de 40 dias (IIQ 40-61 dias), foram transmitidas 271 leituras, verificou-se uma adesão dos doentes de 100% e taxa de transmissão bem-sucedida de 98,1%. A PAP manteve-se dentro do objetivo em três doentes durante o follow-up. Apesar de continuarem assintomáticos, dois doentes apresentaram valores das PAP acima do alvo. Foi feito ajuste dietético e da dose de diurético, sem necessidade de visitas clínicas presenciais, objetivou-se uma redução efetiva das PAP. Durante o seguimento, não se registaram hospitalizações por IC ou óbitos.
ConclusãoA monitoração da IC guiada pela hemodinâmica demonstrou ser segura e eficaz, pode assumir-se como uma ferramenta útil no manejo de doentes com IC, particularmente no contexto da pandemia Covid-19, quando é desejável uma redução do número de consultas presenciais hospitalares.
Heart failure (HF) is a major cause of morbidity and mortality in developed societies and carries a substantial economic burden.1 Despite increasingly effective medical and device therapies, recurrent hospital admissions remain frequent. They are predominantly driven by symptoms related to worsening congestion due to a progressive rise in cardiac filling pressures.2,3 Importantly, each HF-related hospitalization (HFH) is associated with an increase in short-term risk of mortality after discharge.4
In recent decades, different strategies have been analyzed to monitor congestion. Regular physician visits focused on clinical signs and symptoms, blood tests, including natriuretic peptides, and imaging modalities are useful, although not sufficient for optimal control of the risk of HFH.5 Moreover, lockdown periods decreed in Portugal due to the COVID-19 pandemic has forcibly decreased medical contact, leading to late recognition and treatment of episodes of decompensation and missed opportunities for optimization of pharmacological and nonpharmacological therapy. In addition, changes in lifestyle such as diet and decreased physical activity may trigger HF decompensations.6,7 Thus, non-invasive remote monitoring has emerged as a viable option, with continuous supervision of clinical status in between office visits. However, randomized trials on different remote monitoring strategies have not shown a consistent incremental benefit over routine clinic-based care.8–10
Previous research has shown that increases in intracardiac and pulmonary artery pressure (PAP) are the cause of the congestion and begin a few days to weeks before onset of overt symptoms and signs of HF.11 High cardiac filling pressures in patients with HF are associated with a higher risk of hospitalization and mortality.12 These findings HS led to the development of implantable hemodynamic monitoring devices, such as those that can monitor right ventricular (RV) pressure and end-diastolic PAP, although the clinical results of such devices have not been encouraging in patients with HF.13
The CardioMEMS™ remote monitoring system (Abbott Scientific, Atlanta, GA, USA) is a novel wireless system designed to measure PAP continuously and transmit data on a daily basis to health care providers, enabling a timely management of HF.14 Of note, PAP measurements obtained via the CardioMEMS™ system correlate with those obtained by a Swan-Ganz catheter.15
The CHAMPION trial demonstrated that hemodynamic-guided pharmacotherapy reduced HFH risk in New York Heart Association (NYHA) III HF outpatients, irrespective of left ventricular ejection fraction (LVEF).16 A 37% reduction in HFH rates and a 26% relative risk reduction in all-cause hospitalization were observed.17,18 Remote monitoring of PAP by targeting lower filling pressures led to an increased use of cardioprotective therapy and more frequent adjustments in medication.19 In addition to a consistent reduction in HFH and better quality of life,17 the monitoring of PAP might be associated with a mortality benefit.20,21 In the MEMS-HF trial, the first European prospective study, PAP-guided therapy proved to be effective and safe, as freedom from device complications and sensor failure after one year of implantation were 98.3% and 99.6%, respectively.22,23 These findings were also reflected in real-world settings.24,25
In 2014, the CardioMEMS™ system was approved for patients with HF with preserved and reduced ejection fraction by the USA Food and Drug Administration.26 In the 2016 European Society of Cardiology guidelines, invasive remote monitoring of PAP received a class IIb recommendation for targeted management and monitoring tool in selected HF patients.2 Out of the setting of clinical trials, information about PAP, in addition to the clinical signs and symptoms, could improve the overall management of HF, particularly in centers where HF units offer multidisciplinary care.27,28 Indeed, COVID-19 pandemic times have emphasized the importance of telemonitoring, avoiding the spread of infections, as it reduces hospital admissions and in-person medical and nursing visits.
To the best of our knowledge, data about PAP-guided therapy with CardioMEMS™ remote monitoring system in Europe are scarce. Hence, we aimed to describe our first experience with the CardioMEMS™ system in HF patients, including its safety and effectiveness, in the context of the COVID-19 pandemic.
MethodsThis study was approved by the Ethics Committee of Centro Hospitalar Universitário de Lisboa Central. The study was conducted according to the Declaration of Helsinki. All participants signed written informed consent.
Participants and eligibility criteriaConsecutive patients with chronic HF undergoing implantation of the CardioMEMS™ remote monitoring system were selected for participation in this prospective registry. Eligibility criteria for implantation of the CardioMEMS™ were: HF, irrespective of LVEF, NHYA class III despite optimal medical therapy according to published guidelines,2 HFH within previous 12 months, and good patient compliance, as perceived by the health care team.
