Digital health interventions including telehealth, mobile health, artificial intelligence, big data, robotics, extended reality, computational and high-fidelity bench simulations are an integral part of the path toward precision medicine. Current applications encompass risk factor modification, chronic disease management, clinical decision support, diagnostics interpretation, preprocedural planning, evidence generation, education, and training. Despite the acknowledged potential, their development and implementation have faced several challenges and constraints, meaning few digital health tools have reached daily clinical practice. As a result, the Portuguese Society of Cardiology Study Group on Digital Health set out to outline the main digital health applications, address some of the roadblocks hampering large-scale deployment, and discuss future directions in support of cardiovascular health at large.
As ferramentas de saúde digital (por exemplo, telemedicina, mobile health, inteligência artificial, big data, robótica, extended reality, simulações computacionais e simulações de bancada de alta-fidelidade) são parte integrante do caminho rumo à medicina de precisão. A sua aplicação cobre várias áreas, tais como: modificação de fatores de risco, gestão de doenças crónicas, apoio à decisão clínica, interpretação de meios complementares de diagnóstico, planeamento de procedimentos, investigação, educação e formação. Apesar do reconhecido potencial, o seu desenvolvimento e implementação tem enfrentado vários desafios e constrangimentos, o que faz com que o número de ferramentas de saúde digital utilizado na prática clínica diária seja reduzido. Assim, o Grupo de Estudo de Saúde Digital da Sociedade Portuguesa de Cardiologia pretende delinear as principais aplicações das ferramentas de saúde digital na medicina cardiovascular, abordar alguns dos obstáculos que dificultam a sua implantação em larga escala e discutir perspetivas futuras na promoção da saúde cardiovascular.
As the population ages and the burden of cardiovascular (CV) disease continues to grow, increasing strain is placed on healthcare systems.1,2 The diagnostic and therapeutic advances made in recent years are noteworthy, yet their translation into day-to-day practice is often hampered by limited trial generalizability, inequitable access, financial constraints, shortage/uneven distribution of health professionals, and inefficient organization systems.3,4
Digital health interventions (DHI) have the potential to help mitigate some of these shortcomings and improve outcomes across the spectrum of CV care.5 From prevention to treatment, these strategies can contribute to timely high-quality care delivery by extending access,6 enhancing adherence,7 increasing patient engagement,8 providing evidence-based decision support,9 automating tasks,10 and streamlining research.11 Furthermore, through large-volume data mining and analysis, these technologies hold the promise of deepening our understanding of disease processes on an individual basis, leading to refined risk prediction and personalized management choices.12
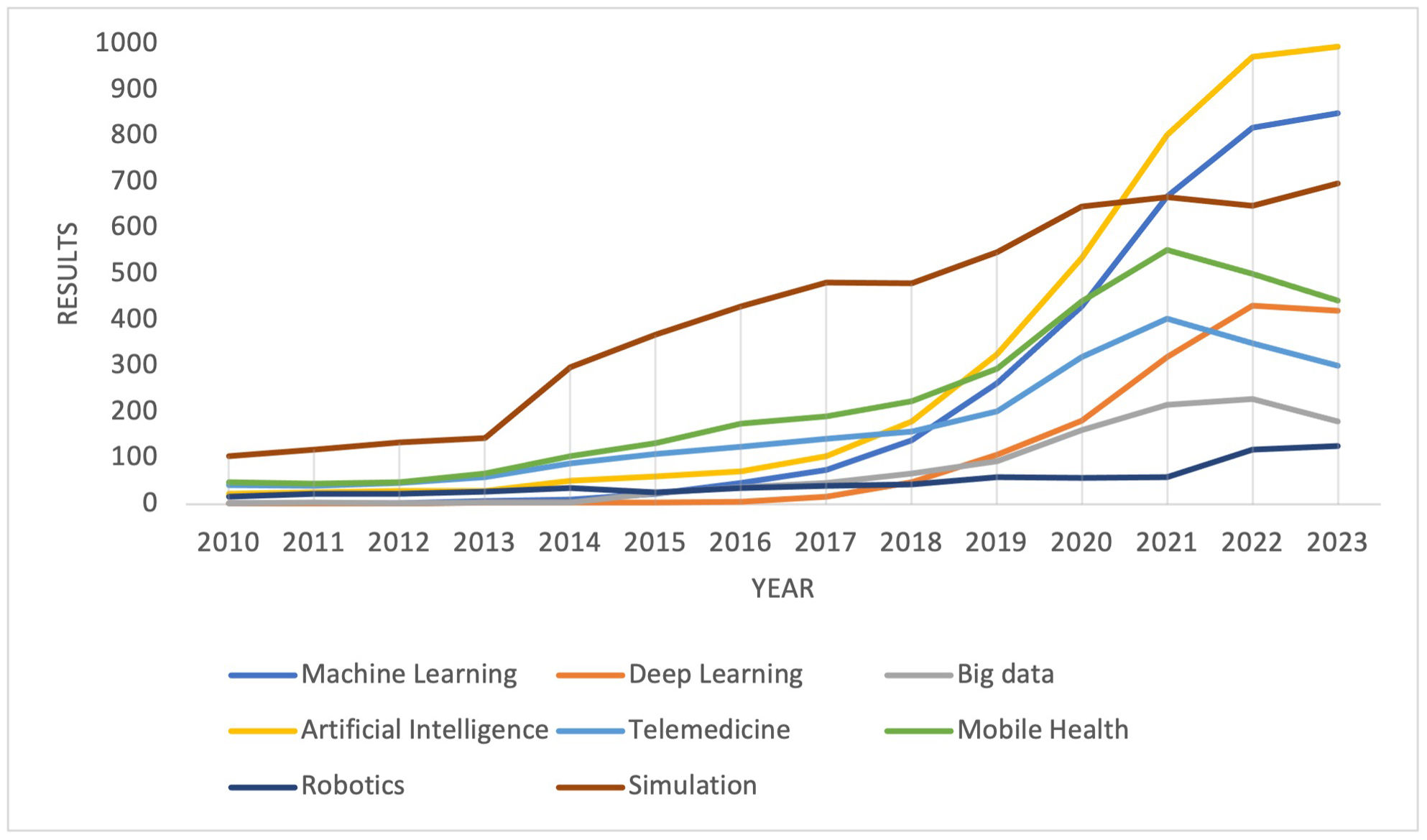
The development of digital health solutions is happening at an unprecedented scale, especially since the COVID-19 pandemic (Figure 1).13,14 However, uptake beyond telemedicine and electronic health records (EHR) has been slow, meaning DHI's immense potential remains largely untapped.15 Concerns over data security, interoperability, cost, and efficacy impede widespread adoption and should be addressed.16,17
