Myocardial performance may be impaired in cytokine-mediated immune reactions. The myocardial performance index (MPI) is a practical parameter that reflects systolic and diastolic cardiac function. We aimed to assess the MPI in patients with COVID-19.
MethodsThe study population consisted of 40 healthy controls and 40 patients diagnosed with COVID-19 who had mild pneumonia and did not need intensive care treatment. All participants underwent echocardiographic examination. First, the MPI and laboratory parameters were compared between healthy controls and patients in the acute period of infection. Second, the MPI and laboratory parameters were compared between the acute infection period and after clinical recovery.
ResultsCompared with healthy controls, patients with COVID-19 had a significantly higher MPI (0.56±0.09 vs. 0.41±0.06, p<0.001), longer isovolumic relaxation time (IRT) (112.3±13.4 vs. 90.6±11.2 ms, p<0.001), longer deceleration time (DT) (182.1±30.6 vs. 160.8±42.7 ms, p=0.003), shorter ejection time (ET) (279.6±20.3 vs. 299.6±34.7 ms, p<0.001) and higher E/A ratio (1.53±0.7 vs. 1.21±0.3, p<0.001). Statistically significantly higher MPI (0.56±0.09 vs. 0.44±0.07, p<0.001), longer IRT (112.3±13.4 vs. 91.8±12.1 ms, p<0.001), longer DT (182.1±30.6 vs. 161.5±43.5 ms, p=0.003), shorter ET 279.6±20.3 vs. 298.8±36.8 ms, p<0.001) and higher E/A ratio (1.53±0.7 vs. 1.22±0.4, p<0.001) were observed during the acute infection period than after clinical recovery. Left ventricular ejection fraction was similar in the controls, during the acute infection period and after clinical recovery.
ConclusionsSubclinical diastolic impairment without systolic involvement may be observed in patients with COVID-19. This impairment may be reversible on clinical recovery.
O desempenho miocárdico pode ser prejudicado em reações imunes mediadas por citocinas. O índice de performance miocárdico (IPM) é um parâmetro que reflete a função cardíaca sistólica e diastólica. O nosso objetivo foi avaliar o IPM em doentes com COVID-19.
MétodosO presente estudo consistiu em analisar 40 casos controlo saudáveis e 40 doentes com diagnóstico de COVID-19 que apresentavam pneumonia ligeira e não necessitavam de tratamento intensivo. Todos os participantes foram submetidos a avaliação ecocardiográfica. Primeiro, o IPM e os parâmetros laboratoriais foram comparados entre os casos controlo saudáveis e os doentes com período agudo de infeção. Em segundo lugar, o IPM e os parâmetros laboratoriais foram comparados entre o período agudo de infeção e após a recuperação clínica.
ResultadosEm comparação com os casos controlo saudáveis, os doentes com COVID-19 tiveram um IPM significativamente maior (0,56±0,09 versus 0,41±0,06, p<0,001), tempo de relaxamento isovolumétrico (TRI) mais longo (112,3±13,4 versus 90,6±11, 2 ms, p<0,001), tempo de desaceleração maior (TD) (182,1±30,6 versus 160,8±42, 7 ms, p=0,003), tempo de ejeção (TE) menor (279,6±20,3 versus 299,6±34, 7 ms, p<0,001) e razão E/A maior (1,53±0,7 versus 1,21±0,3, p<0,001). Um IPM superior estatisticamente significativo (0,56±0,09 versus 0,44±0,07, p<0,001), um TRI mais longo (112,3±13,4 versus 91,8±12, 1 ms, p<0,001), um TD mais longo (182,1±30,6 versus 161,5±43,5 ms, p=0,003), um TE mais curto (279,6±20,3 versus 298,8±36,8 ms, p<0,001) e razão E/A mais elevada (1,53±0,7 versus 1,22±0,4, p<0,001) foram observados durante o período agudo de infeção em comparação com aqueles após a recuperação clínica. A fração de ejeção do ventrículo esquerdo foi semelhante nos casos controlo saudáveis, período agudo de infeção e após a recuperação clínica.