Key exclusion criteria included active infection, history of recurrent pulmonary embolism or deep vein thrombosis, inability to tolerate right heart catheterization (RHC), major cardiovascular events (including myocardial infarction and stroke) within the previous two months, cardiac resynchronization therapy (CRT) implanted within the previous three months, glomerular filtration rate <25 mL/min per 1.73 m2 (measured within two weeks of pressure sensor implant), congenital heart disease or mechanical right heart valve, expected to undergo heart transplantation or require a ventricular assist device within the next six months, known coagulation disorders, and hypersensitivity or allergy to acetylsalicylic acid or clopidogrel.
Description of the systemThe CardioMEMS™ remote monitoring system has been described elsewhere.5,28 Briefly, it consists of an implantable pulmonary artery (PA) sensor, a patient and hospital electronic system, and a digital database. The PA sensor is a small device, with no leads or batteries, designed to be placed permanently in a PA branch. Two nitinol loops at the ends of the capsule serve as anchors in the PA and allow automatic sizing of the device to the PA branch. The patient and hospital electronic system include an electronic unit, antenna, and pillow. The antenna is paddle shaped and pre-assembled inside a pillow to facilitate patient readings (Figure 1). The electronic unit receives the wireless hemodynamic signals from the PA sensor and transmits them automatically to a digital database on a secure website. The PA sensor provides PAP waveform, systolic, diastolic, and mean PAP, and heart rate and daily readings are sent to the healthcare providers. These reading may be accessed at any time via a secure website.
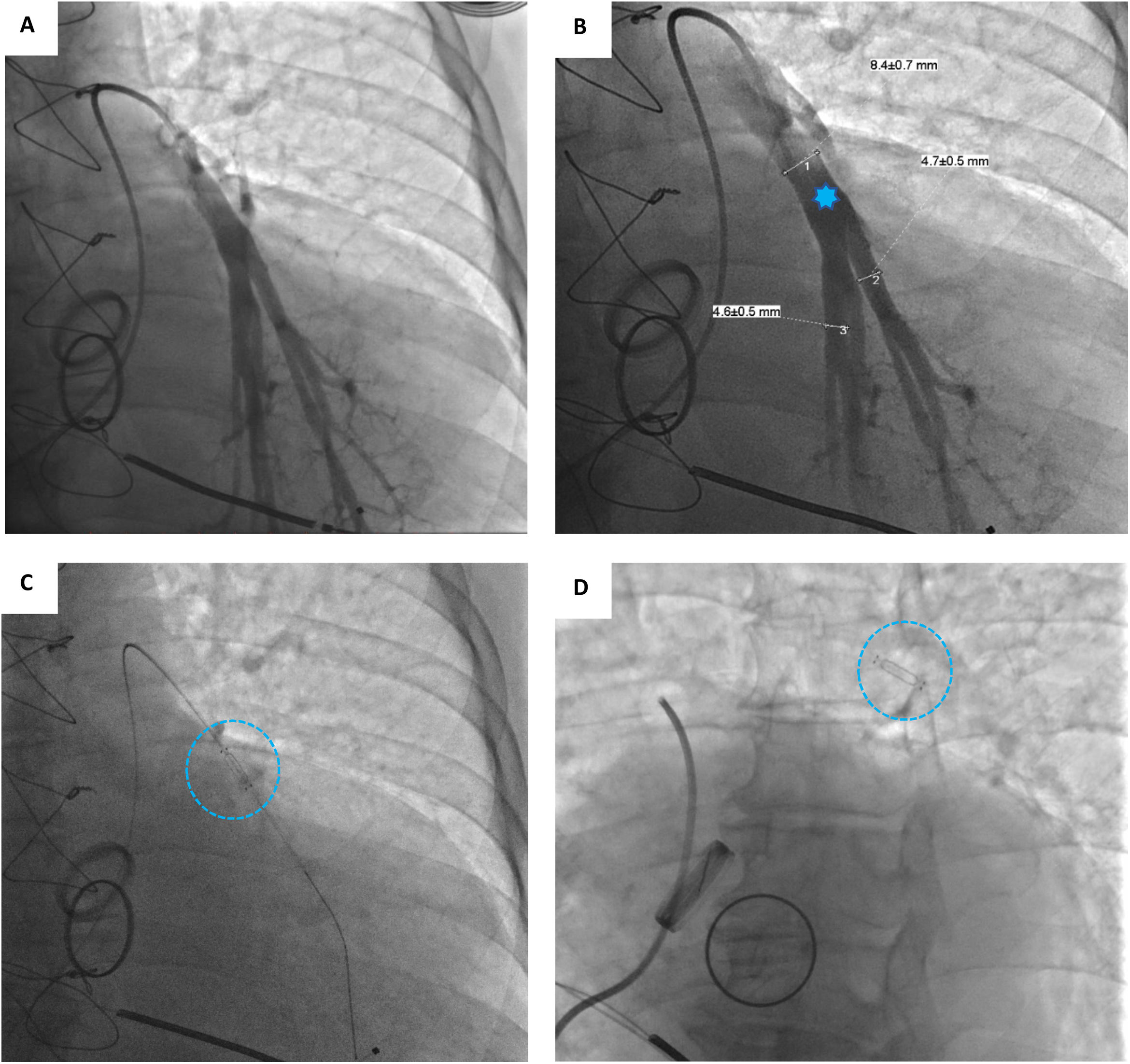
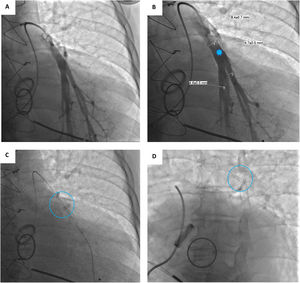
Device implantationPrior to hospital admission, patients were screened for COVID-19 infection. Our methodology for implanting the CardioMEMS™ sensor followed previous reports (Figure 2 and Videos - Supplementary File 1).5 Using sterile technique and local anesthesia, the femoral vein was cannulated with a 7-F sheath and upsized to a 12-F sheath. RHC was performed using a balloon wedge-pressure catheter (BWPC) for pressure assessment and estimation of cardiac output. A limited pulmonary angiogram was performed to identify an appropriate target. Ideally, a left posterior artery was selected, measuring at least 7 mm in diameter. Then, a 0.018-inch stiff guidewire (GW) was placed in the vessel and the BWPC was exchanged for the device delivery system. The CardioMEMS™ sensor, which is tethered to the end of the delivery catheter, was advanced over the GW and released into the target vessel. After removing the delivery catheter, PAP was measured again for device calibration. Hemostasis was achieved using the Perclose ProGlide™ vascular closure device (Abbott Scientific, IL, USA).