In this paper, the Portuguese Society of Cardiology Study Group on Digital Health (‘Grupo de Estudos de Saúde Digital da Sociedade Portuguesa de Cardiologia’) highlights the main digital health applications and their impact on the CV field; reviews the most relevant roadblocks and pitfalls; and discusses future directions for improved CV care, research, and training.
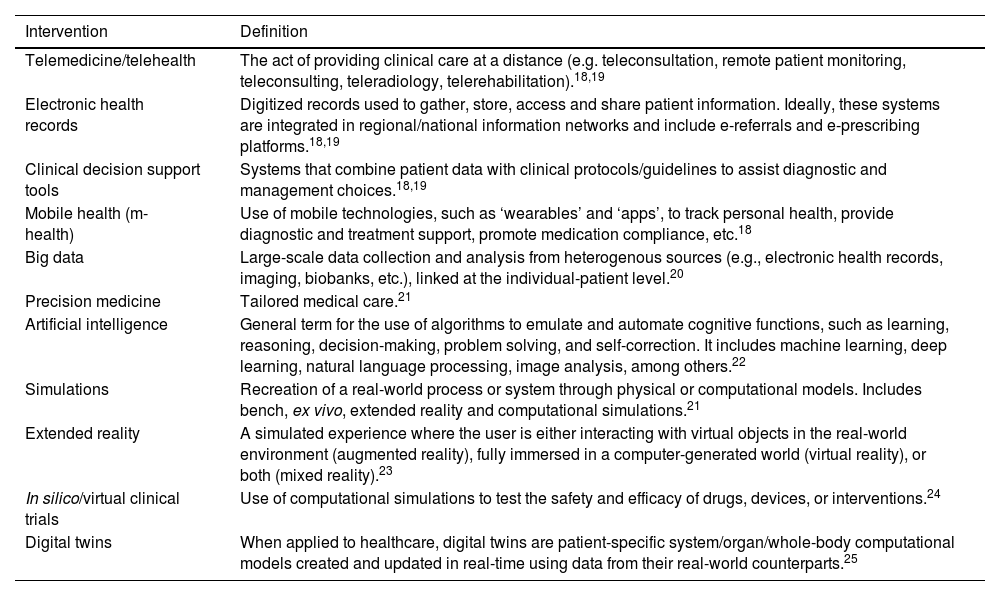
Key terms and definitionsDigital health is an umbrella term for the use of information and communication technologies for health-related matters, including patient care, health surveillance, education, and research.18 It encompasses various services and tools, some of which are highlighted in Table 1. Although we present them individually, it is important to note that these tools are often interdependent and have synergistic effects that will be discussed in the following section.
Examples of digital health interventions.
| Intervention | Definition |
|---|---|
| Telemedicine/telehealth | The act of providing clinical care at a distance (e.g. teleconsultation, remote patient monitoring, teleconsulting, teleradiology, telerehabilitation).18,19 |
| Electronic health records | Digitized records used to gather, store, access and share patient information. Ideally, these systems are integrated in regional/national information networks and include e-referrals and e-prescribing platforms.18,19 |
| Clinical decision support tools | Systems that combine patient data with clinical protocols/guidelines to assist diagnostic and management choices.18,19 |
| Mobile health (m-health) | Use of mobile technologies, such as ‘wearables’ and ‘apps’, to track personal health, provide diagnostic and treatment support, promote medication compliance, etc.18 |
| Big data | Large-scale data collection and analysis from heterogenous sources (e.g., electronic health records, imaging, biobanks, etc.), linked at the individual-patient level.20 |
| Precision medicine | Tailored medical care.21 |
| Artificial intelligence | General term for the use of algorithms to emulate and automate cognitive functions, such as learning, reasoning, decision-making, problem solving, and self-correction. It includes machine learning, deep learning, natural language processing, image analysis, among others.22 |
| Simulations | Recreation of a real-world process or system through physical or computational models. Includes bench, ex vivo, extended reality and computational simulations.21 |
| Extended reality | A simulated experience where the user is either interacting with virtual objects in the real-world environment (augmented reality), fully immersed in a computer-generated world (virtual reality), or both (mixed reality).23 |
| In silico/virtual clinical trials | Use of computational simulations to test the safety and efficacy of drugs, devices, or interventions.24 |
| Digital twins | When applied to healthcare, digital twins are patient-specific system/organ/whole-body computational models created and updated in real-time using data from their real-world counterparts.25 |
Telehealth and m-health technologies allow patients to assume a more proactive role in the management of their health.15 By fading time and space boundaries, they offer the opportunity to continue care outside the clinic or hospital ward, benefiting both patients and healthcare systems.26 Here we provide some examples of their usefulness in four key areas: wellness promotion, risk factor modification, medication compliance and chronic disease management.
