The development of cardiac fibrosis (CF) and hypertrophy (CH) can lead to heart failure. Mesenchymal stem cells (MSCs) have shown promise in treating cardiac diseases. However, the relationship between MSCs and splicing factor arginine/serine rich-3 (SFRS3) remains unclear. In this study, our objectives are to investigate the effect of MSCs on SFRS3 expression, and their impact on CF and CH. Additionally, we aim to explore the function of the overexpression of SFRS3 in angiotensin II (Ang II)-treated cardiac fibroblasts (CFBs) and cardiac myocytes (CMCs).
MethodsRat cardiac fibroblasts (rCFBs) or rat cardiac myocytes (rCMCs) were co-cultured with rat MSCs (rMSCs). The function of SFRS3 in Ang II-induced rCFBs and rCMCs was studied by overexpressing SFRS3 in these cells, both with and without the presence of rMSCs. We assessed the expression of SFRS3 and evaluated the cell cycle, proliferation and apoptosis of rCFBs and rCMCs. We also measured the levels of interleukin (IL)-β, IL-6 and tumor necrosis factor (TNF)-α and assessed the degree of fibrosis in rCFBs and hypertrophy in rCMCs.
ResultsrMSCs induced SFRS3 expression and promoted cell cycle, proliferation, while reducing apoptosis of Ang II-treated rCFBs and rCMCs. Co-culture of rMSCs with these cells also repressed cytokine production and mitigated the fibrosis of rCFBs, as well as hypertrophy of rCMCs triggered by Ang II. Overexpression of SFRS3 in the rCFBs and rCMCs yielded identical effects to rMSC co-culture.
ConclusionMSCs may alleviate Ang II-induced cardiac fibrosis and cardiomyocyte hypertrophy by increasing SFRS3 expression in vitro.
O desenvolvimento de fibrose cardíaca (FC) e hipertrofia (CH) pode levar à insuficiência cardíaca. As células estaminais mesenquimais (MSCs) têm-se mostrado promissoras no tratamento de doenças cardíacas. No entanto, a relação entre as MSCs e a SFRS3 permanece pouco clara. Neste estudo, os nossos objetivos são investigar o efeito das MSCs na expressão de SFRS3 e o seu impacto na fibrose e hipertrofia cardíacas. Além disso, pretendemos explorar a função do excesso de SFRS3 em fibroblastos cardíacos (CFBs) e miócitos cardíacos (CMCs) tratados com angiotensina II (Ang II).
MétodosOs fibroblastos cardíacos de rato (rCFBs) ou os miócitos cardíacos de rato (rCMCs) foram cocultivados com MSCs de rato (rMSCs). A função do SFRS3 nos rCFBs e rCMCs induzidos pela Ang II foi estudada através da sobre-expressão do SFRS3 nessas células, com e sem a presença de rMSCs. Avaliámos a expressão de SFRS3 e avaliámos o ciclo celular, a proliferação e a apoptose de rCFBs e rCMCs. Também medimos os níveis de interleucina (IL)-β, IL-6 e fator de necrose tumoral (TNF)-α e avaliámos o grau de fibrose nos rCFBs e de hipertrofia nas rCMCs.
ResultadosAs rMSCs induziram a expressão de SFRS3 e promoveram o ciclo celular e a proliferação, reduzindo simultaneamente a apoptose de rCFBs e rCMCs tratados com Ang II. A cocultura de rMSCs com essas células também reprimiu a produção de citocinas e atenuou a fibrose dos rCFBs, bem como a hipertrofia das rCMCs desencadeada pela Ang II. A sobreexpressão de SFRS3 nos rCFBs e nas rCMCs produziu efeitos idênticos aos da cocultura de rMSC.
ConclusõesAs MSCs podem aliviar a fibrose cardíaca induzida pela Ang II e a hipertrofia dos cardiomiócitos através do aumento da expressão de SFRS3 in vitro.
Cardiac fibrosis (CF) and cardiac hypertrophy (CH) are two pathological processes that contribute to multiple cardiovascular diseases (CVDs), posing a threat to public health.1 CF is characterized by the excessive accumulation of extracellular matrix (ECM) by cardiac fibroblasts (CFBs), which produce connective tissues to support normal heart function under physiological conditions.2,3 External stimuli such as pro-inflammatory and pro-fibrotic factors, can induce CFBs to proliferate, migrate, and their conversion into α-smooth muscle actin (α-SMA)-expressing myofibroblasts (MFs). These MFs synthesize a large amount of ECM components, leading to the formation of fibrotic heart tissue and a range of heart diseases.4–6
CH is viewed as an adaptive process in response to stressful conditions, such as mechanical stimuli, to enhance heart performance.7,8 Physiological CH occurs as a response to conditions like persistent exercise and pregnancy and is reversible and mild.9,10 However, in pathological circumstances such as hypertension and valvular deficiency, CH becomes chronic, irreversible, and severe if left untreated, leading to heart failure. Therefore, CH is an important indicator of CVD-related morbidity and mortality.11,12 Due to the significant relevance and essential roles of CF and CH in the development of CVDs, considerable efforts have been dedicated to unraveling the underlying mechanisms, which encompass a diverse collection of signaling pathways, transcription factors, and other mediators.13–21 However, a deeper understanding of the pathology of CF and CH is still needed to facilitate the development of more efficient treatments and effective drugs.
Mesenchymal stem cells (MSCs) have demonstrated enormous potential for the treatment of various types of diseases, including CVDs. For instance, bone marrow-derived MSCs promote the healing and regeneration of infarcted myocardium.22–24 Transplantation of MSCs into infarcted heart tissue can substantially reduce scar tissue formation. However, the precise roles of MSCs in relation to CF and CH remain incompletely understood.
SFRS3, also named SRp20 or SRSF3, is a splicing factor that exerts control over alternative splicing, transcriptional termination, protein translation, and regulation of chromatin structure and function.25,26 It plays crucial roles in embryonic development and tumorigenesis.26–29 Studies have shown that miR-486 targeting SRSF3/p21 mediates the aging process of myocardial fibroblasts by improving fibrotic activity, pathological fibrosis and remodeling of ischemic myocardium, thereby promoting regeneration.30 Furthermore, recent studies have revealed that SFRS3 regulates the function of cardiomyocytes by de-capping mRNAs related to contraction and controls mitochondrial activity,31,32 underscoring its significance as an important regulator of cardiomyocytes, but the role of SFRS3 and/or MSCs in CH remains unclear. Because of the importance of SFRS3 in myocardial regulation, we hypothesize that MSCs may influence the development of CF and CH in vivo by regulating SFRS3.
In the present study, we explored the impact of MSCs on SFRS3 expression and angiotensin II (Ang II) induced CF and CH in vitro. Our results revealed that co-culturing MSCs with cardiac fibroblasts (CFBs) or cardiac myocytes (CMCs) resulted in increased expression of SFRS3 in these cells. Moreover, MSCs promoted cell cycle progression and proliferation, while inhibiting apoptosis in Ang II-treated CFBs and CMCs. Additionally, MSCs reduced cytokine secretion by these cells. Notably, MSCs can also mitigate the Ang II-induced fibrosis of CFBs and hypertrophy of CMCs. Similar effects were observed by overexpression of SFRS3 in Ang II-treated cells. Together these data reveal the novel roles of SFRS3 in the regulation of CFBs and CMCs under Ang II treatment and suggest that MSCs may alleviate Ang II-induced CF and CH by upregulating SFRS3 expression.
ObjectivesIn this study, our objectives were to investigate the effect of MSCs on SFRS3 expression, and their impact on CF and CH.