A Selective pulmonary angiogram is required to identify an appropriate-sized pulmonary artery branch. B Measurement of the target vessel (asterisk) at the desired implant location (over 7 mm of diameter). C Sensor on the delivery catheter prior to release (dotted circle). D Sensor (dotted circle) released in the desired location.
Following the HF sensor implant procedure, subjects were hospitalized overnight for observation. Patients on anticoagulants are able to restart anticoagulation after implantation. Patients who were not on anticoagulation were instructed to take 100 mg of acetylsalicylic acid and 75 mg of clopidogrel daily for one month after sensor implantation, followed by single antiplatelet therapy. Before hospital discharge, subjects were trained on how to operate the home electronic monitoring unit and instructed to take PAP measurements daily (Figure 3).
Within the first week of implantation, the goal of the caregiver is to assess PAP trends, address suspicious readings, and develop a specific PAP goal for the patient. We based our goals on diastolic PAP and set prespecified notifications according to the PAP target interval, sudden variation of PAP, heart rate deviations, and absent PAP data (e.g., due to patient non-compliance). Treatment changes were not based on an absolute value but rather on a trend in consecutive readings.
Statistical analysisContinuous variables were expressed as median (interquartile range [IQR]) and categorical data as frequency (percentage). Data was analyzed using SPSS, version 25 (IBM).
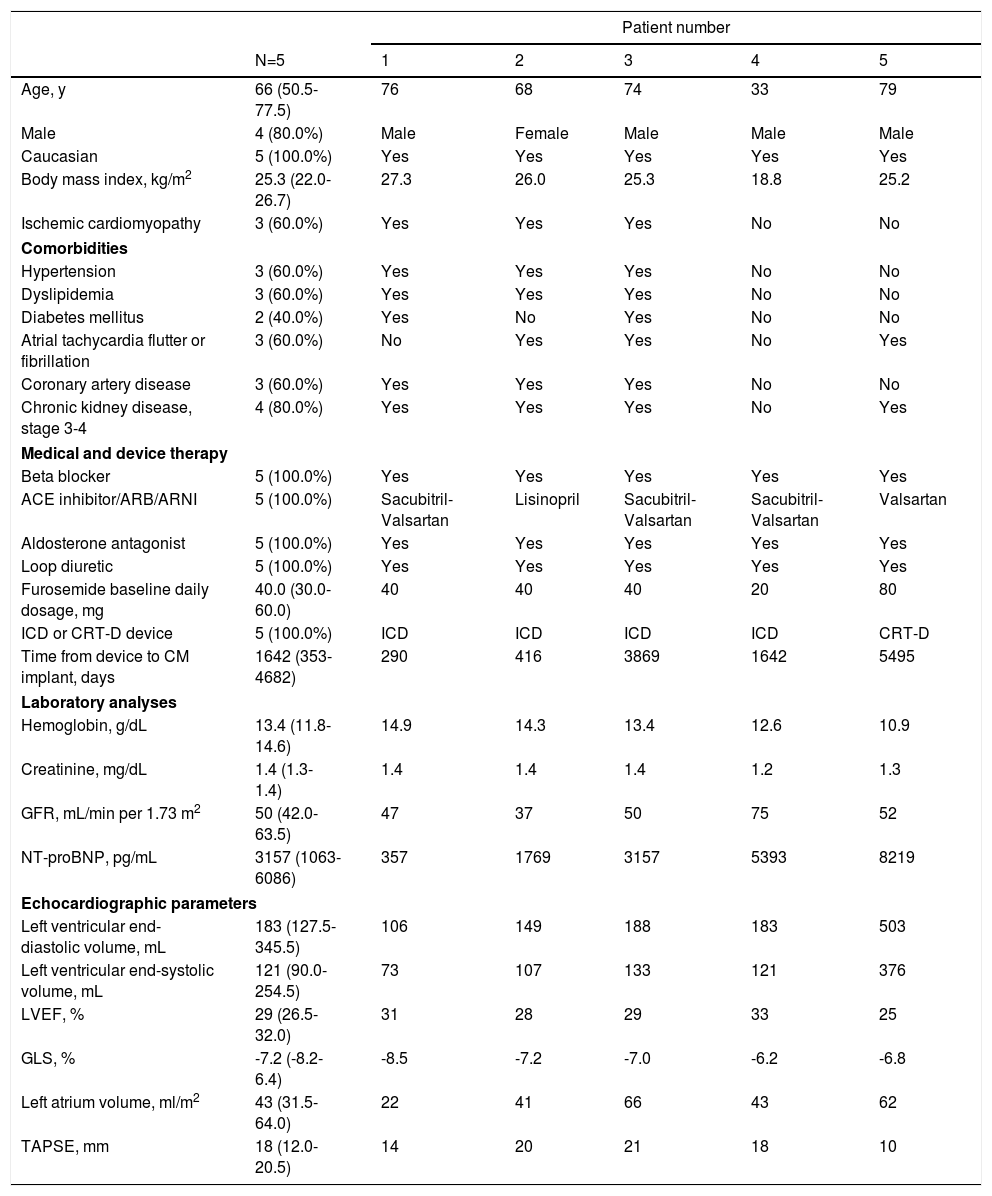
ResultsBaseline characteristicsA total of five consecutive HF patients were included, all of whom presented HF with reduced ejection fraction (Table 1). The median age was 66.0 years (50.5-77.5 years), 80.0% were male and all were Caucasian. The most common HF etiology was ischemic cardiomyopathy, found in three patients with prior myocardial infarction. One patient presented genetic cardiomyopathy (PNPLA2 mutation, Patient 4) and another (Patient 5) had valvular heart disease with prior mitral and aortic mechanical valve replacement, due to rheumatic heart disease.
