Left ventricular global longitudinal strain (LVGLS) is an indicator of myocardial function in patients with heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF). Nevertheless, it is not clear whether LVGLS correlates with filling pressures and cardiac output (CO) in an ambulatory setting. We aimed to assess whether LVGLS is associated with invasive pulmonary artery pressures (PAP) and CO in outpatients using the invasive remote monitoring CardioMEMS™ system.
MethodsThis single-center, prospective observational study included patients with HFrEF undergoing remote monitoring using the CardioMEMS™ system, between January 2020 and December 2022. Repeated transthoracic echocardiography (TTE) studies were performed in each patient and invasive hemodynamic data were obtained during the TTE studies using the CardioMEMS™ system. Univariate and multivariate models were used to assess the potential association between LVGLS and invasive PAP and CO.
ResultsTwelve patients were included and 46 TTE studies were analyzed. LVGLS was correlated with diastolic (d) PAP (r=0.403, p=0.041) and CO (r=−0.426, p=0.039) in the univariate analysis. In multivariate models, LVGLS was an independent predictor of dPAP and CO, but not mean PAP or systolic PAP. The variation of LVGLS between TTE studies was correlated with the variation of dPAP during follow-up (r=0.60, p=0.017).
ConclusionsIn a cohort of HFrEF patients under invasive hemodynamic remote monitoring, LVGLS was independently associated with invasive filling pressures and CO, in an outpatient setting. These findings reinforce the value of LVGLS for the management of outpatients with HFrEF.
O strain longitudinal global do ventrículo esquerdo (SLGVE) é um indicador de função miocárdica em doentes com insuficiência cardíaca com fração de ejeção reduzida (ICFEr) e com fração de ejeção preservada (ICFEp). No entanto, não é totalmente claro se o SLGVE se correlaciona com as pressões de enchimento e débito cardíaco (DC) no contexto ambulatório. Avaliámos a associação entre o SLGVE com as pressões (P) invasivas da artéria pulmonar (AP) e DC utilizando o sistema CardioMEMS™ de monitoração remota invasiva.
MétodosEste estudo unicêntrico, observacional e prospetivo incluiu doentes com ICFEr monitorados remotamente com o sistema CardioMEMS™, entre janeiro 2020 e dezembro 2022. Ecocardiograma transtorácicos (ETT) seriados foram executados em cada doente e os dados invasivos hemodinâmicos foram obtidos no momento de execução do ETT, usando o sistema CardioMEMS™. Modelos uni- e multivariados foram usados para avaliar a potencial associação entre o SLGVE e PAP e DC invasivos.
ResultadosDoze doente foram incluídos e 46 ETT foram analisados. O SLGVE correlacionou-se com a PAP diastólica (r=0,403, p=0,041) e DC (r=−0,426, p=0,039) na análise univariada. Nos modelos multivariados, o SLGVE foi um preditor independente da PAP diastólica e DC, mas não da PAP média e PAP sistólica. A variação do SLGVE entre ETT correlacionou-se com uma variação correspondente de PAP diastólica durante o seguimento (r=0,60, p=0,017).
ConclusõesNuma coorte de doentes com ICFEr sob monitoração remota invasiva, o SLGVE está associado de forma independente com as pressões de enchimento e DC, no contexto ambulatório. Estes achados reforçam o valor do SLGVE na gestão dos doentes ambulatórios com ICFEr.
Heart failure (HF) is a major public health concern worldwide and is responsible for a substantial number of hospitalizations, leading to a significant economic burden.1,2 The pathophysiology of HF is characterized by an increase in filling pressures and/or a reduction of cardiac output (CO) and both have been associated with a higher risk of hospitalization and mortality.3,4 Moreover, previous research has shown that increases in intracardiac pressures and pulmonary artery pressures (PAP) are the cause of congestion and begin a few days to weeks before the onset of overt symptoms and signs of HF.5 Noninvasive diagnostic tools that simultaneously reflect filling pressures and CO may be very useful in clinical practice as they could tailor the management of patients with HF.