Cardiovascular wellness promotionContemporary devices (e.g. smartwatches, chest straps, sensor-embedded clothing, etc.) can measure several physiological parameters, including heart rate, blood pressure, oxygen saturation (photoplethysmography); movement and its intensity (accelerometer, barometer and gyroscope); single and multi-lead electrocardiograms (ECG).27 Advanced signal processing algorithms allow assessment of more complex parameters including sleep, six-minute walk test, maximum oxygen consumption, heart rhythm, falls, and so forth. Through positive reinforcement, gamification and other behavioral science approaches, this data can be leveraged to encourage positive lifestyle changes, such as increasing physical activity, for example.27 Conversely, these consumer-directed products also come with the risk of overmedicalizing normal physiology, which may lead to futile (and even harmful) diagnostics and treatments.28 Moreover, this means an avalanche of data is coming. Although it is good to have more information available for the decision-making process, its utilization within current clinic time constraints is challenging. Further refinements and swift integration in the EHR infrastructure are required.15
Risk factor modification and medication complianceThe same principles apply to risk factor management. Patients and physicians can track relevant data (e.g. blood pressure, weight, physical activity, glycemia, lipid levels, etc.) at a distance and act on it promptly without any visit to the clinic. Mobile phone-based interventions have demonstrated meaningful improvement in blood pressure control,29 smoking cessation rates,30 body mass index29 and medication adherence.31 For example, the MedApp-CHD randomized clinical trial showed that medication reminder apps improved medication adherence in patients with coronary heart disease.32 Interestingly, in this study, advanced app features did not provide incremental value compared to the basic app design.32 Similarly, the HERB-DH1 randomized controlled trial also provided evidence of added value in hypertension management with the use of a personalized non-pharmacologic program generated by a smartphone app.33 On the other hand, in the CONNECT trial, a consumer ‘app’ connected to the primary care EHR failed to increase medication adherence and blood pressure control.34 Taken together, data from preliminary trials and meta-analyses suggest that these interventions benefit people with CV disease, although the effect sizes are modest and their durability remains unknown.35 Importantly, like any other intervention, DHI can only be effective if they are utilized. Factors such as simplicity of use, personalization, and privacy policies should be carefully considered to maximize potential benefit.17
Chronic disease monitoring and managementRemote monitoring is particularly important in chronic conditions, such as heart failure (HF), where early detection of clinical worsening and telemanagement are crucial to improve quality of life, reduce hospitalizations and lower mortality rates.36,37 Virtual consultations, ‘apps’ and ‘wearables’ can be used to track relevant invasive and non-invasive parameters (signs and symptoms,38 thoracic impedance,39,40 pulmonary artery pressure,41,42 lung fluid content,43 etc.) and facilitate timely interventions (e.g. diuretic adjustment). In the IN-TIME and MultiSENSE trials multiparameter remote monitoring via implantable cardiac devices led to significant reductions in hospitalization and symptom improvement.40,44 Similarly, non-invasive lung water content monitoring (via dielectric sensing) bared substantial reduction of the rehospitalization rate in early observational data (87% less hospitalizations compared with the 90 days prior to enrollment).43 A follow-up clinical trial is ongoing (NCT03586336) as the search for the optimal, ideally non-invasive, indicator(s) of volume status continues.
Additional challenges in HF management include rapid initiation and up-titration of guideline-directed medical therapy. For example, the high-intensity care model of the STRONG-HF trial45 is difficult to replicate in real-world clinical settings. Teleconsultations and EHR-based alert systems may also help accelerate the adoption of proven therapies.46 Virtual consultations are one of the most used and one of the most well received DHI (by patients and physicians).47,48 Of note, besides facilitating patient encounters, virtual consultations also enable clinician to clinician interaction for consults and second opinion.15
Importantly, m-health is also playing a meaningful role in research. For example, the authors of the recent ORBITA-2 and the upcoming ORBITA-COSMIC and ORBITA-STAR trials developed an app to assess daily angina burden in a cost and time-effective manner that reduces the recall bias associated with measurement of such outcomes.49,50
Photoplethysmography-based heart rhythm and rate monitoring was used during COVID-19 to remotely follow-up atrial fibrillation (AF) patients.51 This app-based interface enabled 25 European centers to manage the data of 1480 patients remotely, including post-AF ablation. In the future, such technologies, as a supplement to traditional face-to-face consultations, may improve patient surveillance, avoid unnecessary hospital visits, and optimize scarce clinical resources.