Baseline characteristics of each patient.
| Patient number | ||||||
|---|---|---|---|---|---|---|
| N=5 | 1 | 2 | 3 | 4 | 5 | |
| Age, y | 66 (50.5-77.5) | 76 | 68 | 74 | 33 | 79 |
| Male | 4 (80.0%) | Male | Female | Male | Male | Male |
| Caucasian | 5 (100.0%) | Yes | Yes | Yes | Yes | Yes |
| Body mass index, kg/m2 | 25.3 (22.0-26.7) | 27.3 | 26.0 | 25.3 | 18.8 | 25.2 |
| Ischemic cardiomyopathy | 3 (60.0%) | Yes | Yes | Yes | No | No |
| Comorbidities | ||||||
| Hypertension | 3 (60.0%) | Yes | Yes | Yes | No | No |
| Dyslipidemia | 3 (60.0%) | Yes | Yes | Yes | No | No |
| Diabetes mellitus | 2 (40.0%) | Yes | No | Yes | No | No |
| Atrial tachycardia flutter or fibrillation | 3 (60.0%) | No | Yes | Yes | No | Yes |
| Coronary artery disease | 3 (60.0%) | Yes | Yes | Yes | No | No |
| Chronic kidney disease, stage 3-4 | 4 (80.0%) | Yes | Yes | Yes | No | Yes |
| Medical and device therapy | ||||||
| Beta blocker | 5 (100.0%) | Yes | Yes | Yes | Yes | Yes |
| ACE inhibitor/ARB/ARNI | 5 (100.0%) | Sacubitril-Valsartan | Lisinopril | Sacubitril-Valsartan | Sacubitril-Valsartan | Valsartan |
| Aldosterone antagonist | 5 (100.0%) | Yes | Yes | Yes | Yes | Yes |
| Loop diuretic | 5 (100.0%) | Yes | Yes | Yes | Yes | Yes |
| Furosemide baseline daily dosage, mg | 40.0 (30.0-60.0) | 40 | 40 | 40 | 20 | 80 |
| ICD or CRT-D device | 5 (100.0%) | ICD | ICD | ICD | ICD | CRT-D |
| Time from device to CM implant, days | 1642 (353-4682) | 290 | 416 | 3869 | 1642 | 5495 |
| Laboratory analyses | ||||||
| Hemoglobin, g/dL | 13.4 (11.8-14.6) | 14.9 | 14.3 | 13.4 | 12.6 | 10.9 |
| Creatinine, mg/dL | 1.4 (1.3-1.4) | 1.4 | 1.4 | 1.4 | 1.2 | 1.3 |
| GFR, mL/min per 1.73 m2 | 50 (42.0-63.5) | 47 | 37 | 50 | 75 | 52 |
| NT-proBNP, pg/mL | 3157 (1063-6086) | 357 | 1769 | 3157 | 5393 | 8219 |
| Echocardiographic parameters | ||||||
| Left ventricular end-diastolic volume, mL | 183 (127.5-345.5) | 106 | 149 | 188 | 183 | 503 |
| Left ventricular end-systolic volume, mL | 121 (90.0-254.5) | 73 | 107 | 133 | 121 | 376 |
| LVEF, % | 29 (26.5-32.0) | 31 | 28 | 29 | 33 | 25 |
| GLS, % | -7.2 (-8.2-6.4) | -8.5 | -7.2 | -7.0 | -6.2 | -6.8 |
| Left atrium volume, ml/m2 | 43 (31.5-64.0) | 22 | 41 | 66 | 43 | 62 |
| TAPSE, mm | 18 (12.0-20.5) | 14 | 20 | 21 | 18 | 10 |
ACE: angiotensin-converting enzyme; ARA: angiotensin receptor blocker; ARNI: angiotensin receptor/neprilysin inhibitor; ICD: implantable cardioverter defibrillator; CRT (cardiac resynchronization therapy); CM: CardioMEMS; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; TAPSE: tricuspid annular plane systolic excursion.
Data expressed as median (interquartile range).