Left ventricular (LV) global longitudinal strain (GLS) is a simple and powerful echocardiographic tool. Although it is susceptible to changes in load conditions, LVGLS exhibits lower load dependency compared with other indexes, including left ventricle ejection fraction (LVEF), thus providing more accurate information on myocardial function.6–8 LVGLS has been shown to predict outcomes in patients with HF and may be useful in guiding treatment decisions.9–12 Since LVGLS assesses overall LV myocardial performance, it could also be associated with filling pressures and CO. In one study, Kažukauskienė et al., addressed the association between LVGLS and in-hospital invasive hemodynamic parameters obtained by right heart catheterization (RHC), and found a significant positive correlation between GLS and pulmonary capillary wedge pressure (PCWP), mean (m) PAP and pulmonary vascular resistance (PVR).13 Nevertheless, inpatient and outpatient hemodynamics differ substantially for the same patient and there is a lack of evidence regarding the potential association between LVGLS and invasive hemodynamic parameters in an outpatient setting.
CardioMEMS™ (Abbott Scientific, Atlanta, GA, USA) is a remote monitoring system that is approved for reducing the burden of HF hospitalizations (HFH).14 The sensor is implanted in the pulmonary artery (PA) and measures continuously systolic (s) PAP, diastolic (d) PAP and mPAP, and allows for an estimation of CO. Therefore, this system directly assesses the hemodynamic status of HF patients in an ambulatory setting.15,16
ObjectivesIn the present study we aimed to assess whether LVGLS is associated with invasive PAP and CO in HFrEF patients using the CardioMEMS™ system, in the ambulatory setting.
MethodsStudy designThe study was approved by the Ethics Committee of Centro Hospitalar Universitário de Lisboa Central and was conducted according to the Declaration of Helsinki. All participants signed written informed consent. This was a single-center, prospective observational study. Consecutive patients with HFrEF that underwent remote monitoring based on CardioMEMS™ system between January 2020 and December 2022 were included.
Inclusion and exclusion criteriaThe eligibility criteria for CardioMEMS™ system implantation were the diagnosis of HF, NYHA class ≥II despite optimal medical therapy according to the published guidelines,17 at least one HFH in the previous year and good patient compliance as judged by HF team.
Exclusion criteria included a history of recurrent pulmonary embolism or deep vein thrombosis, inability to tolerate RHC, major cardiovascular events (including myocardial infarction and stroke) within the previous three months, cardiac resynchronization therapy (CRT) implanted within the previous three months, glomerular filtration rate <25 mL/min per 1.73 m2 (measured within two weeks of pressure sensor implant), congenital heart disease or mechanical right heart valve, inclusion on the waiting list for heart transplantation, need for ventricular assist device, active infection and known coagulation disorders.
Data collectionBaseline clinical, demographic and laboratory data were collected at the study inclusion, as well as invasive hemodynamic data from the RHC procedure performed during implantation of the CardioMEMS™ sensor.
According to the protocol, a transthoracic echocardiography (TTE) study (as detailed below) was performed at three, six, 12, 18 and 24 months after the CardioMEMS™ system implantation.
Patients performed daily readings using the CardioMEMS™ system, as described below. Invasive hemodynamic data were obtained, including sPAP, dPAP, mPAP, heart rate (HR) and estimated CO. For this study, additional readings were performed immediately before each TTE study, and such data were used for the analysis.
Transthoracic echocardiographyA routine protocol from the echocardiographic laboratory at our center for conventional M-mode, two-dimensional, Doppler, and tissue Doppler measurement was used.
LV diameters were measured from the parasternal long-axis view. LV end-systolic and end-diastolic volumes were measured and indexed to body surface area (BSA). LVEF was calculated using the Simpson biplane method. Left atrium (LA) volume was measured by a biplane area-length method from the apical four and two-chamber views and indexed to the BSA. Mitral E and A peak velocity were measured and the ratio of early diastolic LV inflow velocity to atrial-systolic velocity (E/A) was calculated. The average tissue Doppler-derived early diastolic mitral annular velocity (e′) was obtained from the mitral annulus’ septal and lateral sides. The average ratio of early diastolic LV inflow velocity to early diastolic mitral annular velocity (E/e′) was calculated. Tricuspid annular plane systolic excursion (TAPSE) was measured by M-mode and sPAP was estimated based on the tricuspid regurgitant jet velocity.