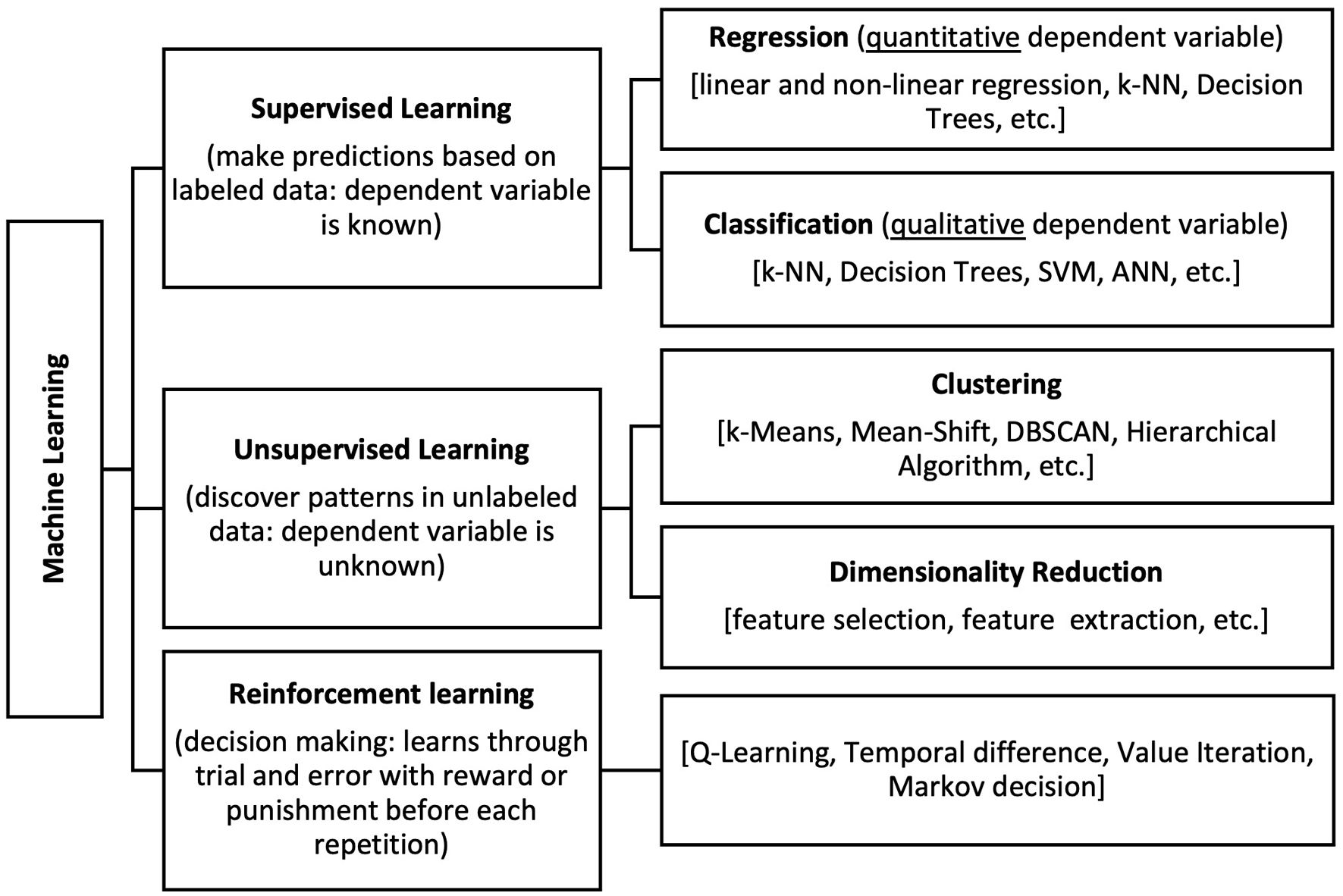
Artificial intelligence and big dataBig data is often defined by the five “vs”: volume (up to yottabytes), velocity (rapid generation and accumulation of new data), variety (diverse data formats and sources, e.g. clinical, omics, infodemiological, etc.), veracity (indeterminable; datasets are too vast to be verified), and value (what it adds to current knowledge).52 The latter is largely dependent on the use of advanced analytics, such as artificial intelligence (AI)/machine learning (Figure 2). Its ability to detect signals and patterns, beyond what a human can recognize, offers the opportunity to extract actionable information from these ever-growing datasets. Here we focus on some examples of the intersections between big data and AI, and their roles in cardiovascular medicine.