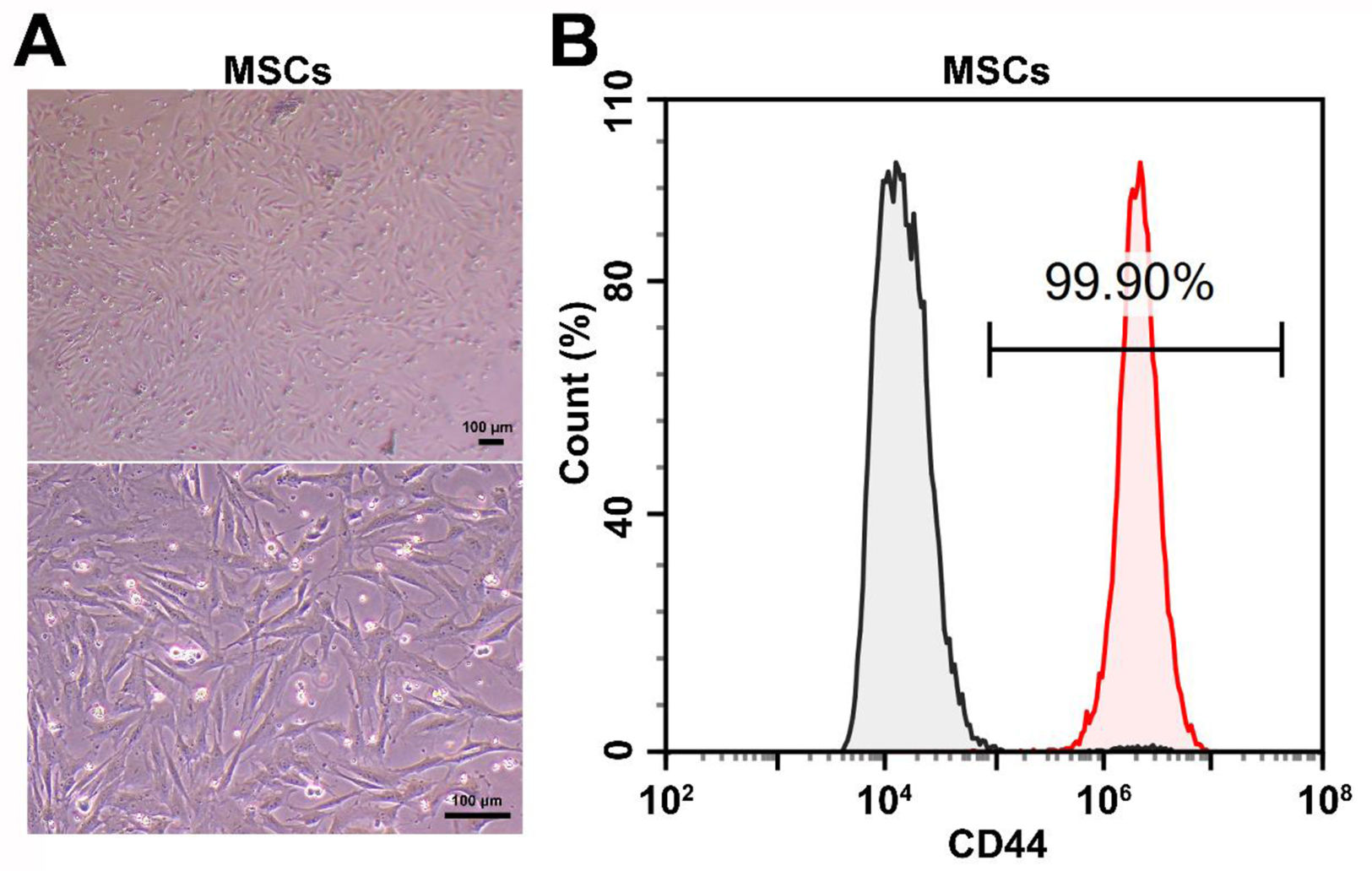
Material and methodsCell cultureAll cell lines and culture media used in this study were purchased from Immocell Biotechnology (Xiamen, China). Rat MSCs (rMSCs, #IMP-R150) were cultured in rat bone marrow MSC complete medium (#IMP-R150-1), rat cardiac myocytes (rCMCs, #IMP-R011) were cultured in rat cardiomyocyte complete medium (#IMP-R011-1), and rat cardiac fibroblasts (rCFBs, #IMP-R012) were cultured in rat myocardial fibroblast complete medium (#IMP-R012-1), all medium which used contained 10% fetal bovine serum (FBS). The cells were maintained in an incubator at 37°C with 5% atmospheric CO2, as well as in a 95% humified environment. The identity of rMSCs was further confirmed by evaluating CD44 expression using a CD44 antibody (ab243894, Abcam, UK). Stained cells were analyzed using a NovoCyte® flow cytometer (Agilent, USA).
Plasmid construction and transfectionThe coding sequence of rat SFRS3 cDNA was cloned into the pLV-CMV-MCS-pGK-puro vector, using the following primers: SFRS3-CDS-Forward: 5′-ATTCACGCGTGCGGCCGCATGCATCGTGATTCCTGTC-3′ and SFRS3-CDS-Reverse: 5′-AGGGATCCGGGCCCGGGCTATTTCCTTTCATTTGACCTAG-3′. For transfection, the control or SFRS3 overexpression plasmids were transfected into rCFBs or rCMCs using Lipofectamine 2000 (Invitrogen, USA) following the manufacturer's instructions.
Cell co-cultureTo assess the impact of rMSCs on rCFBs or rCMCs, rCFBs or rCMCs were seeded at a density of 2×104 cells/cm2 in 6-well plates and cultured with 0.1 μM Ang II (#A9290; Solarbio, China). Simultaneously, rMSCs (4×104 cells/cm2) were seeded onto polyester membrane Transwell-clear inserts (pore size 0.4 μm; Corning, USA), which were placed into each well of the 6-well plates for co-culturing with rCFBs or rCMCs. After 48 hours, both rCFBs and rCMCs, along with their culture medium, were collected for subsequent assays.
Cell cycle assayTo detect the cell cycle, the harvested cells were fixed with 95% ethanol at −20°C for 12 hours. Following fixation, the cells were washed with phosphate-buffered saline (PBS) and subsequently incubated with 20 μg/mL 7-aminoactinomycin D (7-AAD) (Beyotime, China) in the dark at 37°C for 30 min. After staining, the cells were rinsed once again with PBS and then subjected to analysis using a NovoCyte® flow cytometer (Agilent, USA).
Proliferation assayThe upper chambers of Transwell 96-well plates were seeded with 3.13×104 rMSCs/cm2. Control and SFRS3-overexpressing rCFBs or rCMCs were seeded in the lower chambers at a density of 3.13×104 cells/cm2 and cultured with 0.1 μM Ang II. After 48 hours, 10 μL of methyl thiazolyl tetrazolium (MTT) solution was added to each well in the lower chambers, followed by an additional 4-hour incubation. Subsequently, the culture medium was removed, and 150 μL of dimethyl sulfoxide (DMSO) was added to each lower chamber. The optical density (OD) values at 490 nm (OD490) were measured using a spectrophotometer.
Apoptosis assayApoptosis was assessed using the Annexin V-FITC kit (#C1062S, Beyotime, China) following the manufacturer's instructions. Briefly, the cells were digested, resuspended, pelleted, and washed twice with PBS. Subsequently, the cells were incubated with Annexin V-FITC/PI staining solution and binding buffer in the dark for 20 min at room temperature. Finally, the stained cells were analyzed using a NovoCyte® flow cytometer (Agilent, USA).