Left ventricular global longitudinal strain analysis was performed with two-dimensional speckle tracking-echocardiography with imaging acquisition at 50–70 frames/s. LV endocardial border was manually traced at the end-diastole frame (first frame of mitral valve coaptation) from three apical views (four-, two- and three-chamber views). To ensure accurate tracking, the TTE operator assessed the detected region of interest (ROI) and manually modified the ROI as appropriate. LVGLS was calculated by averaging all available segments at the time of peak systolic strain.
Remote invasive monitoring using the CardioMEMS™ systemBriefly, the CardioMEMS™ system consists of three main components: an implantable PA sensor, a patient and hospital electronic system, and a digital database. The PA sensor is a small device placed permanently in a PA branch and features nitinol loops for secure positioning. It provides hemodynamic data, including PAP waveform, sPAP, dPAP, mPAP, HR and CO estimation.
The CardioMEMS™ sensor was implanted in the PA according to manufacturer instructions16 and calibrated with a RHC using a balloon wedge-pressure catheter and indirect Fick CO calculation. The CardioMEMS™ system measures PAP, which is highly correlated with PAP obtained using a Swan-Ganz catheter.18 The CO was estimated using a proprietary algorithm (Abbott Scientific, Atlanta, GA, USA) based on the PAP waveform, mPAP, HR, and a reference CO measured at the implant.19 Even though estimated CO is not currently approved for regular clinical use, the algorithm had undergone prior development, refinement, and retrospective testing using clinical RHC data. These tests showed that the estimated CO is non-inferior to currently employed CO measurement methods.20
Statistical analysisCategorical variables are presented as frequency and percentages, and continuous variables as means and standard deviations or medians and interquartile ranges, as appropriate. Normal distribution was checked using the Shapiro–Wilk test.
We performed uni- and multivariate analyses to assess whether LVGLS was associated with hemodynamic parameters (sPAP, dPAP, mPAP and CO), irrespective of other echocardiographic parameters. First, we performed a univariate analysis using Spearman's correlation to assess which echocardiographic parameters, including LVGLS, were associated with each of the hemodynamic parameters. We then performed a multivariable analysis (backward stepwise technique) for the hemodynamic parameters that had a significant correlation with LVGLS in the previous analysis, adjusting for other echocardiographic covariates. Variables with a p value <0.05 in the univariate analyses were included in the multivariable model. For covariate adjustment, the following echocardiographic parameters were considered: LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), indexed LV end-diastolic volume (LVEDV), indexed LV end-systolic volume (LVESV), interventricular septum (IVS) thickness, LA volume, LVEF, TAPSE, sPAP, mean E/e′ ratio, E-wave velocity, A-wave velocity and E/A ratio.
In addition, after the multivariate regression models had been performed, we computed a post hoc power analysis for each model obtained. We used the G*Power 3.1.9 software for this purpose. We used a F test for linear multiple regression fixed model, R2 deviation from zero to perform a post hoc power analysis, assuming a medium effect size f2 of 0.15, a type α error probability of 0.05, a sample size of 46 and the number of predictors according to each final model. The power (1−β error probability) was then calculated for each model.
Finally, an exploratory analysis was carried out to assess the potential association between the variation in left ventricular global longitudinal strain (LVLGS) and variation in the hemodynamic parameters (sPAP, dPAP, mPAP and CO) during follow-up. The variation in LVGLS and hemodynamic parameters was calculated as the absolute difference between two consecutive measurements for each patient. The Spearman's correlation was used to test the correlations between the mean variation in LVGLS between consecutive assessments and the mean variation (corresponding to the same follow-up period) in hemodynamic parameters.
IBM SPSS Statistics® (version 26) was used for statistical analyses. A p value <0.05 was considered significant.
ResultsBaseline characteristicsA total of 12 patients were included in the study and a total of 46 TTE were performed during a median follow-up time of 20.5 months. Table 1 summarizes the baseline characteristics of the sample. The mean age was 67±11 years, 11 (92%) were male and 8 (67%) had ischemic dilated cardiomyopathy. Mean LVEF was 28.5±8%, mean LVGLS was −7.3±3% and mean NT-proBNP was 5032±4518.1 pq/mL. Five (42%) patients had past medical history of atrial fibrillation (AF), but only four out of 46 TTE studies were performed in AF rhythm. All patients were on optimized guideline-directed medical therapy. Nine (75%) patients had an implantable cardiac device (ICD) and 2 (16.7%) had CRT. Five patients had more than one HFH and 4 (33%) had received a levosimendan infusion in the previous year.