Widespread electrocardiogram (ECG) digitization and the development of these new algorithms that can sieve through massive amounts of raw data have revived the interest in automated ECG interpretation.53 Unlike initial versions that relied on discrete measurements and rule-based approaches, current models focus on pattern recognition at large. There are numerous successful examples in the literature, particularly with deep neural networks trained on large datasets of single and 12-lead ECGs, that at least match and even outperform experts.53,54 Moreover, these neural networks are also being used to uncover additional subtleties that allow them to determine the ejection fraction,55 hemoglobin value,56 age and sex,57 AF risk (while in sinus rhythm),58 presence of hypertrophic cardiomyopathy,59 pulmonary embolism,60 among many other conditions. In doing so, these algorithms augment the ECG's diagnostic scope and power. Given its wide availability, inexpensiveness and relative simplicity, AI-augmented ECG can be particularly useful in triage and follow-up, for example, of patients at risk for ventricular dysfunction, such as heart transplant recipients and those receiving cardiotoxic therapies. This strategy was successfully tested in the EAGLE trial, which demonstrated an increased detection of reduced ejection fraction in a primary care setting with AI-ECG.61 Furthermore, there is also room for the integration of these algorithms in mobile devices and wearables. Their ability to detect AF was demonstrated in the Apple Heart Study.62
Image acquisition, segmentation, and analysisArtificial intelligence models can systematize the acquisition, processing, and interpretation of both invasive and non-invasive cardiovascular images to boost diagnostic accuracy and reduce interobserver/interscan variability. Mor-Avi et al. demonstrated that an AI-embedded imaging tool can help novices acquire similar quality images to expert sonographers by providing instant feedback on the images’ diagnostic quality and suggesting specific gestures to mend it in real-time.63 Whereas software like Us2.AI© (Us2.AI, Singapore) can produce a complete report with accurate measurements and guideline-based interpretation of ultrasound images.64 Operator-dependency is one of imaging's main pitfalls. A paradigmatic example of this is the visual assessment of coronary stenosis.65,66 Automatic registration and segmentation of angiography images has been shown to reduce interobserver variability and improve the operators’ estimation of lesion severity, thus refining patient/lesion selection for revascularization.67–70 Moreover, AI-driven analysis can also obviate the need for further testing. For example, fractional flow reserve and instantaneous wave-free ratio derived from both invasive and non-invasive coronary angiography may abridge the need to use a pressure wire or invasive angiography all-together.71–74 Lastly, AI-based systems can also accurately merge different imaging modalities to create high-fidelity three-dimensional (3D) reconstructions of different structures that can improve our understanding of complex anatomies, assist procedural planning and guide interventions in real-time. The best-known example is probably the EchoNavigator® (Philips, The Netherlands) software that fuses fluoroscopy and echocardiography images to enhance the perception of the position of the catheters and devices being deployed relative to the surrounding heart structures.75
Natural language processing and chatbotsElectronic health records are increasingly recognized as a powerful tool to enhance the quality of medical care and clinical research.76 However, a significant portion of EHR involves free text that is not readily accessible for data abstraction and the intricate nature of plain language makes conventional rule-based methods prone to misclassification and bias.77 Deep learning-based natural language processing (NLP) models are emerging as more dependable alternatives to interrogate large volumes of free text and leverage its’ data for predictive analytics,78 patient identification/recruitment,79 among others. HF with preserved ejection fraction (HFpEF) is a challenging and often-missed diagnosis. Recently, a group from King's College London used an NLP pipeline to identify patients who were likely to have HFpEF.80 While only 311 patients (8.3%) had a formal clinical diagnosis of HFpEF, the NLP model identified 2811 more patients (75.4%) who met diagnostic criteria from the European guidelines.80 Although this group was younger and had fewer comorbidities, a higher five-year mortality rate was observed.80
In addition to understanding natural language, these models also produce it, usually in the form of chatbots. Two main capabilities of these chatbots apply to the medical field.81 The first is their ability to act as a scribe and produce clinical notes based on the transcript of the physician–patient encounter. The clinician still must proofread the chatbot's output, but a significant portion of the process is done automatically.81 Secondly, and more broadly, the ability to analyze the patient's data and come up with a diagnosis and treatment plan. In this case, the bots’ answer would be viewed as a ‘second opinion’, either validating one's decision or prompting revision or further investigation when there is no consensus. Although current iterations are far from being able to provide such support, this is the ultimate goal.82