Quantitative PCR (qPCR)Total RNA from the cells was extracted and purified using an RNA extraction kit (#9767, Takara, Japan). The quality and quantity of the RNA were assessed using spectrometry and electrophoresis. Reverse transcription was performed using a cDNA synthesis kit (#R111-01, Vazyme, China). Quantitative PCR (qPCR) was conducted on a LightCycler® 96 PCR system (Roche, Switzerland) using a qPCR kit (#Q411-02, Vazyme, China), the results were normalized using the internal reference gene β-actin. The expression levels of the target genes were calculated using the 2^(−ΔΔCT) method. The following primers were used: SFRS3-F: 5′-TTATGTAGGTAATCTTGG-3′; SFRS3-R: 5′-ATAGTGTTCTTCCATCTA-3′; IL-1β-F: 5′-CCTGAACTCAACTGTGAA-3′; IL-1β-R: 5′-TGGAAGCAATCCTTAATCT-3′; TNF-α-F: 5′-AACAAGGAGGAGAAGTTC-3′; TNF-α-R: 5′-TTGAGAAGATGATCTGAGT-3′; IL-6-F: 5′-GGAAATGAGAAAAGAGTTGTG-3′; IL-6-R: 5′-AGAAGACCAGAGCAGATT-3′; RNA18S5N-F: 5′-AGGCGCGCAAATTACCCAATCC-3′; RNA18S5N-R: 5′-GCCCTCCAATTGTTCCTCGTTAAG-3′; Galectin-3-F: 5′-GAGTACTAGAAGCGGCCGAG-3′; Galectin-3-R: 5′-CTGTGCCGCTCACCTGATTA-3′; Stanniocalcin-2 (STC2)-F: 5′-GGGAATGCTACCTCAAGCAC-3′; STC2-R: 5′-GGTCCACGTAGGGTTCGT-3′; ANF-F: 5′-GGCTCCTTCTCCTCACCAA-3′; ANF-R: 5′-CTCTGAGACGGGTTGACTTCC-3′; β-MHC-F: 5′-CCGAGTCCCAGGTCAACAA-3′; β-MHC-R: 5′-CTTCACGGGCACCCTTGGA-3′; BNF-F: 5′-CGTCAGTCGTTTGGGCTGTA-3′; BNF-R: 5′-GCAGCCAGGCGGTCTTCCT-3′; β-actin-F: 5′-GGCTGTATTCCCCTCCATCG-3′; β-actin-R: 5′-CCAGTTGGTAACAATGCCATGT-3′.
Western blotTotal protein was extracted from rCMCs or rCFBs by lysing the cells with RIPA lysis buffer (Beyotime, China) containing protease and phosphatase inhibitors. The protein concentration was determined using a BCA kit. Subsequently, the protein samples were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (Bio-Rad, USA). The membranes were then blocked with 5% skimmed milk for one hour at room temperature. Afterward, the membranes were incubated overnight at 4°C with primary antibodies diluted in blocking solution. On the following day, the membranes were washed three times with TBS+0.1% Tween (TBST) and incubated with secondary antibodies diluted in blocking solution for two hours at room temperature. After three additional washes with TBST, the signal was developed using an HRP chemiluminescence kit (Immun-StarTM, Bio-Rad, USA) and detected using an ImageQuant LAS 4000 system (GE Healthcare, USA). Image J software (NIH, USA) was utilized for quantification of band intensity. The following antibodies were used: anti-SFRS3 (#10916-1-AP; Proteintech, China), anti-Fibronectin (#15613-1-AP; Proteintech, China), anti-Collagen I (#14695-1-AP; Proteintech, China), anti-alpha-SMA (#23081-1-AP; Proteintech, China), anti-β-actin (#20536-1-AP; Proteintech, China) and HRP-conjugated goat anti-rabbit IgG (#SA00001-2; Proteintech, China).
Enzyme-linked immunosorbent assay (ELISA)For the evaluation of cytokine expression, an enzyme-linked immunosorbent assay (ELISA) was performed using kits obtained from Elabscience Biotechnology (USA) according to the manufacturer's instructions. The following ELISA kits were utilized: rat IL-1β ELISA kit (#E-EL-R0012c), rat TNF-α ELISA kit (#E-EL-R2856c), and rat IL-6 ELISA kit (#E-EL-R0015c). OD values were detected at the wavelength of 450 nm and the expression levels of cytokines were calculated.
Hematoxylin and eosin stainingThe cells were fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature. Afterward, the cells were stained in hematoxylin dye solution for 5 min, washed with tap water, treated with hematoxylin differentiation solution for 3 s, and fully washed with running water, then, hematoxylin blue return solution was used to return blue for 3 s, as well as fully washed with running water, and dehydrated with 85% and 95% gradient ethanol for 5 min each, and then dyed in eosin dye solution for 5 min, after 2 times of dehydration with anhydrous ethanol for 5 min each, then dehydrated with fresh anhydrous ethanol for 5 min, xylene transparent for 5 min, and finally add neutral gum to seal the tablet. Hematoxylin and eosin (H&E) staining was performed using an H&E staining kit (#G1005, Servicebio, China). A light microscope (BX51, Olympus, Japan) was used to observe and photograph the cells. Image J (National Institutes of Health) was used to count the relative cell areas.
Masson's stainingAfter being fixed with 4% PFA, the cells were stained using a Masson's trichrome kit (#G1006, Servicebio, China) according to the manufacturer's instructions. In brief, the cells were incubated in Masson A solution at 65°C for 30 min, washed with tap water for 30 s, and Masson B solution was mixed with Masson C solution in equal volume, the cells were immersed in the mixed solution for 1 min, then washed with running water, the cells were differentiated in 1% hydrochloric acid ethanol solution (concentrated hydrochloric acid:anhydrous ethanol=1:100) for 1 min, washed under running water, drained excess water, and soaked in Masson D solution for 3 min, rinsed under running water for 20 s, and then soaked in Masson E solution for 1 min, dyed directly in Masson F solution for 30 s, rinsed and differentiated by three consecutive tanks of 1% acetic acid solution for 7 s each time, as well as dehydrated by three consecutive tanks of anhydrous ethanol for 3 s, 5 s and 5 s, then finally transparent by xylene for 5 min, and sealed by neutral gum. The stained samples were observed and photographed using a light microscope (BX51, Olympus, Japan).
Statistical analysisAll statistical analyses were conducted using GraphPad Prism software (version 3.0). Data between two groups were compared using the unpaired Student's t-test, and data normality was confirmed using the Shapiro–Wilk test. Statistical significance was defined as p<0.05. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; ns, no significance. All cell experiments were independently repeated at least three times.