Baseline characteristics of included patients.
| n=12 | |
|---|---|
| Demographics | |
| Age, years | 67±11 |
| Male sex | 11 (92%) |
| Clinical findings | |
| Body mass index, kg/m2 | 26±4 |
| Creatinine levels, mg/dL | 1.4±0.4 |
| Glomerular filtration rate, mL/min/1.73 m2 | 46.5 (16) |
| NT-proBNP, pg/mL | 5032±4518 |
| Troponin T levels, ng/L | 26±18 |
| Glycosylated hemoglobin, % | 6.3±1 |
| NYHA class II | 4 (33%) |
| NYHA class III | 8 (67%) |
| Medical history | |
| Hypertension | 10 (83%) |
| Diabetes mellitus | 8 (67%) |
| Dislipidemia | 10 (83%) |
| Active smoking | 3 (25%) |
| Chronic kidney disease | 9 (75%) |
| Atrial fibrillation | 5 (42%) |
| Etiology | |
| Ischemic | 8 (67%) |
| Non-ischemic dilated | 2 (17%) |
| Valvular | 1 (8%) |
| Ischemic and valvular | 1 (8%) |
| Medical treatment history | |
| Loop diuretic agent | 12 (100%) |
| Beta-blocker | 11 (92%) |
| Spironolactone | 11 (92%) |
| RAS-A | 11 (92%) |
| ACE-I | 1 (8%) |
| ARB | 1 (8%) |
| Sacubitril/valsartan | 9 (75%) |
| SGLT-2 inhibitor | 4 (33%) |
| Statin | 11 (92%) |
| Ezetimibe | 3 (25%) |
| Anti-platelet therapy | 6 (50%) |
| Oral anti-coagulation | 7 (58%) |
| Anti-arrhythmic drugs | 6 (50%) |
| Devices | |
| Implantable cardiac defibrillator | 9 (75%) |
| Cardiac resynchronization therapy with defibrillator | 2 (17%) |
| Echocardiographic parameters | |
| LVEDD (mm) | 69±9 |
| LVESD (mm) | 52±15 |
| LVEDV (mL/m2) | 97±35 |
| LVESV (mL/m2) | 72±30 |
| Interventricular septum thickness (mm) | 8.5±1.8 |
| Left atrium volume (mL/m2) | 50±22 |
| LVEF (%) | 28.5±8 |
| LVGLS (%) | −7.3±3 |
| TAPSE (mm) | 17±4 |
| Mitral regurgitation (≥moderate) | 5 (42%) |
| Systolic pulmonary artery pressure (mmHg) | 47±14 |
| Mean E/e′ ratio | 20±11 |
| Peak E-wave velocity (cm/s) | 91±32 |
| Peak A-wave velocity (cm/s) | 72±30 |
| E/A ratio | 1.6±0.6 |
ARB: angiotensin II receptor blockers; HF: heart failure; LVEDD: left ventricle end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; LVGLS: left ventricular global longitudinal strain; NT-proBNP: n terminal pro B type natriuretic peptide; NYHA: New York Heart Association; RAS-A: renin-angiotensin-system acting agents; SGLT: sodium glucose cotransporter-2; SPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion.