Risk prediction and deep phenotypingWhereas traditional risk scoring systems only incorporate a limited set of variables, AI algorithms can integrate all physiologic information available for that patient. Between EHR, biobanks, imaging and ECG repositories, there is a wealth of data available to characterize each patients’ phenotype, determine individual risk of adverse outcomes, and the likelihood of suitable treatment response. HFpEF affects a very diverse group of patients. This heterogeneity has been proposed as one of the likely contributors to the multiple neutral trials in this patient subset.83 Hence, it has been a major focal point of several ‘phenomapping’ studies. Based on the TOPCAT cohort, Segar and colleagues used a finite mixture-model-based clustering to identify three phenogroups of HFpEF patients with distinct long-term outcomes.84 Patients in phenogroup one had significantly worse cardiometabolic features (higher body mass index, higher burden of diabetes mellitus, dyslipidemia, etc.) and outcomes, including all-cause mortality.84 Also within the HF sphere, similar approaches have been used, for example, to forecast the risk of rehospitalization85 and response to cardiac resynchronization therapy.86,87 From an adaptive boosting model, the PRAISE authors derived a score to predict major events (all-cause death, myocardial infarction, and major bleeding) at one-year post-acute coronary syndrome.88
SimulationsComputational, extended reality and high-fidelity bench simulations are emerging precision medicine enablers. These simulations allow for testing of virtually infinite treatment strategies in a timely and cost-effective way with zero risk for the patient. Potential applications include procedural planning/decision support, training, and research.89
Procedural planning and guidancePatient-specific simulations of different devices and/or procedures may help determine device size, optimal implantation technique, as well as predict the likelihood of future complications. For example, in left atrial appendage closure the incidence of peri-device leaks is not negligible and a recent meta-analysis found that, regardless of size, residual leaks detected by transesophageal echocardiography were associated with higher risk of thromboembolism, major bleeding, and all-cause mortality.90 Patient-specific 3D printing and computational modeling techniques may be useful to determine optimal device position and size.90,91 The recent PREDICT-LAA trial tested this strategy via HEARTguide (FEops, Belgium), a digital twin generator.92 Although the trial did not meet its primary endpoint (presence of grade 3–4 leak and/or device related thrombus) a trend toward less appendage patency and a significantly higher complete closure rate were observed. A similar software (TAVIguide, FEops, Belgium) has been tested in transcatheter aortic valve implantation (TAVI) planning.93 While it did not have a significant effect on valve sizing, it did affect the target depth of implantation. Future iterations may help abate other TAVI pitfalls (paravalvular leaks and conduction abnormalities).93
Regarding procedural guidance per se, we highlight the possible use of extended reality to guide catheter ablation (CommandEP™, SentiAR Inc., United States of America)94 and vascular access either as a stand-alone tool95 or in combination with ultrasound.96,97 Potential advantages include better ergonomics, improved accuracy, less damage to adjacent tissues and lower radiation exposure.95–97
Although promising, thus far, most of the evidence in this area comes from relatively small proof-of-concept studies that are insufficient for now to support their systematic use to guide decision-making and improve clinical outcomes.
Education and trainingSimulations can also play a significant role in education and training. Extended reality and high-fidelity bench simulations may improve trainees’ understanding of complex anatomies/clinical scenarios and provide a safe and reproducible training environment before engaging in actual patient management.23,98 Small prospective trials have shown simulation training's positive influence on trainee-centered outcomes consistently across several procedures in the CV sphere. Young et al. showed that simulation-trained fellows performed better on intra-aortic balloon pump and transvenous pacing placement.99 De Ponti and colleagues obtained similar results for transeptal puncture100 and catheter placement, with the added benefit of reduced fluoroscopy times.101 Despite being increasingly recognized as a valuable teaching method,102 its systematic incorporation in CV training programs is still rare. Lack of dedicated training time, good quality equipment and funding are some of the barriers preventing the shift toward simulation-enhanced education.103
In silico clinical trials and digital twinsComputer simulations are also evolving as a tool to evaluate the safety and efficacy of new drugs, devices, or procedures. They outperform conventional studies in terms of cost, allotted time, need for the use of animal models and patient representativeness. Hence, major regulatory bodies, such as the United States’ Food and Drug Administration (FDA), now support the use of these novel approaches throughout the life cycle of CV interventions, from prototyping to post-market surveillance, to inform better clinical trials.104 Recently, Aguado-Sierra et al. validated Alya (ELEM Biotech, Spain), a computational platform to perform in silico/virtual cardiac trials.105 For example, using hydroxychloroquine and azithromycin, they evaluated the platform's ability to assess different drugs’ proarrhythmic potential and the results were very close to those of traditional clinical trials (21% vs 21.8%).
In silico clinical trials are essentially an extension of the above-mentioned ‘digital twin’ concept.25 Digital twins’ main applications are individual risk prediction and tailored decision support (all available management options can be tested on the digital twin first to select the best possible one). This technology is relatively new and evidence for improved outcomes is lacking. Proof-of-concept studies, such as the Virtual-heart Arrhythmia Risk Predictor (VARP) study used virtual heart models to stratify post-infarction arrhythmia risk.106 The model's risk assessment was superior to current metrics and has the potential to refine patient selection for implantable cardioverter-defibrillators. Regarding treatment strategies specifically, a group from the Cleveland Clinic is running a randomized controlled trial (NCT05181449) to determine whether a digital twin-based treatment plan could improve blood glucose control and, possibly, lead to disease remission in patients with type 2 diabetes.