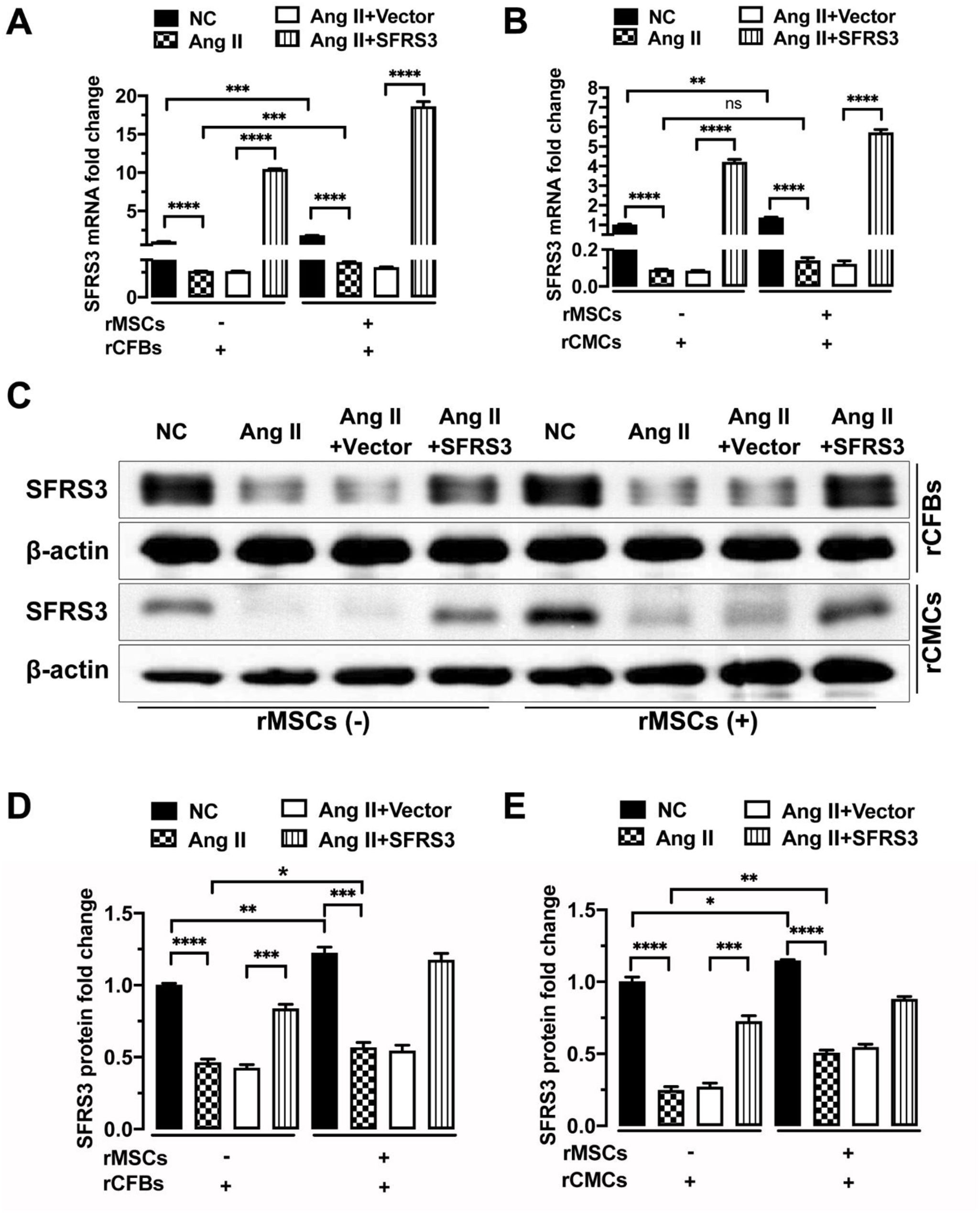
ResultsrMSCs induced the expression of SFRS3 in normal and Ang II-treated rCFBs and rCMCsTo investigate the potential effect of MSCs on SFRS3 expression in CFBs and CMCs, we obtained rMSCs and verified their identity through CD44 staining. Our analysis revealed that 99.9% of the tested cells were CD44+, confirming their MSC nature (Figure 1). Subsequently, we conducted co-cultures of these rMSCs with rCFBs or rCMCs and observed a significant increase in SFRS3 mRNA levels in both cell types (Figure 2A and B). To further explore the expression of SFRS3 during fibrosis of CFBs and hypertrophy of CMCs, we treated rCFBs and rCMCs with Ang II, a commonly utilized inducer of CF and CH.33,34 Remarkably, our findings demonstrated that Ang II treatment considerably reduced SFRS3 mRNA levels in both rCFBs and rCMCs (Figure 2A and B). Consistently, the protein levels of SFRS3 in rCFBs and rCMCs were also upregulated by co-culturing with rMSCs but decreased upon Ang II treatment (Figure 2C–E). Notably, we observed that rMSCs were capable of increasing SFRS3 mRNA and protein levels even in Ang II-treated rCFBs and rCMCs (Figure 2A–E). These results suggest that rMSCs are positive regulators of SFRS3 expression in both rCFBs and rCMCs, regardless of the underlying physiological or pathological conditions.
rMSCs increased SFRS3 expression in normal and Ang II-treated rCFBs and rCMCs. (A) qPCR data showing SFRS3 mRNA levels in rCFBs. (B) qPCR data showing SFRS3 mRNA levels in rCMCs. (C) Western blot data showing SFRS3 protein levels in rCFBs and rCMCs. (D) Quantification of the Western blot data of rCFBs. (E) Quantification of the Western blot data of rCMCs. β-Actin was used to normalize the expression levels of SFRS3. The NC group indicated that the cells did not receive any treatment, the Ang II group indicated that the cells were treated with Ang II, the Ang II+Vector group indicated that the cells were transferred to empty plasmid based on the Ang II treatment and the Ang II+SFRS3 group indicated that the SFRS3 plasmid was transferred after Ang II treatment. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; ns, no significance.
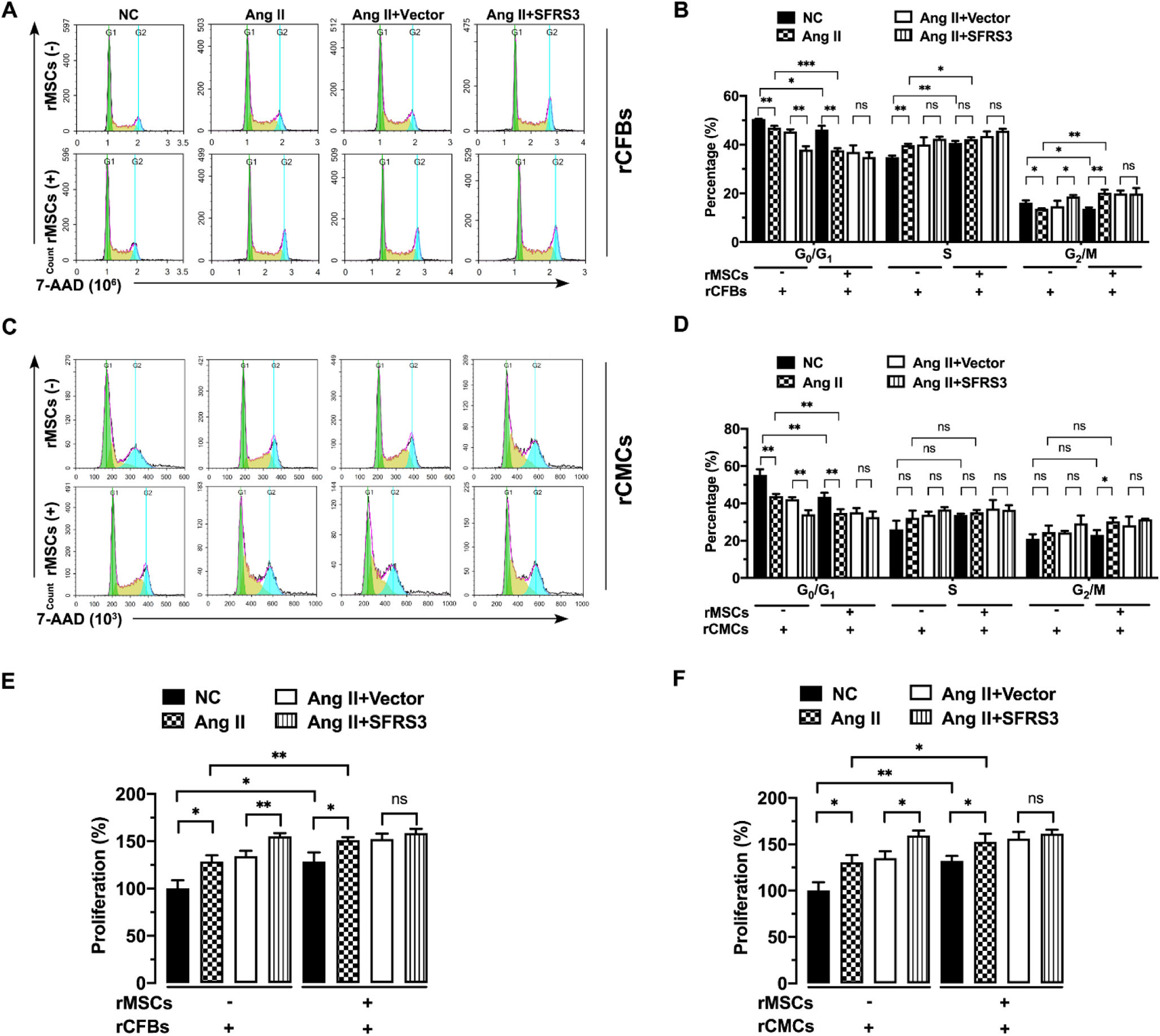
To assess the impact of rMSCs on normal and Ang II-treated rCFBs and rCMCs, we initially examined the cell cycle of these cells in the presence or absence of MSCs. Our results revealed that co-culturing rMSCs significantly decreased the percentage of rCFBs and rCMCs in the G0/G1 phase while increasing the proportion of cells in the S and G2/M phases, as evidenced by 7-AAD staining, although their impact on rCMCs in the S and G2/M phase was less evident (Figure 3A–D). A similar effect was observed in Ang II-treated cells (Figure 3A–D), indicating that rMSCs facilitated cell cycle progression in both rCFBs and rCMCs. Given that rMSCs can upregulate SFRS3 in these cells, we investigated the impact of increase SFRS3 levels on normal and Ang II-treated rCFBs and rCMCs. We overexpressed SFRS3 in these cells, resulting in significant upregulation of both SFRS3 mRNA and protein levels (Figure 2A–E). Notably, overexpression of SFRS3 also promoted cell cycle progression in Ang II-treated rCFBs and rCMCs when rMSCs were not present (Figure 3A–D). However, in the presence of rMSCs, the overexpression of SFRS3 had minimal effect on cell cycle progression in these cells (Figure 3A–D). Consistent with this, we discovered that rMSCs facilitated the proliferation of control and Ang II-treated rCFBs and rCMCs, as indicated by MTT assay results (Figure 3E and F). Conversely, overexpression of SFRS3 only induced additional proliferation in these cells when rMSCs were absent (Figure 3E and F), possibly because of the higher SFRS3 expression in rCFBs and rCMCs co-cultured with rMSCs, which desensitizes these cells to SFRS3 overexpression.