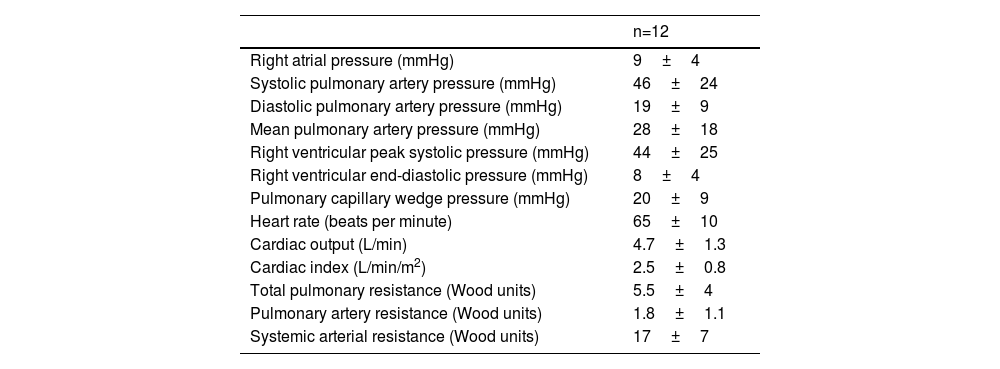
At the time of CardioMEMS™ implantation, mean dPAP was 19±9 mmHg, mean sPAP was 46±24 mmHg, mean mPAP was 28±18 mmHg, mean PCWP was 21±9 mmHg and, mean HR was 65±10 beats per minute and mean CO was 4.8±1.3 L/min (Table 2).
Right heart catheterization at baseline (CardioMEMS™ implantation).
| n=12 | |
|---|---|
| Right atrial pressure (mmHg) | 9±4 |
| Systolic pulmonary artery pressure (mmHg) | 46±24 |
| Diastolic pulmonary artery pressure (mmHg) | 19±9 |
| Mean pulmonary artery pressure (mmHg) | 28±18 |
| Right ventricular peak systolic pressure (mmHg) | 44±25 |
| Right ventricular end-diastolic pressure (mmHg) | 8±4 |
| Pulmonary capillary wedge pressure (mmHg) | 20±9 |
| Heart rate (beats per minute) | 65±10 |
| Cardiac output (L/min) | 4.7±1.3 |
| Cardiac index (L/min/m2) | 2.5±0.8 |
| Total pulmonary resistance (Wood units) | 5.5±4 |
| Pulmonary artery resistance (Wood units) | 1.8±1.1 |
| Systemic arterial resistance (Wood units) | 17±7 |
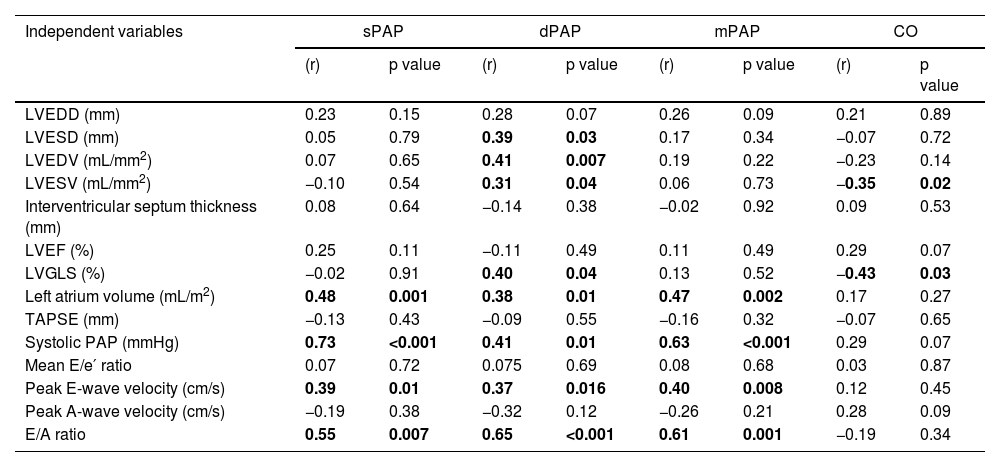
A univariate analysis was performed to assess the correlation between echocardiographic parameters, including LVGLS, and invasive hemodynamic parameters obtained using the CardioMEMS™ (Table 3).