The median LVEF and global longitudinal strain (GLS) were 29.0% (26.5-32.0%) and -7.2% (-8.2-6.4%), respectively, and all were receiving a beta blocker, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker or angiotensin receptor/neurolysin inhibitors, and an aldosterone antagonist. Two patients were on the hospital program for the ambulatory six hour infusion of levosimendan.
An implantable cardioverter defibrillator (ICD) was previously implanted in all patients, and one had previously received an upgrade to a cardiac resynchronization therapy device system. The median time from ICD to CardioMEMS™ system implantation was 1642 (353-4682) days. Sustained ventricular arrhythmias were documented in two patients in the previous 12 months. All patients had HFH in the previous 12 months and one required three hospitalizations.
Implantation, effectiveness and safety endpointsThe PA sensor was placed in a left PA branch in all patients. There were no major procedural complications, including PA injury, hemoptysis, pulmonary embolism, device thrombosis, access site-related bleeding, or infection. There was a mild backward migration of one device during removal of the delivery catheter and 0.018-inch GW, which required repositioning with the BWPC. There were no pressure sensor failures or device malfunction.
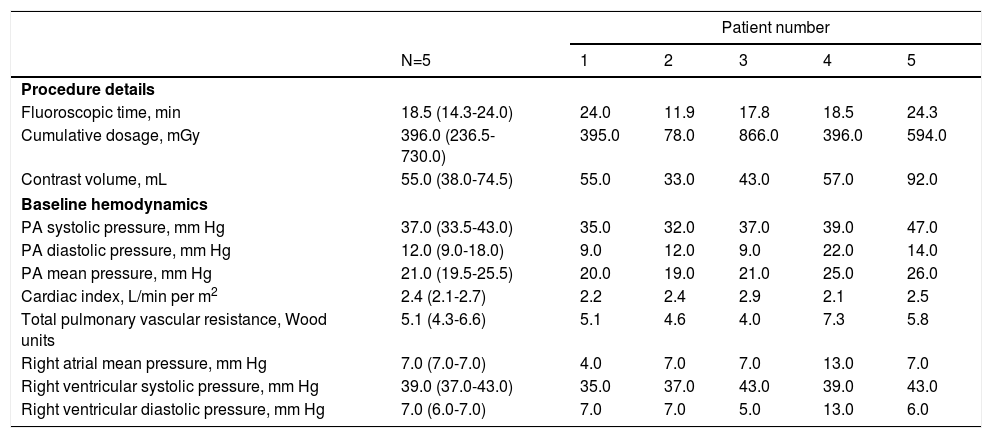
The median fluoroscopic time was 18.5 minutes and the median contrast volume used was 55 mL. Hemodynamic data in the cardiac catheterization laboratory at the time of sensor implant are presented in Table 2.
Procedure details and baseline hemodynamic data at sensor implant.
| Patient number | ||||||
|---|---|---|---|---|---|---|
| N=5 | 1 | 2 | 3 | 4 | 5 | |
| Procedure details | ||||||
| Fluoroscopic time, min | 18.5 (14.3-24.0) | 24.0 | 11.9 | 17.8 | 18.5 | 24.3 |
| Cumulative dosage, mGy | 396.0 (236.5-730.0) | 395.0 | 78.0 | 866.0 | 396.0 | 594.0 |
| Contrast volume, mL | 55.0 (38.0-74.5) | 55.0 | 33.0 | 43.0 | 57.0 | 92.0 |
| Baseline hemodynamics | ||||||
| PA systolic pressure, mm Hg | 37.0 (33.5-43.0) | 35.0 | 32.0 | 37.0 | 39.0 | 47.0 |
| PA diastolic pressure, mm Hg | 12.0 (9.0-18.0) | 9.0 | 12.0 | 9.0 | 22.0 | 14.0 |
| PA mean pressure, mm Hg | 21.0 (19.5-25.5) | 20.0 | 19.0 | 21.0 | 25.0 | 26.0 |
| Cardiac index, L/min per m2 | 2.4 (2.1-2.7) | 2.2 | 2.4 | 2.9 | 2.1 | 2.5 |
| Total pulmonary vascular resistance, Wood units | 5.1 (4.3-6.6) | 5.1 | 4.6 | 4.0 | 7.3 | 5.8 |
| Right atrial mean pressure, mm Hg | 7.0 (7.0-7.0) | 4.0 | 7.0 | 7.0 | 13.0 | 7.0 |
| Right ventricular systolic pressure, mm Hg | 39.0 (37.0-43.0) | 35.0 | 37.0 | 43.0 | 39.0 | 43.0 |
| Right ventricular diastolic pressure, mm Hg | 7.0 (6.0-7.0) | 7.0 | 7.0 | 5.0 | 13.0 | 6.0 |
Data expressed as median (IQR). PA: pulmonary artery.
The mean follow-up time was 47.6 days (median 40, 40-61). A total of 271 pressure transmissions were collected during the study period with a reading compliance of 100%. Patient 2 experienced a failure in data transmission due to connection problems with the home electronic monitoring unit, which was solved after resetting the system. Freedom from sensor failure was, therefore, 98.1%.