rMSCs or higher levels of SFRS3 promoted cell cycle progression and proliferation in Ang II-treated rCFBs and rCMCs. (A) Flow cytometric analysis results of rCFBs stained with 7-AAD. (B) Quantification of the 7-AAD staining results of rCFBs. (C) Flow cytometric analysis results of rCMCs stained with 7-AAD. (D) Quantification of the 7-AAD staining results of rCMCs. (E) MTT assay data indicating the proliferation of rCFBs. (F) MTT assay data indicating the proliferation of rCMCs. The NC group indicated that the cells were treated without any treatment, the Ang II group indicated that the cells were treated with Ang II, the Ang II+Vector group indicated that the cells were transferred to empty plasmid based on the Ang II treatment and the Ang II+SFRS3 group indicated that the SFRS3 plasmid was transferred after Ang II treatment. *, p<0.05; **, p<0.01; ***, p<0.001; ns, no significance.
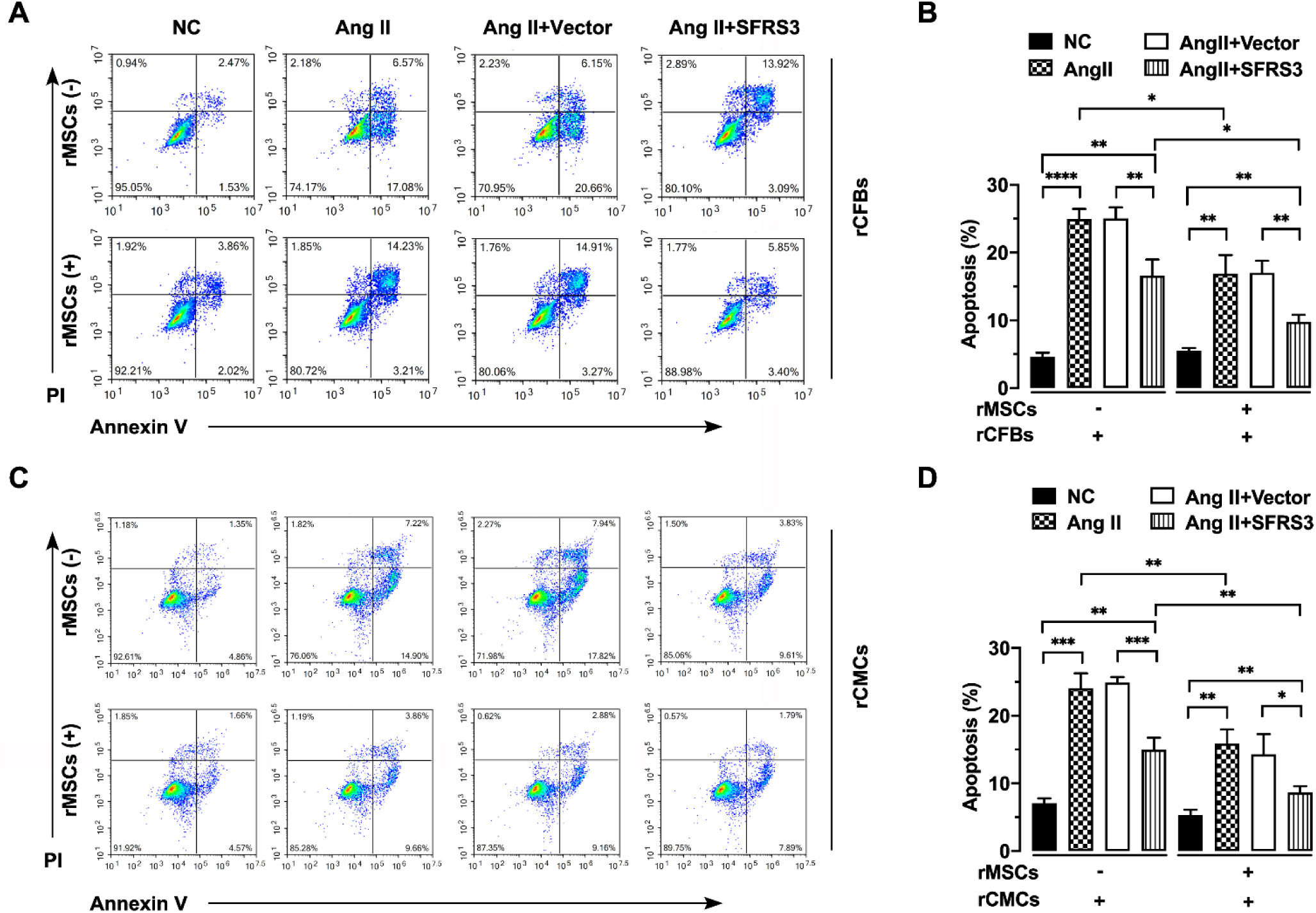
Furthermore, we assessed the apoptosis of these cells using Annexin V/PI staining. Our findings demonstrated that rMSCs significantly reduced apoptosis in Ang II-treated rCFBs and rCMCs (Figure 4A–D). Similarly, overexpression of SFRS3 partially rescued apoptosis in Ang II-treated rCFBs and rCMCs, both in the presence and absence of rMSCs (Figure 4A–D). Interestingly, co-culturing rMSCs enhanced the rescue effect of increased SFRS3 levels on Ang II-induced apoptosis in rCFBs and rCMCs (Figure 4A–D), indicating that rMSCs can attenuate apoptosis triggered by Ang II through mechanisms other than inducing SFRS3 expression. Collectively, these findings suggest that rMSCs or higher levels of SFRS3 can stimulate cell cycle progression and proliferation while repressing apoptosis in Ang II-treated rCFBs and rCMCs.