Association between echocardiographic and hemodynamic parameters in the univariate analysis (n=46).
| Independent variables | sPAP | dPAP | mPAP | CO | ||||
|---|---|---|---|---|---|---|---|---|
| (r) | p value | (r) | p value | (r) | p value | (r) | p value | |
| LVEDD (mm) | 0.23 | 0.15 | 0.28 | 0.07 | 0.26 | 0.09 | 0.21 | 0.89 |
| LVESD (mm) | 0.05 | 0.79 | 0.39 | 0.03 | 0.17 | 0.34 | −0.07 | 0.72 |
| LVEDV (mL/mm2) | 0.07 | 0.65 | 0.41 | 0.007 | 0.19 | 0.22 | −0.23 | 0.14 |
| LVESV (mL/mm2) | −0.10 | 0.54 | 0.31 | 0.04 | 0.06 | 0.73 | −0.35 | 0.02 |
| Interventricular septum thickness (mm) | 0.08 | 0.64 | −0.14 | 0.38 | −0.02 | 0.92 | 0.09 | 0.53 |
| LVEF (%) | 0.25 | 0.11 | −0.11 | 0.49 | 0.11 | 0.49 | 0.29 | 0.07 |
| LVGLS (%) | −0.02 | 0.91 | 0.40 | 0.04 | 0.13 | 0.52 | −0.43 | 0.03 |
| Left atrium volume (mL/m2) | 0.48 | 0.001 | 0.38 | 0.01 | 0.47 | 0.002 | 0.17 | 0.27 |
| TAPSE (mm) | −0.13 | 0.43 | −0.09 | 0.55 | −0.16 | 0.32 | −0.07 | 0.65 |
| Systolic PAP (mmHg) | 0.73 | <0.001 | 0.41 | 0.01 | 0.63 | <0.001 | 0.29 | 0.07 |
| Mean E/e′ ratio | 0.07 | 0.72 | 0.075 | 0.69 | 0.08 | 0.68 | 0.03 | 0.87 |
| Peak E-wave velocity (cm/s) | 0.39 | 0.01 | 0.37 | 0.016 | 0.40 | 0.008 | 0.12 | 0.45 |
| Peak A-wave velocity (cm/s) | −0.19 | 0.38 | −0.32 | 0.12 | −0.26 | 0.21 | 0.28 | 0.09 |
| E/A ratio | 0.55 | 0.007 | 0.65 | <0.001 | 0.61 | 0.001 | −0.19 | 0.34 |
LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; LVGLS: left ventricular global longitudinal strain; PAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion.
Left ventricular global longitudinal strain was significantly correlated with both dPAP (r=0.40, p=0.04) and CO (r=−0.43, p=0.03), but neither with sPAP nor with mPAP. Considering such results, the echocardiographic variables correlated with dPAP and/or CO, other than LVGLS, are presented.
LVESD (r=0.39, p=0.03), LVEDV (r=0.41, p=0.007), LVESV (r=0.31, p=0.04), LA volume (r=0.39, p=0.01), echocardiographic-derived sPAP (r=0.41, p=0.01), peak E-wave velocity (r=0.37, p=0.016) and E/A ratio (r=0.65, p<0.001) were significantly correlated with dPAP. Only LVESV showed a significant correlation with CO (r=−0.35, p=0.02), in addition to LVGLS.
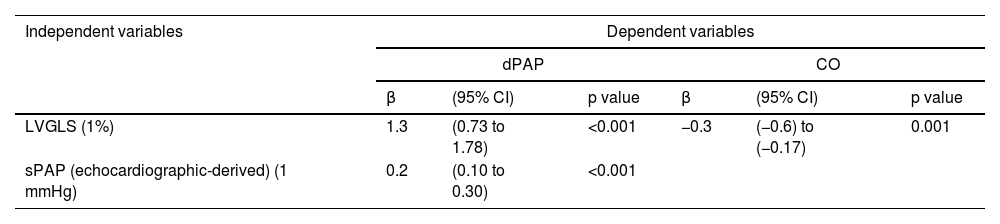
Considering the results obtained from the univariate analysis, multiple linear regression models were performed for dPAP and CO as the dependent variables (Table 4).
Multivariate linear regression analysis.
| Independent variables | Dependent variables | |||||
|---|---|---|---|---|---|---|
| dPAP | CO | |||||
| β | (95% CI) | p value | β | (95% CI) | p value | |
| LVGLS (1%) | 1.3 | (0.73 to 1.78) | <0.001 | −0.3 | (−0.6) to (−0.17) | 0.001 |
| sPAP (echocardiographic-derived) (1 mmHg) | 0.2 | (0.10 to 0.30) | <0.001 | |||
CI: confidence interval; LVGLS: left ventricular global longitudinal strain; sPAP: systolic pulmonary artery pressure.