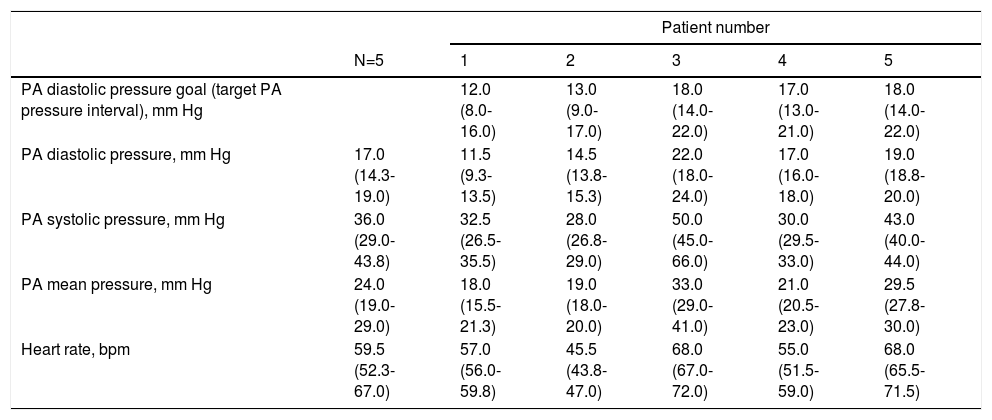
Ambulatory pressure readings during the first week at home were higher than those at the time of sensor implant (Table 3). A diastolic PAP goal was defined for each patient considering baseline values and measurements during the first week.
Ambulatory pulmonary artery pressures during first week after implantation of the remote monitoring system.
| Patient number | ||||||
|---|---|---|---|---|---|---|
| N=5 | 1 | 2 | 3 | 4 | 5 | |
| PA diastolic pressure goal (target PA pressure interval), mm Hg | 12.0 (8.0-16.0) | 13.0 (9.0-17.0) | 18.0 (14.0-22.0) | 17.0 (13.0-21.0) | 18.0 (14.0-22.0) | |
| PA diastolic pressure, mm Hg | 17.0 (14.3-19.0) | 11.5 (9.3-13.5) | 14.5 (13.8-15.3) | 22.0 (18.0-24.0) | 17.0 (16.0-18.0) | 19.0 (18.8-20.0) |
| PA systolic pressure, mm Hg | 36.0 (29.0-43.8) | 32.5 (26.5-35.5) | 28.0 (26.8-29.0) | 50.0 (45.0-66.0) | 30.0 (29.5-33.0) | 43.0 (40.0-44.0) |
| PA mean pressure, mm Hg | 24.0 (19.0-29.0) | 18.0 (15.5-21.3) | 19.0 (18.0-20.0) | 33.0 (29.0-41.0) | 21.0 (20.5-23.0) | 29.5 (27.8-30.0) |
| Heart rate, bpm | 59.5 (52.3-67.0) | 57.0 (56.0-59.8) | 45.5 (43.8-47.0) | 68.0 (67.0-72.0) | 55.0 (51.5-59.0) | 68.0 (65.5-71.5) |
Data expressed as median (interquartile range). PA: pulmonary artery.
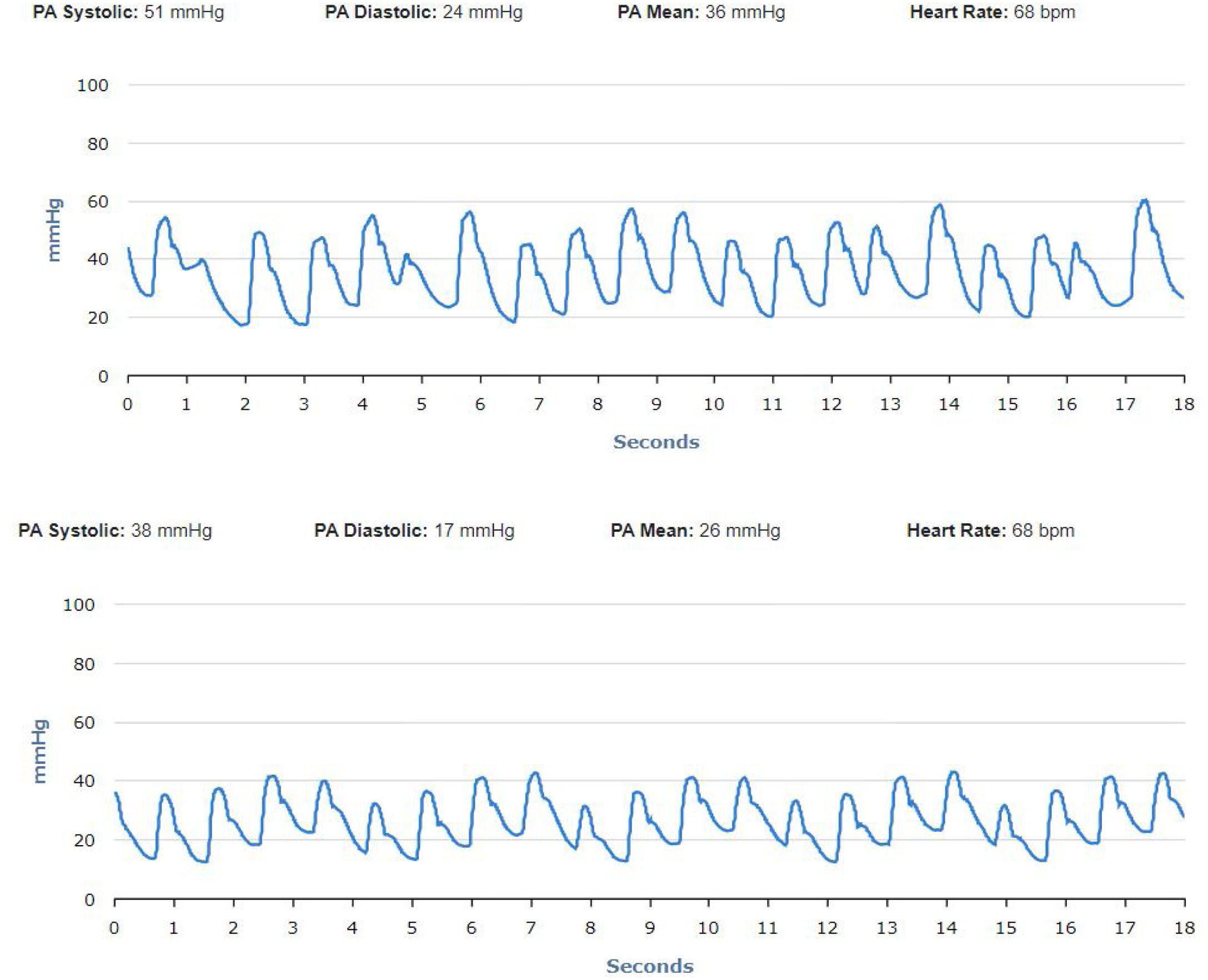
Patient 3 had increased PA diastolic pressure during follow-up and one notification was sent (three consecutive readings above optimal range).