rMSCs or SFRS3 overexpression inhibited apoptosis of rCFBs and rCMCs triggered by Ang II treatment. (A) Flow cytometric analysis results of rCFBs stained with Annexin V/PI. (B) Quantification of the Annexin V/PI staining results of rCFBs. (C) Flow cytometric analysis results of rCMCs stained with Annexin V/PI. (D) Quantification of the Annexin V/PI staining results of rCMCs. The NC group indicated that the cells were treated without any treatment, the Ang II group indicated that the cells were treated with Ang II, the Ang II+Vector group indicated that the cells were transferred to empty plasmid based on the Ang II treatment and the Ang II+SFRS3 group indicated that the SFRS3 plasmid was transferred after Ang II treatment. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
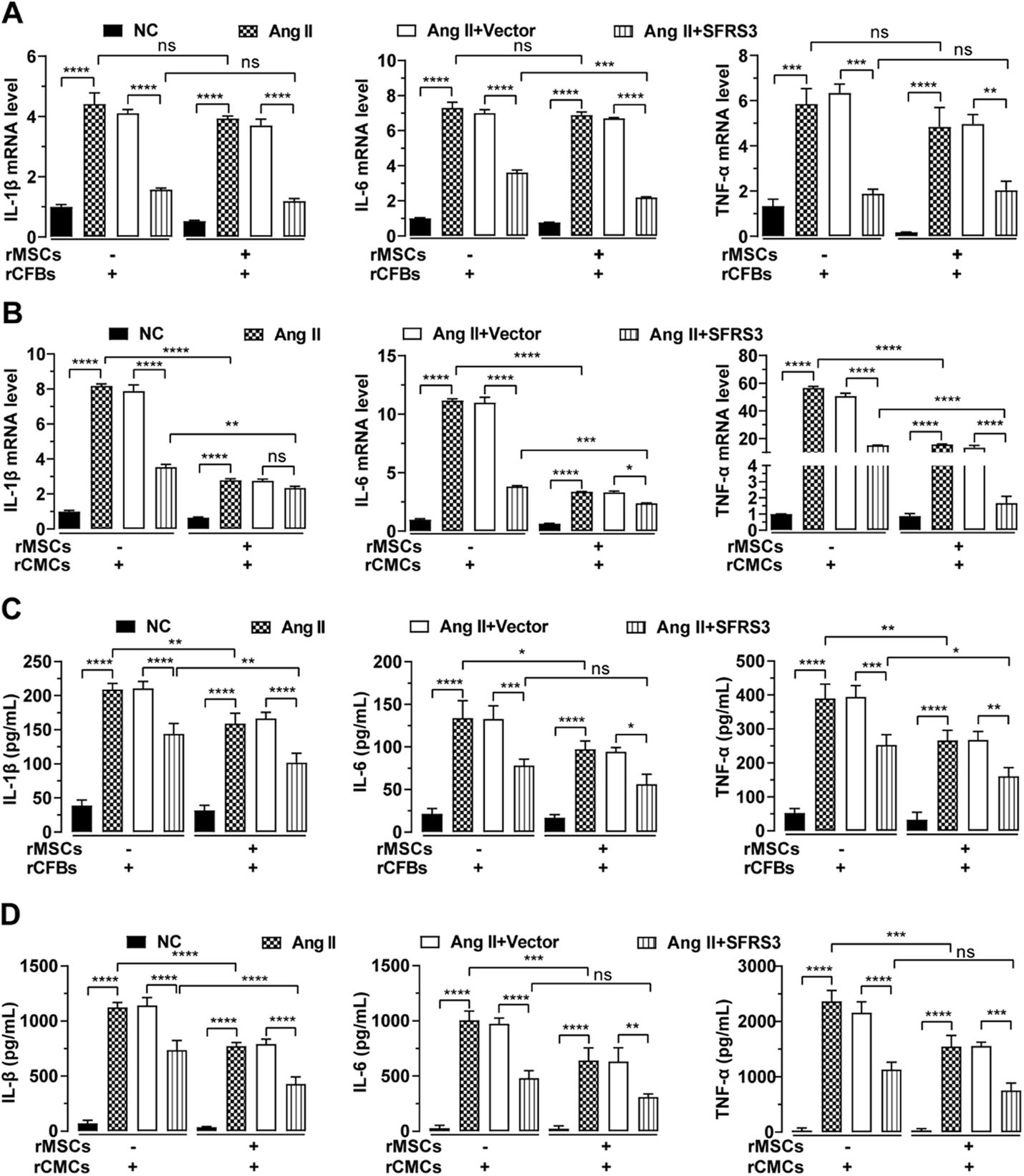
Since Ang II promotes fibrosis in CFBs and hypertrophy in CMCs by stimulating their cytokine secretion,35–37 we investigated the inhibitory effect of rMSCs on fibrosis in rCFBs and hypertrophy in rCMCs which treated by Ang II. Through qPCR analysis, we observed that Ang II significantly increased the mRNA levels of IL-1β, IL-6, and TNF-α in both cell types (Figure 5A and B). However, in the presence of rMSCs, the mRNA levels of these cytokines in Ang II-treated rCFBs exhibited a slight reduction, albeit not statistically significant (Figure 5A). In contrast, rMSCs markedly suppressed the mRNA expression of these cytokines in Ang II-treated rCMCs (Figure 5B). Consistently, ELISA data confirmed that rMSCs significantly downregulated cytokine secretion by Ang II-treated rCFBs and rCMCs (Figure 5C and D). Interestingly, overexpression of SFRS3 also effectively downregulated both mRNA expression and secretion of IL-1β, IL-6, and TNF-α in Ang II-treated rCFBs and rCMCs, regardless of the presence or absence of rMSCs (Figure 5A–D). Notably, rMSCs significantly enhanced the inhibitory effect of higher levels of SFRS3 on TNF-α and IL-1β secretion in Ang II-treated rCFBs, as well as IL-1β secretion in rCMCs (Figure 5C and D). Together these findings demonstrate that rMSCs or higher levels of SFRS3 can reduce cytokine secretion by Ang II-treated rCFBs and rCMCs.
rMSCs or SFRS3 overexpression repressed secretion of cytokines by Ang II-treated rCFBs and rCMCs. (A) qPCR data showing the mRNA levels of IL-1β, IL-6, and TNF-α in rCFBs with indicated treatments. (B) qPCR data showing the mRNA levels of IL-1β, IL-6, and TNF-α in rCMCs with indicated treatments. (C) ELISA results indicating the concentrations of IL-1β, IL-6, and TNF-α in conditioned media from rCFBs. (D) ELISA results indicating the concentrations of IL-1β, IL-6, and TNF-α in conditioned media from rCMCs. The NC group indicated that the cells were treated without any treatment, the Ang II group indicated that the cells were treated with Ang II, the Ang II+Vector group indicated that the cells were transferred to empty plasmid based on the Ang II treatment and the Ang II+SFRS3 group indicated that the SFRS3 plasmid was transferred after Ang II treatment. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; ns, no significance.