RoboticsThe use of medical robots has been steadily rising, particularly in the surgical field, due to their potentially lower invasiveness and high precision.107 Initial experience in the cardiovascular field mainly concerned minimally invasive cardiac surgeries, such as coronary artery bypass grafting and mitral valve repair.108,109 Nonetheless, there is increasing interest in using these devices in electrophysiology, structural and coronary interventions to streamline procedures and reduce occupational hazards, such as radiation exposure and musculoskeletal strain.110 Small clinical studies, such as the PRECISE and CORA-PCI studies have demonstrated feasibility and safety in increasingly complex anatomies.111,112 A 2021 meta-analysis of 13 studies (1348 patients) comparing the standard approach and magnetic navigation system-guided ventricular tachycardia ablation showed higher early success rates, less complications and reduced fluoroscopy time with the latter.113 Likewise, in a propensity-score analysis from a Portuguese cohort, magnetic AF ablation required significantly less fluoroscopy time to achieve similar procedural results.114 Robust data, with large-scale randomized controlled trials, is lacking though. Regarding future perspectives, AI-driven robotics is likely the next step for enhanced precision and efficiency.89 The same applies to the imaging domain, where robots are bound to democratize access to echocardiography.115
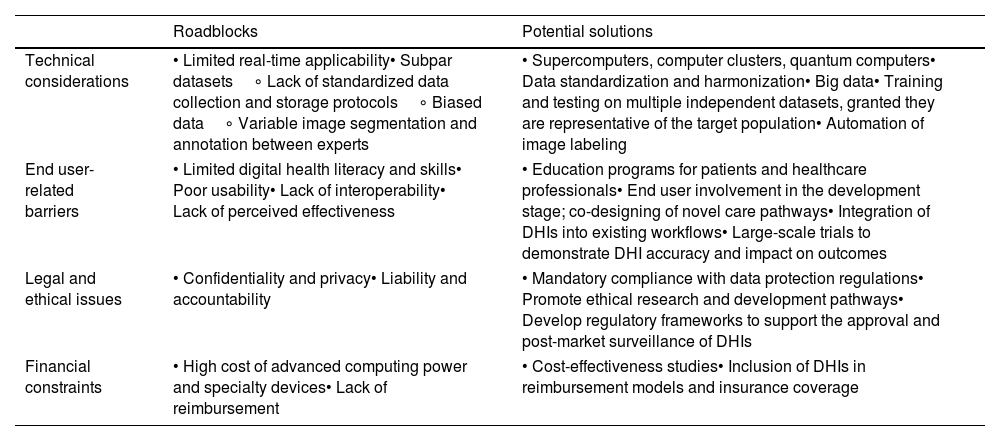
Implementation into clinical practice – roadblocks and limitationsHealthcare's digital transformation has been hampered by several factors that require addressing to ensure we leverage DHIs full potential. This was the main focal point of recent position statements by the European Society of Cardiology, World Heart Federation, American College of Cardiology and American Heart Association.5,16,116,117 Based on their frameworks we have summarized some digital health-related challenges, as well as proposed solutions in Table 2.
Examples of digital health-related roadblocks and potential solutions.
| Roadblocks | Potential solutions | |
|---|---|---|
| Technical considerations | • Limited real-time applicability• Subpar datasets∘ Lack of standardized data collection and storage protocols∘ Biased data∘ Variable image segmentation and annotation between experts | • Supercomputers, computer clusters, quantum computers• Data standardization and harmonization• Big data• Training and testing on multiple independent datasets, granted they are representative of the target population• Automation of image labeling |
| End user-related barriers | • Limited digital health literacy and skills• Poor usability• Lack of interoperability• Lack of perceived effectiveness | • Education programs for patients and healthcare professionals• End user involvement in the development stage; co-designing of novel care pathways• Integration of DHIs into existing workflows• Large-scale trials to demonstrate DHI accuracy and impact on outcomes |
| Legal and ethical issues | • Confidentiality and privacy• Liability and accountability | • Mandatory compliance with data protection regulations• Promote ethical research and development pathways• Develop regulatory frameworks to support the approval and post-market surveillance of DHIs |
| Financial constraints | • High cost of advanced computing power and specialty devices• Lack of reimbursement | • Cost-effectiveness studies• Inclusion of DHIs in reimbursement models and insurance coverage |
DHI: digital health intervention.
The algorithms we previously mentioned are only as good as the data they are trained on. First, any intrinsic biases on the training dataset (e.g. exclusion of an ethnic group, different image acquisition protocols, conflicting data annotation) will negatively affect the models’ output and lead to false generalizations.118 To minimize this risk, the algorithms must be tested on multiple independent datasets and carefully monitored for any partialities to ensure accurate representation of the population they are intended for.118 Second, another common criticism of these models is the lack of ‘interpretability’ and ‘explainability’, the so-called black-box AI. A significant portion of AI algorithms do not provide insight on how they arrived at a given conclusion, preventing proper reliability assessment.119 To address this concerns, major efforts are being made to ‘open the black box’ and/or construct intrinsically explainable/interpretable algorithms (e.g. ECG heatmaps).119 Third, these systems often require large datasets (big data) for training and validation. Although the data exists, it is often not accessible and/or of subpar quality. Heterogeneous data collection and storage methods, lack of universal quality control metrics and issues regarding data ownership render a significant portion of available data unusable. In 2022, the European Commission published a regulation proposal (52022PC0197) to create a European Health Data Space which will hopefully improve health information structures within the European Union and help mitigate some of these limitations.120 Fourth, patient-specific simulations are time-consuming. Advanced computing (e.g. supercomputers, computer clusters or quantum computing) will likely be needed to provide real-time guidance, which could entail a substantial financial burden.121 In this regard, cost-effectiveness studies should not be overlooked.
In an effort to minimize the aforementioned biases and raise the quality of cardiovascular AI-based studies, the European Heart Journal recently published an article recommending careful consideration of five major quality criteria: reproducibility, clear identification of intended use, appropriate sample sizes, software/code availability, and rigorous internal and external validation.122 These guidelines were developed specifically for AI-based products, but their principals also apply to other DHI.