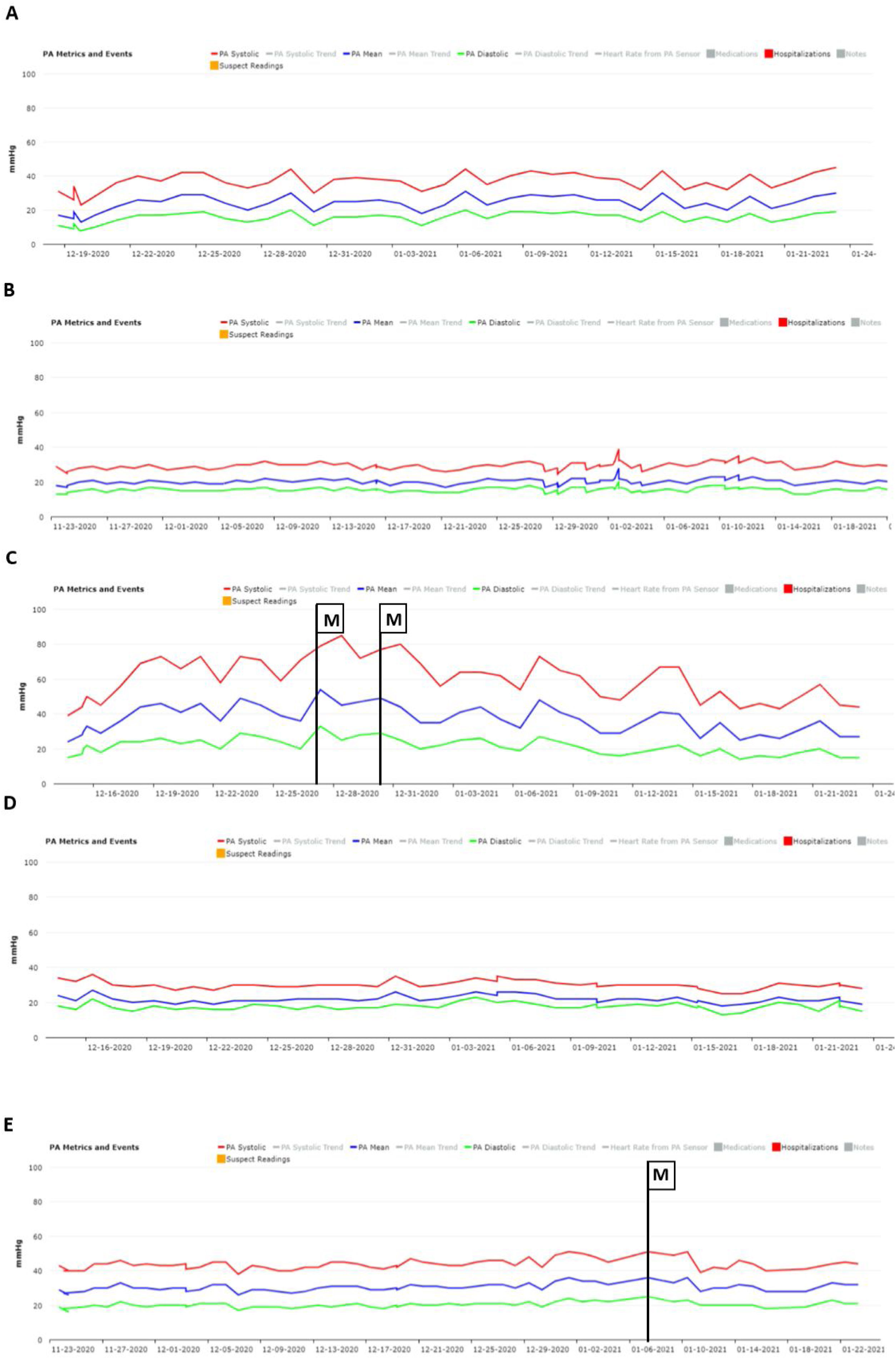
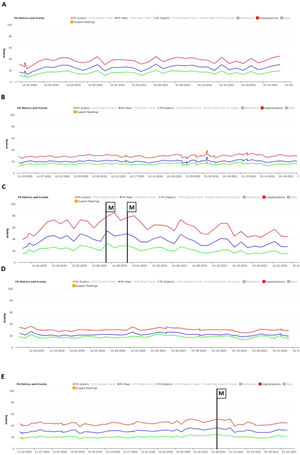
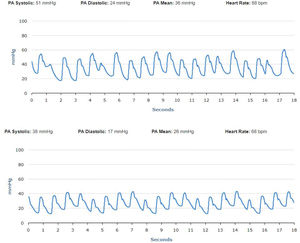
No significant oscillations in PAP parameters occurred during follow-up in patients 1,2, and 4 (4A, 4B and 4D) and diastolic PAP remained within the goal interval. Patient 3 experienced a marked increase in PAP during the Christmas vacation period (Figure 4C). This patient was contacted by the HF team and, although being asymptomatic, he was advised to double the daily furosemide dosage (40 to 80 mg per day). However, PAP did not lower to reach the goal and new therapeutic adjustment was required, including a diuretic dosage (120 mg per day) and more restrictive dietary and water ingestion. Afterwards, an effective reduction in PAP was observed (Figure 5). Patient 5 triggered a single notification (three consecutive readings above the optimal range - Figure 4E). As this patient was also asymptomatic, this triggered dietary counseling and adjustment of medication by the attending physician, leading to PAP normalization. All cases were managed via telephone contact, without the need for outpatient visits. No HFH or deaths occurred during the follow-up period.