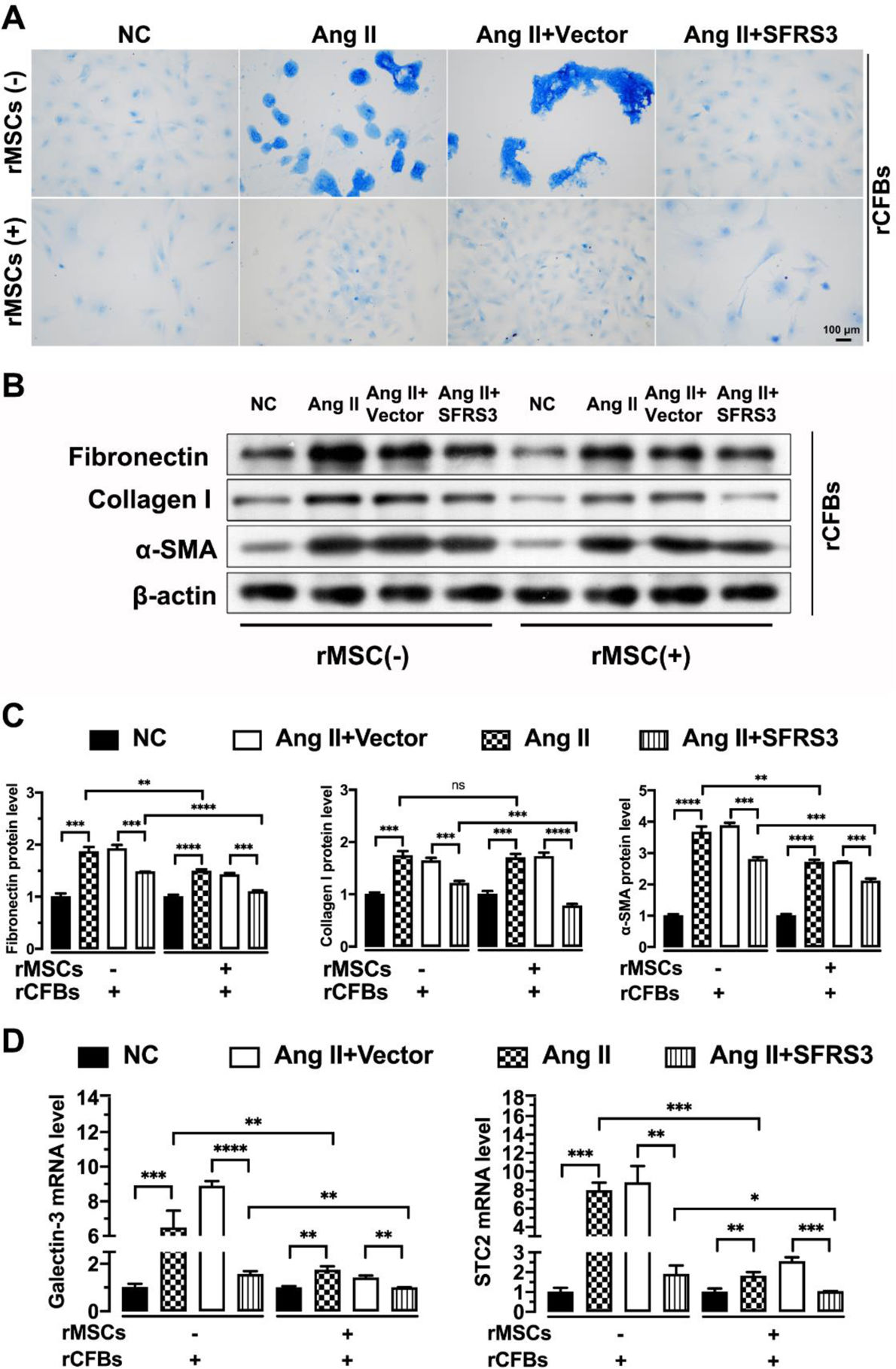
To investigate the direct inhibitory effect of rMSCs on Ang II-induced fibrosis in rCFBs, we examined collagen synthesis in these cells. Masson's trichrome staining results revealed that rMSCs effectively attenuated the extra collagen synthesis induced by Ang II in rCFBs (Figure 6A). Supporting this, rMSCs significantly decreased the protein levels of fibrosis markers Fibronectin and α-SMA in Ang II-treated rCFBs, although their effect on Collagen I expression was limited (Figure 6B and C). Furthermore, rMSCs also significantly downregulated the mRNA levels of Galectin-3 and STC2 (Figure 6D), which are strongly induced by Ang II treatment and associated with CF.38,39 Galectin-3, a member of the lectin family, is involved in cell adhesion, apoptosis, pre-mRNA splicing and immunity in vivo as a galactoside-binding protein.38 The STC family of glycoproteins, consisting of STC1 and STC2, is thought to play an important role in regulating calcium and Pi homeostasis.39 Similarly, overexpression of SFRS3 abolished the excessive collagen formation in Ang II-treated rCFBs, regardless of the presence or absence of rMSCs (Figure 6A). Additionally, higher levels of SFRS3 reduced the protein levels of Fibronectin, α-SMA, and even Collagen I in Ang II-treated rCFBs (Figure 6B and C). Moreover, higher levels of SFRS3 downregulated the mRNA levels of Galectin-3 and STC2 in these cells (Figure 6D). Importantly, the inhibitory effect of rMSCs on the expression of these marker genes was further enhanced by SFRS3 overexpression (Figure 6B–D). Together, these observations indicate that both rMSCs and higher levels of SFRS3 exert a negative regulatory effect on fibrosis in rCFBs induced by Ang II treatment.
The fibrosis of Ang II-treated rCFBs was inhibited by rMSCs or higher levels of SFRS3. (A) Masson's trichrome staining results of rCFBs with indicated treatments. The collagen fibers are stained with blue color. (B) Western blot indicating the expression of Fibronectin, Collagen I, and α-SMA in rCFBs with indicated treatments. β-Actin was used to normalize the expression levels of these proteins. (C) Quantification of the Western blot data. (D) qPCR data showing the mRNA levels of Galectin-3 and STC2 in rCFBs with indicated treatments. The NC group indicated that the cells did not receive any treatment, the Ang II group indicated that the cells were treated with Ang II, the Ang II+Vector group indicated that the cells were transferred to empty plasmid based on the Ang II treatment and the Ang II+SFRS3 group indicated that the SFRS3 plasmid was transferred after Ang II treatment. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; ns, no significance.