LVGLS and echocardiographic-derived sPAP were the two independent predictors of invasive dPAP. This model explains 60% of dPAP value (R2=0.60, adjusted R2=0.56, p<0.001). A 1% higher LVGLS absolute value is associated with a 1.3 mmHg higher dPAP (p<0.001), when adjusted for echocardiographic-derived sPAP. LVGLS was the only independent predictor of CO. The model explains 36% of CO value (R2=0.362, p=0.001). A 1% higher LVGLS absolute value is associated with a lower 0.38 L/min CO.
Considering the two final models mentioned above, the statistical power analysis of the dPAP regression model was determined to be 0.62 and for the CO regression model was 0.73.
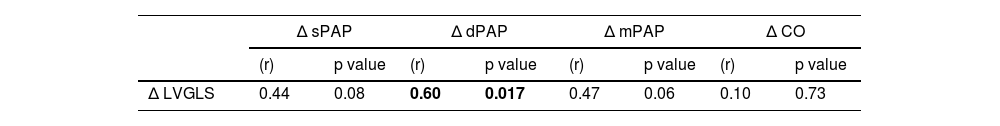
In the exploratory analysis, the variation in LVGLS was significantly correlated with a corresponding variation with invasive dPAP (r=0.60, p=0.017) (Table 5).
Association between the variation in LVGLS and hemodynamic parameters.
| Δ sPAP | Δ dPAP | Δ mPAP | Δ CO | |||||
|---|---|---|---|---|---|---|---|---|
| (r) | p value | (r) | p value | (r) | p value | (r) | p value | |
| Δ LVGLS | 0.44 | 0.08 | 0.60 | 0.017 | 0.47 | 0.06 | 0.10 | 0.73 |
CO: cardiac output; dPAP: diastolic pulmonary artery pressure; LVGLS: left ventricle global longitudinal strain; mPAP: mean pulmonary artery pressure; sPAP: systolic pulmonary artery pressure; Δ: variation.
We assessed the potential association between LVGLS and invasive measurement of filling pressures and CO using the CardioMEMS™ system in an outpatient setting, in patients with HFrEF. LVGLS was independently associated with dPAP and CO values. To the best of our knowledge, this is the first study to address the association between LVGLS and invasive hemodynamic parameters in outpatients with HF, using the CardioMEMS™ system.
Given the higher risk of HFH and mortality associated with increased filling pressures and reduction in CO in patients with HFrEF,3,4 it is valuable for clinical practice to have both invasive and noninvasive diagnostic tools that allow a correct estimation of these hemodynamic parameters. In fact, noninvasive approaches have been already utilized in daily practice to monitor congestion and reduce HFH, but still have limitations and may not be entirely effective for optimal HFH prevention.21 Furthermore, given the high cost and limited availability of CardioMEMS™, we believe that recognizing a semi-automated echocardiographic method that demonstrates a good correlation with invasive dPAP and CO measurements in an outpatient setting would be of significant value for clinical practice.
In the univariate analysis there was a significant correlation between LVGLS and both dPAP and CO values. After multiple linear regression, the LVGLS-based prediction model showed that LVGLS and echocardiographic-derived sPAP estimation were independent predictors of invasive dPAP measured by CardioMEMS™ system, thus explaining the 60% of dPAP value. Although it is not a strong prediction model, we consider the independent association between LVGLS and dPAP particularly relevant for clinical practice, since dPAP is a major surrogate of filling pressures and changes in dPAP are independent predictors of mortality in HF.22–24 Moreover, the importance of monitoring dPAP in clinical practice is highlighted by the fact that invasive remote monitoring using the CardioMEMS™ is mainly based on dPAP pre-specified target values, and such strategy is associated with a reduction in HFH and improvement in quality of life.24–26 Another observation from our study that reinforces the value of LVGLS was that a variation in LVGLS value between consecutive TTE studies was significantly correlated with a variation in corresponding dPAP values. To the best of our knowledge, these data had not been reported previously. This correlation emphasizes the dynamic nature of LVGLS in reflecting changes in LV function and its direct impact on PA pressures, making it a valuable indicator for monitoring and managing this condition.