We present a case series of five HF patients who received the CardioMEMS™ remote monitoring system. To the best of our knowledge, this is the first experience with this system in Portugal.
Most research has been performed within the USA healthcare system and, recently, a European prospective multicenter trial, which was conducted in 31 centers in Germany, the Netherlands, and Ireland, was published. The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) included 234 NYHA class III patients with at least one HFH in the preceding year, in whom a CardioMEMS™ system was implanted. During follow-up, the median patient adherence to daily PAP transmissions was 87.6% and mean PAP decreased by 5.1±7.4 mmHg. At 12 months after implant, a 62% reduction in HFH (0.60 vs. 1.55 events/patient-year) was observed and patient-reported quality life indices improved.22,23 Moreover, remote PAP monitoring was shown to improve exercise capacity, compared with the standard-of-care HF management.29 Further studies are needed to address this important issue, given that exercise capacity has a positive impact on morbidity and quality of life.
Basic differences in HF disease management strategies between countries might influence the clinical results of hemodynamically guided HF treatment. A low rate of utilization of these systems is still found, regardless of the positive preliminary cost-effectiveness analysis in clinical practice.25 Additional national and international studies/registries are required to determine whether the clinical benefits of CardioMEMS™ are reproducible in a real-world cohort of patients.
Despite the small size of the sample in our single-center study and the short follow-up, our findings have important clinical implications. We describe a successful real-world experience of patient selection, technical system implantation and follow-up in a country with relatively limited resources, and even more constrained during the COVID-19 pandemic. The results were in line with the CHAMPION trial18 and MEMS-HF,22,23 as CardioMEMS™ proved to be a safe and effective solution. In our sample there were no device- or system-related complications.
In our first experience, CardioMEMS™ enabled closer non-invasive hemodynamic monitoring of patients, successfully guiding the extent of therapeutic interventions, while supporting clinical decision-making. The patients reported greater convenience and confidence as they were monitored daily.
The real progress relies on evidence that effective prevention of HF exacerbation is based on aggressive treatment of an early rise in diastolic PAP, regardless of the underlying cause. The timely adjustment of HF treatment enables fast intravascular unloading and more precise neurohormonal drug titration with an expected favorable impact on short- and long-term patient outcomes.24 This new feature has changed the paradigm of outpatient clinic management, as the physician can provide immediate and truly individualized therapy.
Remote PAP monitoring also has the potential to lower overall hospital burden (number of outpatient visits, admissions and resources used) of HF in an attempt to keep the stable patient out of hospital and hospitalize the unstable patient, only if refractory to remote interventions at home. This could be of special interest in the COVID-19 pandemic, as HF patients are a high-risk group with potentially higher mortality if infected. These technologies require dedicated HF units, including professional coaching to supervise and motivate patients to follow therapeutic recommendations and timely PAP reassessment.
Patient education about the risks, benefits and compliance is also critical for informed decision on system implantation, emphasizing not only the need for daily routine monitoring but also the expected reduced number of hospitalizations and improved quality of life. In our center, a multidisciplinary HF unit was previously available, including remote monitoring program with implantable electronic devices.
There are some limitations to unrestricted use of the CardioMEMS™ in patients with HF. Beyond the cost constraints and the need for dedicated multidisciplinary teams, clinical conditions such as severe chronic kidney disease and mechanical right heart valves may hinder the use of this remote monitoring system. Nevertheless, a large number of patients with HF are expected to benefit from the CardioMEMS™, irrespective of LVEF, especially more symptomatic patients. As remote hemodynamic monitoring systems become more accessible and more experience is gained, the role of these new tools in HF care pathways will be unveiled, such as in congenital heart disease and pulmonary artery hypertension.30–32
ConclusionsIn our first experience, PAP-guided management was safe, with no major device or system-related complications, and effective, with a low rate of pressure sensor failure. This hemodynamic-guided remote monitoring enabled early individualized care and may be a useful adjunctive tool to the standard-of-care management in selected HF patients, particularly in the context of COVID-19 pandemics, where a reduction in the number of health care visits is desirable.
Conflicts of interestThe authors have no conflicts of interest to declare.