ConclusãoA disfunçao diastólica subclínica sem difunção sistólica pode ser observada em doentes com COVID-19. Esta deficiência pode ser reversível na recuperação clínica.
Wuhan, China, became the center of an epidemic of “unknown pneumonia” detected in December 2019.1,2 Blood samples, oral swabs, bronchoalveolar lavage fluid, and anal swabs collected from patients with severe pneumonia were analyzed. This novel pathogen, an enveloped RNA beta-coronavirus, was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by this virus was called coronavirus disease 2019 (COVID-19). The COVID-19 pandemic has emerged as a public health crisis of global proportions.3 COVID-19 typically presents with respiratory tract symptoms including fever, dry cough, and dyspnea. The disease can progress to pneumonia and acute respiratory distress syndrome.4 The pathophysiology of COVID-19 infection is not clear. However, an extreme immune response and cytokine storm are thought to play pivotal roles in severe cases of the disease.4
Our knowledge of the cardiovascular effects of COVID-19 is limited. Studies have reported that left and right ventricular ejection fraction may be reduced by a cytokine-mediated systemic response to infection through increases in the end-diastolic and end-systolic volumes of both ventricles.5 However, it is not known whether left ventricular (LV) systolic and diastolic dysfunction develops in patients with COVID-19.
Two-dimensional and Doppler echocardiography provide important information about LV diastolic and systolic function. The myocardial performance index (MPI) is a reliable echocardiographic index that combines diastolic and systolic measurements.6 In this study, we aimed to assess left ventricular systolic and diastolic function in COVID-19 patients using MPI.
MethodsStudy populationThe present study enrolled 40 healthy control subjects and 40 patients diagnosed with COVID-19 who had mild pneumonia (hospitalized, requiring supplemental oxygen but not needing intensive care, invasive or non-invasive ventilation or high-flow oxygen devices). Patients with atrioventricular conduction disturbances, non-sinus rhythm on ECG, ventricular extrasystoles, valvular heart disease, or a history of chronic heart failure, chronic rheumatic heart disease, atrial dysrhythmia, permanent cardiac pacemaker, or acute coronary syndrome, were excluded from the study. The clinical history and laboratory data of patients and results of reverse transcription-polymerase chain reaction (RT-PCR) of oropharyngeal swabs were assessed. All patients had a positive RT-PCR result. All patients with COVID-19 were treated with vitamin C, hydroxychloroquine, and/or favipiravir. All participants were recruited from our hospital.