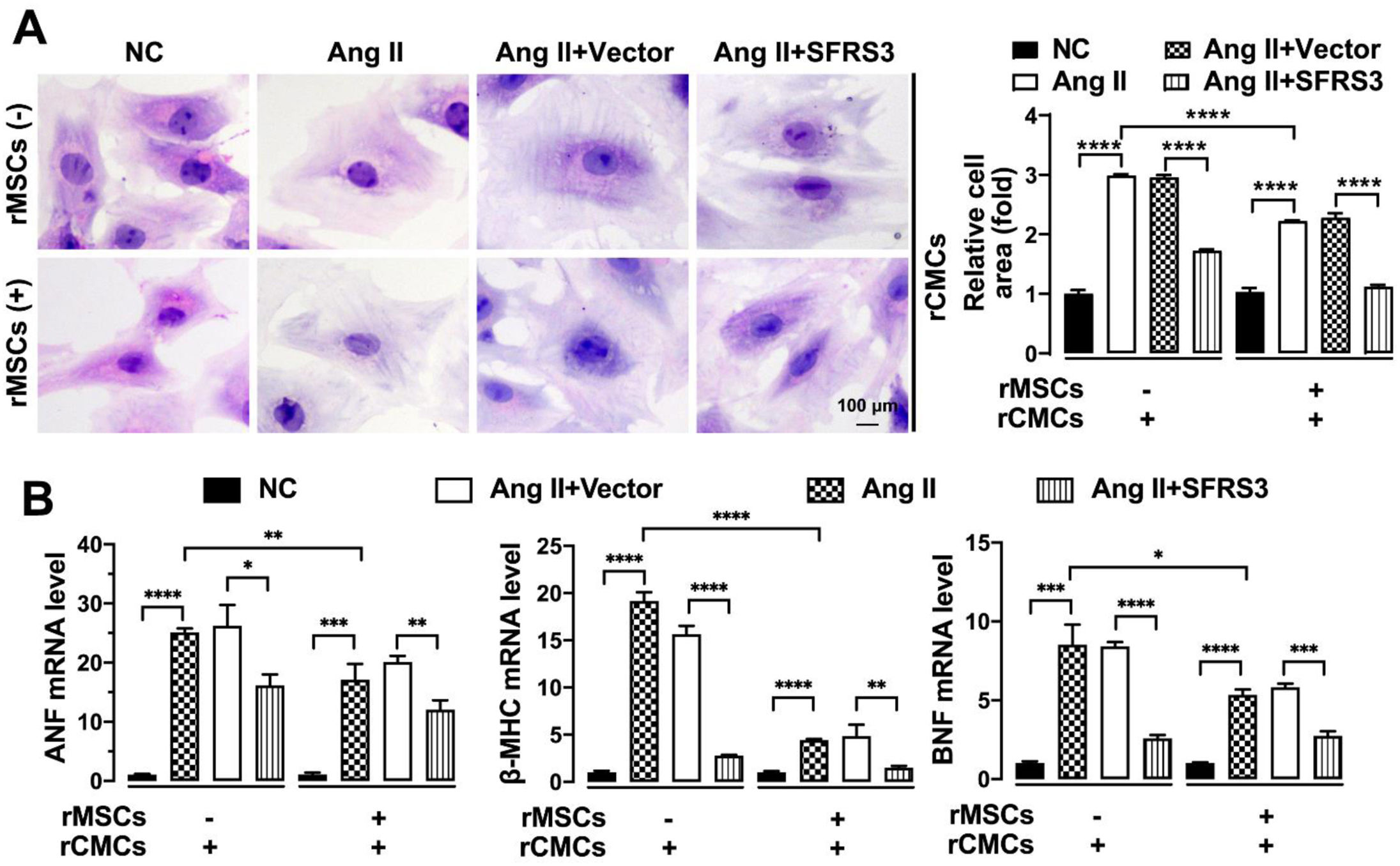
To further investigate the impact of rMSCs or SFRS3 overexpression on the hypertrophy of Ang II-treated rCMCs, we conducted experiments to assess the features related to hypertrophy. H&E staining results demonstrated that the hypertrophy induced by Ang II in rCMCs was rescued when co-cultured with rMSCs (Figure 7A). Consistently, the expression of genes associated with CH, such as ANF, β-MHC, and BNF,40,41 was significantly reduced in Ang II-treated rCMCs in the presence of rMSCs (Figure 7B). Moreover, we observed that overexpression of SFRS3 effectively rescued the hypertrophy of Ang II-treated rCMCs and attenuated the expression of ANF, β-MHC, and BNF, regardless of co-culturing with rMSCs (Figure 7A and B). These findings indicate that both rMSCs and SFRS3 overexpression can rescue the hypertrophy of Ang II-treated rCMCs.
Ang II-triggered hypertrophy of rCMCs was mitigated by rMSCs or SFRS3 overexpression. (A) H&E staining showing the morphology of rCMCs with indicated treatments. (B) qPCR results showing the expression of ANF, β-MHC, and BNF in rCMCs with indicated treatments. The NC group indicated that the cells were treated without any treatment, the Ang II group indicated that the cells were treated with Ang II, the Ang II+Vector group indicated that the cells were transferred to empty plasmid based on the Ang II treatment and the Ang II+SFRS3 group indicated that the SFRS3 plasmid was transferred after Ang II treatment. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
CVDs are still a leading cause of death and continue to be a significant burden on global public health for decades.42,43 Although significant progress has been made in the prevention, diagnosis, and treatment of CVDs, there is an urgent need for novel drugs and therapies to enhance the efficacy of treatment for these fatal diseases.44,45
MSCs are a type of stem cell that can differentiate into a broad range of cell types, showing great potential in the field of regenerative medicine.46 Stem cell therapy utilizing MSCs is also being clinically tested for treating fibrotic heart diseases, and accumulating evidence show that MSCs can attenuate the phenotypes associated with CH.47,48 However, a more comprehensive understanding of the precise function of MSCs during CF and CH, as well as the underlying molecular mechanisms, is still required to further enhance and optimize these therapeutic approaches.
In the current study, we investigated the effect of MSCs on both control and Ang II-induced CFBs and CMCs in vitro. Our data showed that rMSCs can promote cell cycle progression and proliferation in both control and Ang II-treated CFBs and CMCs. Additionally, rMSCs significantly attenuated Ang II-induced apoptosis in both cell types. These results are consistent with the known function of MSCs in other contexts.49 Furthermore, our data indicated that rMSCs exerted a protective effect on rCFBs and rCMCs, preventing Ang II-induced fibrosis and hypertrophy, respectively. However, the precise mechanisms underlying these effects need further investigation.
Meanwhile, we assessed the impact of rMSCs on SFRS3 expression in these cardiac cells in the absence or presence of Ang II. Our findings revealed that rMSCs could induce the expression of SFRS3 under both conditions. Increased SFRS3 expression is commonly observed in various types of tumors. Given the oncogenic roles of SFRS3, its expression in normal tissues is tightly regulated. However, the mechanism by which rMSCs upregulate SFRS3 expression in rCFBs and rCMCs remains unclear. Considering that MSCs often secrete exosomes containing mRNA to regulate recipient cells, it is plausible that rMSCs elevate the SFRS3 mRNA level by delivering such exosomes, which are captured and internalized by co-cultured rCFBs and rCMCs. Alternatively, rMSCs may promote SFRS3 expression by modulating other factors, such as HnRNP L, known for its capability to upregulate SFRS3 expression.50 Investigating these possibilities would be crucial to clarify the relationship between MSCs and SFRS3.
The oncogenic functions of SFRS3 in promoting proliferation and cell cycle progression, while repressing apoptosis of tumor cells,25 highly overlap with the functions of rMSCs in regulating rCFBs and rCMCs. This prompted us to explore the impact of SFRS3 overexpression in these cardiac cells. As anticipated, our data revealed that overexpression of SFRS3 can promote cell cycle progression and proliferation in Ang II-treated rCFBs and rCMCs. This finding aligns with the fact that SFRS3-deficient cardiomyocytes exhibit reduced proliferation in vivo.31 Additionally, similar to rMSCs, higher levels of SFRS3 effectively attenuated the increased apoptosis in rCFBs and rCMCs induced by Ang II treatment. Although no ectopic apoptosis was identified in the cardiomyocytes of SRSF3 loss-of-function mutant mice, our results are consistent with the anti-apoptotic function of SFRS3 observed in other contexts.51 One possibility for this inconsistency is that SFRS3 may selectively repress apoptosis in certain types of cardiac cells by targeting specific genes in a context-dependent manner. Therefore, it would be meaningful to investigate the mechanism by which SFRS3 inhibits apoptosis in rCFBs and rCMCs.
Despite the high functional redundancy between rMSCs and SFRS3 overexpression in counteracting the effects of Ang II on rCFBs and rCMCs, it is noteworthy that overexpression of SFRS3 only partially rescues the apoptosis triggered by Ang II in these cardiac cells. This indicates that rMSCs may influence rCFBs and rCMCs through additional mechanisms other than promoting SFRS3 expression. Further investigations are necessary to clarify the anti-apoptotic role of rMSCs in Ang II-treated rCFBs and rCMCs.
ConclusionData from our in vitro experiments demonstrate that SFRS3 expression is induced by rMSCs while repressed by Ang II treatment in rCFBs and rCMCs. Both rMSCs and higher levels of SFRS3 can promote the cell cycle progression and proliferation of rCFBs and rCMCs. Co-culturing with rMSCs or overexpressing SFRS3 attenuates the apoptosis of Ang II-treated rCFBs and rCMCs, reduces their cytokine secretion, and mitigates the fibrosis of rCFBs and hypertrophy of rCMCs induced by Ang II treatment. These observations expand our understanding of the roles of MSCs and SFRS3 in CF and CH and may provide potential benefits for improving MSC-based therapy to treat CVDs.
Informed consentNo human studies were carried out by the authors for this article.
FundingThis work was supported by Medical Innovation Projects of Fujian Province's Health and Scientific Research Talent Training Project (Grant No. 2019-CXB-25).
Conflict of interestThe authors declare that they have no conflict of interest.