End user-related barriersA 2021 systematic scoping review found that difficult-to-use tools, poor internet connection, and fear of using technology were the most common patient-level barriers, followed by impersonal care delivery, user characteristics (e.g. older age, cognitive impairment, language, values, socioeconomic status, etc.), time spent, and technical concerns.17 On the other hand, physician-related barriers included perceived increase in workload, weak interoperability, unclear benefits, cybersecurity issues, and financial concerns.17 In many cases, development of DHI is not based on the end user's expectations and necessities (i.e. patients and physicians). Their involvement in the research and development stage is rare with implications on their acceptance and usability. A multidisciplinary effort among all stakeholders (patients, clinicians, developers, ethicists, industry, and regulatory/governmental agencies, etc.) should facilitate the creation of user-friendly tools.17 Additionally, DHI implementation should be supported by education programs for patients, caregivers, healthcare professionals and students alike to demystify these novel care pathways and assist them throughout the whole process.123 The lack of integration of DHI into existing systems and processes can be a major deterrent to their use.124 If not fully incorporated into current workflows they will increase the workload rather than lighten it. Therefore, clinician engagement will largely be dependent on the interoperability of DHI. As previously mentioned, most of the evidence in this area comes from small observational studies.125 Large clinical trials focusing on hard outcomes, such as the ongoing HEARTLINE study (NCT04276441), are warranted to increase end user's confidence in these tools. Low socioeconomic status may be the hardest hurdle to overcome in DHI adoption. The development of appropriate reimbursement models for DHI and expansion of internet coverage and speed are key to ensure equitable access.126 In spite of these challenges, a recent nationwide cross-sectional survey found that, in general, Portuguese cardiovascular healthcare professionals have a positive outlook on these tools and are welcoming of the digital transformation.127 Likewise, these technologies are usually very well received by the patients. For example, satisfaction levels with the MESSAGE-HF monitoring strategy were excellent (net promoting score at 180 days: 78.5).47 Nevertheless, clinical outcomes were not improved.
Ethical and legal concernsTesting and use of DHI raises several complex ethical and legal questions about confidentiality and liability. As previously stated, the development and implementation of DHI in clinical practice requires the exchange and analysis of sensitive data. Therefore, strong regulatory oversight is required to ensure data minimization for the intended use and proper anonymization, following the applicable directives, namely the European Union's General Data Protection Regulation (32016R0679)128 and the Privacy and Electronics Communication Directive (02002L0058-20091219).129 Recently, the European Council and Parliament published the first regulatory framework for safe AI utilization, the AI Act. It defines four risk categories: unacceptable, high, limited, and minimal or no risk. Systems that meet the unacceptability criteria will be prohibited. Among the remaining categories, the higher the risk the stricter the legal requirements and supervision. Another key point of discussion relates to what will happen when DHIs fail and the associated legal conundrums. For example, in a case of ECG misinterpretation, would legal liability for an unintended complication be distributed between the physician and the software developer/distributer? In the case of a data privacy breech in a mobile phone app, who is accountable? The software developer, the cloud management service, the healthcare system/institution? Others? All the above? These are just some of the questions that may arise. Accountability issues should be explored to establish legal liability upfront.
On another note, the DHI regulatory landscape is still not fully established. Both the European Medicines Agency and the FDA have published guidelines and regulations on their classification and certification. However, in this ever-growing field, regulations will need to evolve continuously to ensure they are fit to deal with the growing demands. Of note, the number of DHI that reaches the “market” is significantly lower than the number of developed DHI. This may be (at least partially) explained by the long and taxing bureaucratic process. In an attempt to lessen this burden, the FDA created the Digital Health Center of Excellence Services which is meant to guide stakeholders through the process and facilitate timely certification.
Digital health in cardiovascular medicine – Quo Vadis?Despite these limitations, digital health is bound to revolutionize CV care. According to the American Medical Association's Playbook, the key steps to implement a DHI are: (1) Identify a need; (2) Construct a team; (3) Define success/goals; (4) Select the right technology; (5) Get funding; (6) Budget; (7) Workflow integration/design; (8) Care team preparation/education; (9) Patient engagement; (10) Implementation; (11) Evaluation; (12) Scaling.130,131 Nonetheless, many challenges remain, particularly in ensuring data quality and security, and dealing with variations that impact the effectiveness of DHI. These issues highlight the need for continuous research and cooperation among a diverse group of stakeholders, such as medical professionals, engineers, and patients. Addressing these challenges demands standardized data formats, strong encryption for privacy, interpretability to foster trust, extensive training, and improved collaboration among stakeholders. In summary, in ChatGPT's (OpenAI, USA) words: “The fusion of digital technologies with cardiovascular medicine is reshaping healthcare, offering real-time monitoring and personalized treatment plans. This integration has revolutionized diagnosis, management, and prevention of cardiovascular diseases, leveraging wearable devices, advanced imaging, and telemedicine. However, challenges like data security, interoperability, and ethical concerns persist, necessitating careful navigation. The future of cardiovascular digital health hinges on collaborative efforts to address these obstacles and ensure widespread access to innovative technologies. The direction it takes relies on collective commitment from healthcare professionals, technology developers, and policymakers to navigate complexities and ensure progress.”
ConclusionCardiology is undergoing a remarkable transformation, with digital health playing a pivotal role in reshaping CV disease prevention, diagnosis, and management. These technologies will help deliver high-quality patient-centered care in a timely, equitable and cost-conscious way. However, as with any other medical tool, they must withstand rigorous testing and validation before integrating a contemporary cardiologist's armamentarium.
FundingThe authors have no financial relationships.
Conflicts of interestThe authors have no other conflicts of interest to declare.