This study was conducted prospectively according to the principles of the Declaration of Helsinki and the study protocol was approved by the institutional medical ethics committee (2020/5-25). Written informed consent was obtained from all participants.
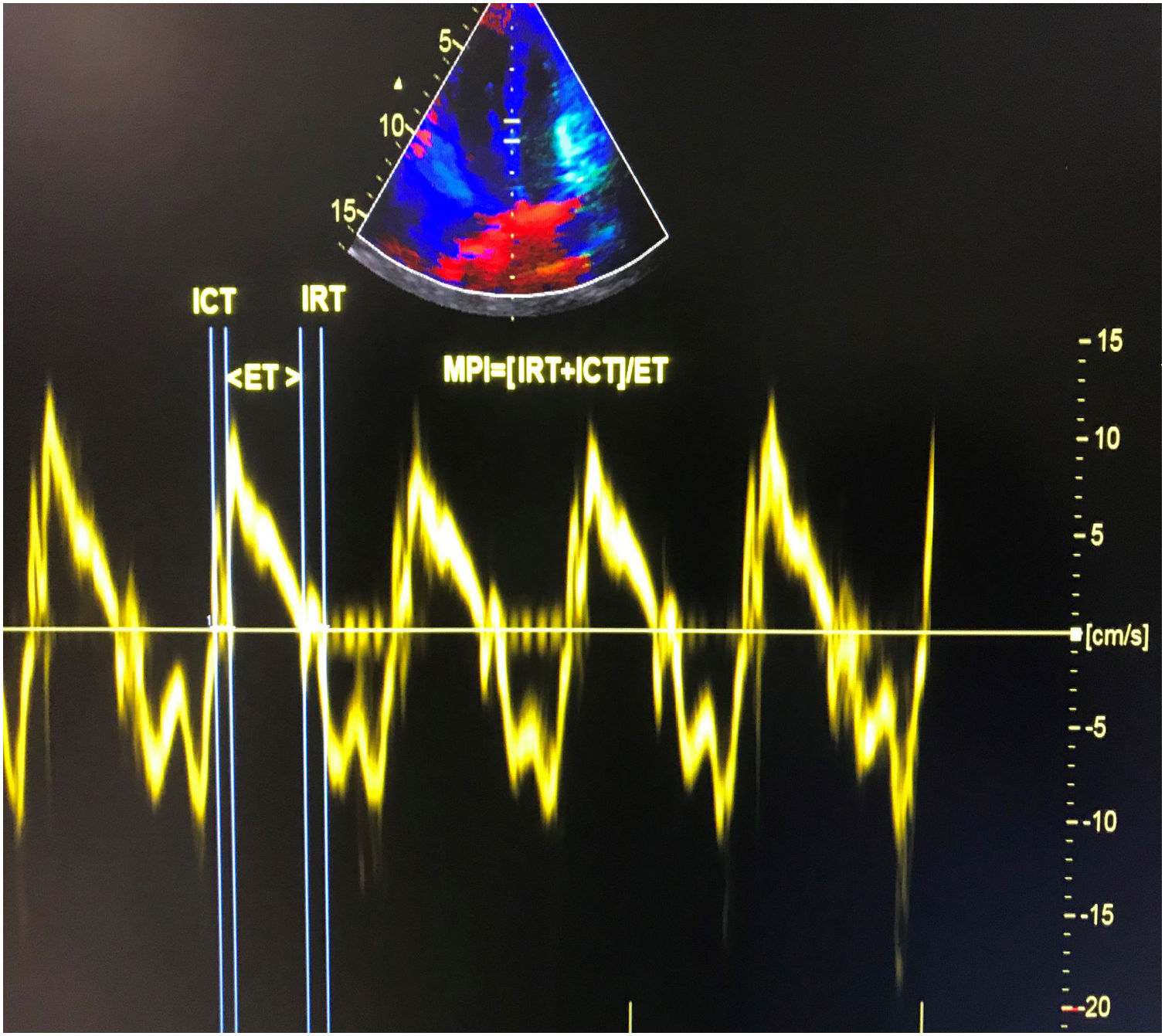
Echocardiographic examinationTransthoracic echocardiographic examinations were performed in all subjects using a Vivid 5S (GE Healthcare Systems, Horten, Norway) with a 2.0-3.5 MHz transducer. The exam was performed at the acute stage of infection and after clinical recovery in patients with COVID-19. The recovery echocardiography exam was performed on patients who were still hospitalized but had recovered both symptomatically and according to laboratory findings. The mean time between baseline and recovery echocardiography exams was 28±3.4 days. Doppler and two-dimensional echocardiography were performed in standard views according to the guidelines of the American Society of Echocardiography.7 The modified Simpson's method was used to calculate left ventricular ejection fraction (LVEF).8 Apical 4-chamber views were obtained by positioning the sample volume between the tips of the mitral leaflets for pulsed wave Doppler recordings of mitral inflow velocities. Peak late (A) and early (E) transmitral filling velocities and the E/A ratio were measured as conventional Doppler indices. Isovolumic relaxation time (IRT) was defined as the time between closure of the aortic valve and opening of the mitral valve. Isovolumic contraction time (ICT) was defined as the time between closure of the mitral valve and opening of the aortic valve. Ejection time (ET) was defined as the time between aortic valve opening and closure on the LV outflow velocity profile. Pulsed wave tissue Doppler imaging was performed in apical 4-chamber view with a sample volume of 2 mm placed on the medial wall of the mitral annulus. High-frequency signals were excluded by adjusting filter settings. Doppler ultrasound scanning intervals were measured from the mitral annular velocity intervals. MPI was calculated using the equation: MPI=(ICT+IRT)/ET, where IRT is isovolumic relaxation time, ICT is isovolumic contraction time, and ET is ejection time.9 Tissue Doppler measurements were obtained from a mean of five consecutive cardiac cycles. The calculation of tissue Doppler-derived MPI is presented in Figure 1.