It is noteworthy that although the primary aim of our analysis was to evaluate the association between LVGLS and invasive hemodynamic parameters, we also observed that other echocardiographic parameters commonly used to estimate LV filling pressures were correlated with dPAP: LA volume, echocardiographic-derived sPAP, peak E-wave velocity and E/A ratio (Table 3).27 The exception to this observation was the E/e′ ratio, which has been widely used to predict LV filling pressures, although in our sample this parameter was not correlated with invasive hemodynamic measurements. The presence of mitral valve disease with heavy annular calcification, coronary artery disease with regional dysfunction at the sampled segments and conduction abnormalities or ventricular pacing are common in HFrEF patients and may explain the lack of association between E/e′ and invasive hemodynamics in our sample.27,28 Tolia et al.29 demonstrated a significant correlation between dPAP measured using the CardioMEMS™ and estimated left atrial pressure (LAP), calculated by taking the E/e′ and applying the Nagueh formula (LAP=1.24×(E/e′)+1.9) in 17 subjects. Nevertheless, in this cohort, patients with the presence of mitral prosthesis, severe annular calcification and permanent AF were excluded, which may affect the generalizability of the results and utility of this approach in non-selected HFrEF patients. Furthermore, in our sample only four patients were on AF rhythm during TTE study, although 42% of our patients had a previous history of AF. This is particularly relevant, given that the presence of AF may have an impact on the variability of different echocardiographic measurements, including diastolic function parameters. These findings highlight the importance of LVGLS as an easy, reliable, and reproducible alternative tool to assess filling pressures in this setting.30,31
Cardiac output is a very important measure in the pathophysiology of HFrEF patients and a major prognostic determinant in HF.17 Kažukauskienė et al.13 demonstrated a non-significant trend of correlation between LVGLS and invasive measurement of cardiac index in patients hospitalized with non-ischemic dilated cardiomyopathy. However, our study was focused on a different clinical scenario. In fact, CO invasive measurements were taken in an outpatient setting using the CardioMEMS™ system in our study, as opposed to in-hospital RHC. In addition, our sample included a higher proportion of patients with ischemic dilated cardiomyopathy (75%), which may be more representative of outpatient HFrEF patients. Although LVGLS was a weak predictor of invasive CO, thus explaining only 36% of its value, the association between LVGLS and CO provides novel insights into the clinical and noninvasive assessment of patients with HFrEF and reinforces the value LVGLS in such a clinical context. The results of this study contribute to a better understanding of the relationship between LVGLS and outpatient hemodynamics in HF patients, including both LV filling pressures and CO. As LVGLS is a major marker of myocardial systolic function, it appears that it not only provides valuable information regarding CO, but also regarding LV filling pressures. Moreover, repeated LVGLS measurements may be valuable in an outpatient setting as they may be associated with changes in filling pressures in patients with HFrEF.
This study has some limitations that should be acknowledged. The sample size was small and further studies with larger sample sizes are needed to confirm our findings. In fact, both of our final models had a statistical power <0.80, which may have been influenced by the small sample size. Still, the dPAP model had a 62% chance and the CO model had a 73% chance that the statistical test would detect a true effect in our population, which we believe is relevant for our clinical practice, and serves as hypothesis generator for future research. Nevertheless, we acknowledge it is essential to interpret these findings cautiously considering the limitations of the study analysis. Moreover, this was a single-center study, which may limit the generalizability of the results. The results could be extrapolated only to patients with HFrEF with prior HFH, which may represent a more severe subgroup of patients with HF. In addition, we opted to test only echocardiographic parameters for model prediction, as we did not include other potential relevant clinical or laboratory variables in the analysis. However, we wanted to assess the value of LVGLS, among a variety of other echocardiographic variables, to predict invasive hemodynamic parameters.
ConclusionsIn a cohort of patients with HFrEF, LVGLS was independently associated with invasive dPAP and CO, as assessed using the CardioMEMS™ system, in an outpatient setting.
Ethical approvalEthics Committee of Centro Hospitalar Universitário de Lisboa Central Observational study.
Conflict of interestsThe authors have no conflicts of interest to declare.