Laboratory parametersBlood samples were studied on day 0 for controls and patients in the acute period of infection and on days 7, 14, and 28 for RT-PCR testing and other laboratory parameters in patients with COVID-19. An immunofluorescence assay was used to obtain COVID-19 antibody titers and respiratory secretions were sent for RT-PCR testing. Cardiac troponin I (cTnI) was studied with Immulate (DPC, Bio) from blood samples. Lactate dehydrogenase (LDH), creatinine kinase (CK), and creatinine levels were analyzed using an Architect c8000 Chemistry analyzer (Abbott Diagnostics, Abbott Park, IL, USA) with commercial kits (Abbott Diagnostics). Hemoglobin and complete white blood cell counts (WBC), including neutrophil and lymphocyte counts, were determined using an automated CELL-DYN Ruby Hematology Analyzer (Abbott Diagnostics) and expressed as ×103 cells/mm3.
Statistical analysisIBM SPSS v. 22.0 (Chicago, IL, USA) was used for the analyses. Categorical variables are presented as numbers and percentages, and were compared with the chi-square test. Continuous variables were tested for normality of distribution with the Kolmogorov-Smirnov test. Continuous variables are presented as mean ± standard deviation. An independent t test or Mann-Whitney U test was used to compare variables between controls and patients in the acute infection period. Similarly, an independent t test or Mann-Whitney U test was used to compare variables between the acute infection period and after clinical recovery, as appropriate. The acute period of infection and clinical recovery values were compared using the paired t test if the data were normally distributed. A p-value below 0.05 was considered statistically significant.
ResultsThis study enrolled 40 consecutive patients (26 males (65%); mean age, 54±11 years) and 40 healthy control subjects (24 males (60%); mean age, 52±13 years). The patients were admitted to the hospital with fever >38°C, cough, sore throat, rhinorrhea, shortness of breath, headache, myalgia, and/or diarrhea. The PCR test was positive in all patients. The mean interval between the first echocardiogram and symptom onset was 9.3±5.5 days.
Clinical characteristics including age, gender, smoking status, hypertension, diabetes, systolic blood pressure, diastolic blood pressure, and heart rate were similar between patients and controls (p>0.05 for all). Compared with controls, COVID-19 patients had a significantly higher MPI (0.56±0.09 vs. 0.41±0.06, p<0.001), longer IRT (112.3±13.4 vs. 90.6±11.2 ms, p<0.001), longer deceleration time (182.1±30.6 vs. 160.8±42.7 ms, p=0.003), shorter ET (279.6±20.3 vs. 299.6±34.7 ms, p<0.001) and higher E/A ratio (1.53±0.7 vs. 1.21±0.3, p<0.001). LVEF was similar in COVID-19 patients and controls (60.3±3.2% vs. 61.9±4.8%, p>0.05), as was ICT (44.3±7.8 vs. 41.8±8.7 ms, p>0.05). Compared with controls, patients in the acute infection period had significantly higher cTnI (0.96±0.12 vs. 0.10±0.01 ng/ml, p<0.001), LDH (486.8±118.6 vs. 316.4±108.9 IU/l, p=0.002), WBC (12.3±4.7 vs. 7.0±2.6 103/μl, p<0.001) and neutrophil count (9.7±6.2 vs. 4.2±1.5 103/μl, p<0.001), and lower lymphocyte count (0.9±0.2 vs. 2.8±0.6 103/μl, p<0.001). Creatinine, CK and hemoglobin levels were similar in patients and controls (p>0.05 for all). Table 1 summarizes the clinical characteristics, laboratory and echocardiographic findings of patients in the acute period of COVID-19 infection and controls.
Clinical characteristics and laboratory and echocardiographic findings of the groups.
| COVID-19 patients (n=40) | Control group (n=40) | p | |
|---|---|---|---|
| Age, years | 54±11 | 52±13 | NS |
| Gender, male, n (%) | 26 (65) | 24 (60) | NS |
| Smoking, n (%) | 12 (30) | 10 (25) | NS |
| Hypertension, n (%) | 8 (20) | 6 (15) | NS |
| Diabetes, n (%) | 6 (15) | 4 (10) | NS |
| Systolic BP, mmHg | 124.6±8.2 | 123.6±6.8 | NS |
| Diastolic BP, mmHg | 73.6± 6.0 | 72.5±4.6 | NS |
| Heart rate, beats/min | 75.4±12 | 73.6± 16 | NS |
| LVEF, % | 60.3± 3.2 | 61.9±4.8 | NS |
| MPI | 0.56±0.09 | 0.41±0.06 | <0.001 |
| IRT, ms | 112.3±13.4 | 90.6±11.2 | <0.001 |
| ICT, ms | 44.3±7.8 | 41.8±8.7 | NS |
| E/A | 1.53±0.7 | 1.21±0.3 | <0.001 |
| ET, ms | 279.6±20.3 | 299.6±34.7 | <0.001 |
| DT, ms | 182.1±30.6 | 160.8±42.7 | 0.003 |
| Hb, g/dl | 13.2±1.6 | 13.8±1.7 | NS |
| CK, IU/l | 216.3±125.4 | 198.2±113.6 | NS |
| LDH, IU/l | 486.8±118.6 | 316.4±108.9 | 0.002 |
| cTnI, ng/ml | 0.96±0.12 | 0.10±0.01 | <0.001 |
| Creatinine, mg/dl | 1.2±0.6 | 1.0±0.7 | NS |
| WBC, 103/μl | 12.3±4.7 | 7.0±2.6 | <0.001 |
| Neutrophil count, 103/μl | 9.7±6.2 | 4.2±1.5 | <0.001 |
| Lymphocyte count, 103/μl | 0.9±0.2 | 2.8±0.6 | <0.001 |
BP: blood pressure; CK: creatinine kinase; cTnI: cardiac troponin I; DT: deceleration time; E/A:transmitral early to late peak velocity ratio; ET: ejection time; ICT: isovolumic contraction time; IRT: isovolumic relaxation time; Hb: hemoglobin; LDH: lactate dehydrogenase; LVEF: left ventricular ejection fraction; MPI: myocardial performance index; NS: not significant; WBC: white blood cell count.
Systolic and diastolic blood pressure and heart rate were similar between the acute period of infection and after clinical recovery (p>0.05 for all). Compared with the acute infection period, after clinical recovery there was significantly lower MPI (0.44±0.07 vs. 0.56±0.09, p<0.001), shorter IRT (91.8±12.1 vs. 112.3±13.4 ms, p<0.001), shorter deceleration time (161.5±43.5 vs. 182.1±30.6 ms, p=0.003), longer ET (298.8±36.8 vs. 279.6±20.3 ms, p<0.001) and lower E/A ratio (1.22±0.6 vs. 1.53±0.7, p<0.001). LVEF was similar in the acute period of infection and after clinical recovery (60.3±3.2% vs. 60.7±3.8%, p>0.05), as was ICT (44.3±7.8 vs. 40.6±9.7ms, p>0.05). Compared with the acute period of COVID-19 infection, after clinical recovery there was significantly lower cTnI (0.11±0.08 vs. 0.96±0.12 ng/ml, p<0.001), LDH (334.5±110.9 vs. 486.8±118.6 IU/l, p=0.004), WBC (7.2±3.1 vs. 12.3±4.7 103/μl, p<0.001) and neutrophil count (4.5±1.8 vs. 9.7±6.2 103/μl, p<0.001) and higher lymphocyte count (2.1±0.4 vs. 0.9±0.2 103/μl, p<0.001). Creatinine, CK and hemoglobin levels were similar between the acute period of infection and after clinical recovery (p>0.05 for all). Table 2 presents the clinical characteristics, laboratory and echocardiographic findings of patients in the acute period of COVID-19 infection and after clinical recovery.
Clinical characteristics and laboratory and echocardiographic findings.
| Acute period of infection (n=40) | After clinical recovery (n=40) | p | |
|---|---|---|---|
| Systolic BP, mmHg | 124.6±8.2 | 126.5±7.1 | NS |
| Diastolic BP, mmHg | 73.6±6.0 | 74.7±4.2 | NS |
| Heart rate, beats/min | 75.4±12 | 72.8±14 | NS |
| LVEF, % | 60.3±3.2 | 60.7±3.8 | NS |
| MPI | 0.56±0.09 | 0.44±0.07 | <0.001 |
| IRT, ms | 112.3±13.4 | 91.8±12.1 | <0.001 |
| ICT, ms | 44.3±7.8 | 40.6±9.7 | NS |
| E/A | 1.53±0.7 | 1.22±0.6 | <0.001 |
| ET, ms | 279.6±20.3 | 298.8±36.8 | <0.001 |
| DT, ms | 182.1±30.6 | 161.5±43.5 | 0.003 |
| Hb, g/dl | 13.2±1.6 | 13.4±1.8 | NS |
| CK, IU/l | 216.3±125.4 | 195.7±115.2 | NS |
| LDH, IU/l | 486.8±118.6 | 334.5±110.9 | 0.004 |
| cTnI, ng/ml | 0.96±0.12 | 0.11±0.08 | <0.001 |
| Creatinine, mg/dl | 1.2±0.6 | 1.1±0.4 | NS |
| WBC, 103/μl | 12.3±4.7 | 7.2±3.1 | <0.001 |
| Neutrophil count, 103/μl | 9.7±6.2 | 4.5±1.8 | <0.001 |
| Lymphocyte count, 103/μl | 0.9±0.2 | 2.1±0.4 | <0.001 |
BP: blood pressure; CK: creatinine kinase; cTnI: cardiac troponin I; DT: deceleration time; E/A:transmitral early to late peak velocity ratio; ET: ejection time; Hb: hemoglobin; ICT: isovolumic contraction time; IRT: isovolumic relaxation time; LDH: lactate dehydrogenase; LVEF: left ventricular ejection fraction; MPI: myocardial performance index; NS: not significant; WBC: white blood cell count.
This is the first echocardiographic follow-up study to assess left ventricular systolic and diastolic function in COVID-19 patients. Our data showed that LV performance was impaired subclinically during the acute period of COVID-19 and that this impaired performance recovered with clinical improvement.
Since the first case was reported at the end of 2019, the COVID-19 outbreak has become a pandemic. The high transmission ratio of the virus has made it a threat to public health globally. Coronaviruses are non-segmented positive-sense RNA viruses that are broadly distributed in humans and other mammals, such as camels, bats, mice, dogs, and cats.10 Coronaviruses can cause respiratory, enteric, cardiovascular, and neurological disease.11–14 A 2020 report by the China Medical Treatment Expert Group for COVID-19 showed the spectrum of clinical and diagnostic features associated with SARS-CoV-2 infection among Chinese patients.15 According to this report, the most common symptoms in patients with COVID-19 were fever (in 88.7% of patients during hospitalization) and cough (in 67.8% of patients), as well as headache, fatigue, or shortness of breath. In our study, in agreement with previous data, fever was present in 84.2% of the patients on admission. The second most common symptom was cough (60.4% of patients). The overall mortality rate is 2.3% and those with pre-existing comorbid conditions have higher case mortality rates: diabetes 7.3%, chronic respiratory disease 6.3%, hypertension 6.0% cardiovascular disease 10.5%, and cancer 5.6%.16
We assessed left ventricular diastolic and systolic function using several echocardiographic parameters. Although systolic and diastolic dysfunction appear together, there are few Doppler echocardiographic variables that combine systolic and diastolic measurements. We used the MPI, which includes the LV systolic contraction and diastolic relaxation periods.17 This index was first used by Tei et al. to predict LV systolic and diastolic function noninvasively.9 MPI is not significantly affected by preload, afterload, sample volume location, age, or rhythm18,19 and is therefore a reliable index. The value of MPI in LV dysfunction has been confirmed in patients with symptomatic heart failure of non-ischemic or ischemic etiology and idiopathic dilated cardiomyopathy.17,18 We aimed to assess LV systolic and diastolic function in COVID-19 patients using MPI. In the present study, MPI was higher in the acute disease period in patients with COVID-19, but regressed significantly after clinical recovery. Previous reports showed that systolic function is affected in COVID-19 patients. However, there is limited information about LV diastolic function in these patients. Our study showed that not only systolic but also diastolic function may be impaired in COVID-19 patients. In other words, COVID-19 patients may develop subclinical diastolic impairment without systolic involvement.
The pathophysiological and cardiovascular effects of COVID-19 are not clear. However, the disease is thought to be associated with an excessive immune response triggered by the virus. Studies have found that COVID-19 patients have high levels of interleukin (IL)-1 beta, IL-6, interferon gamma, and monocyte chemoattractant protein-1, which probably leads to an activated type 1 helper T cell response.4 Studies of coronavirus and influenza outbreaks suggest that viral infections can trigger a systemic inflammatory response that causes acute coronary syndromes, arrhythmias, and heart failure.20–23 Similarly, underlying cardiovascular disease may be exacerbated or new cardiac disease may be induced during COVID-19. Acute coronary events may be due to increased myocardial demand triggered by infections that accelerate myocardial damage or infarction. Alternatively, circulating cytokines released during severe systemic inflammatory stress may cause instability and rupture of atherosclerotic plaque. Patients with heart failure are also prone to hemodynamic decompensation during the stress of severe infections. During most influenza outbreaks, more patients die of cardiovascular causes than from pneumonia.24 COVID-19 causes myocardial inflammation and myocarditis.25,26 The elevated cTnI in our patients might reflect myocardial damage. cTnI is part of the contractile apparatus in cardiomyocytes and is expressed only in the heart.27-29 As a result of its organ specificity, cTnI can be used to diagnose cardiomyocyte damage.28,29 The elevated LDH observed may reflect tissue damage and inflammation.
We assumed that the temporary impairment in echocardiographic parameters in the acute phase was caused by proinflammatory mediators, such as tumor necrosis factor (TNF) and the IL-1 and -6 families of cytokines, which have significant negative inotropic effects.30 Myocardial function may also be impaired through direct COVID-19 infection of myocytes, leading to active myocarditis. Reported data on the severity, extent, and short- and long-term cardiovascular effects of COVID-19 are limited.
Our study has some limitations, the main one of which is its small sample size. Another limitation is that circulating levels of cytokines such as IL-1, IL-6, and TNF could not be measured. In addition, the lack of clarity concerning the effects of hydroxychloroquine and favipiravir on cardiac function are also limitations of the study. Further studies that measure the circulating levels of these cytokines and echocardiographic variables are needed based on the current hypothesis of cytokine-mediated temporary impairment of left ventricular performance.
In conclusion, subclinical diastolic impairment without systolic involvement may be observed in patients with COVID-19. This could be due to isolated subclinical diastolic dysfunction. The underlying mechanism and its clinical significance can be established by further studies.
FundingThis study received no grant funding from any agency in the public, commercial or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.